Cultivating Microalgae in Desert Conditions: Evaluation of the Effect of Light-Temperature Summer Conditions on the Growth and Metabolism of Nannochloropsis QU130
Abstract
:Highlights:
- ➢
- Nannochloropsis QU130 showed a high level of acclimation to high light and temperature
- ➢
- Light and temperature effects on growth and metabolism were found to be related
- ➢
- Biomass productivity improved under temperature cycles with constant light
- ➢
- Light and temperature regimes induced a combined stress with 45% productivity loss
- ➢
- Nannochloropsis QU130 demonstrated benefits for outdoor culture in harsh desert conditions
1. Introduction
2. Materials and Methods
2.1. Photobioreactor
2.2. Strain Cultivation
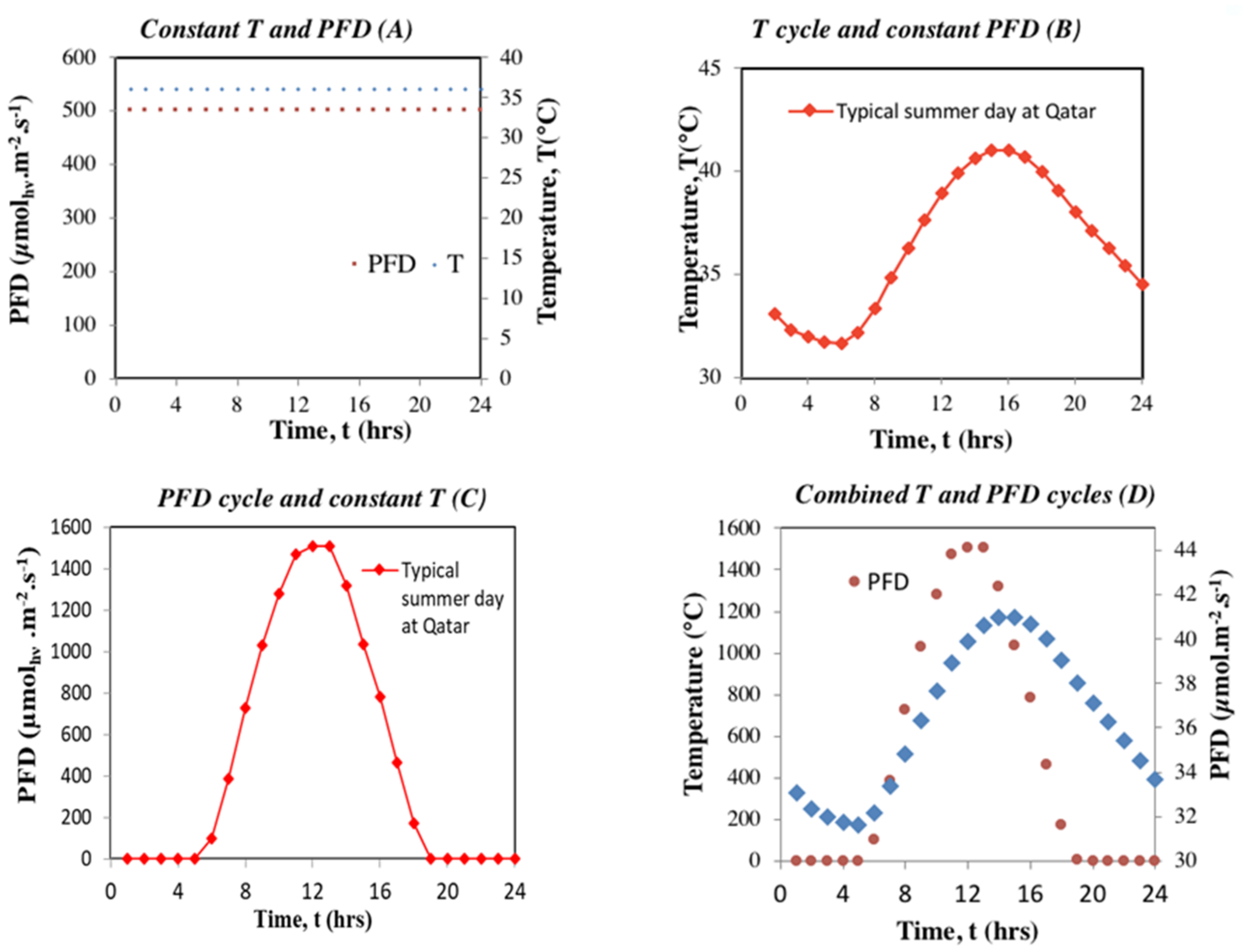
2.2.1. Overview of the Approach
- ➢
- Regime B: Temperature fluctuation, corresponding temperature cycle over 24 h between 32 °C and 41°C with light constant at 500 μmolhν.m−2s−1 (Figure 2)
- ➢
- Regime C: Light fluctuation, corresponding day/night cycle over 24 h between 0 and 1500 μmolhν.m−2 s−1 with temperature constant at 36 °C (Figure 2).
- ➢
- Regime D: Combined light and temperature fluctuations, corresponding light and temperature cycles over 24 h (Figure 2).
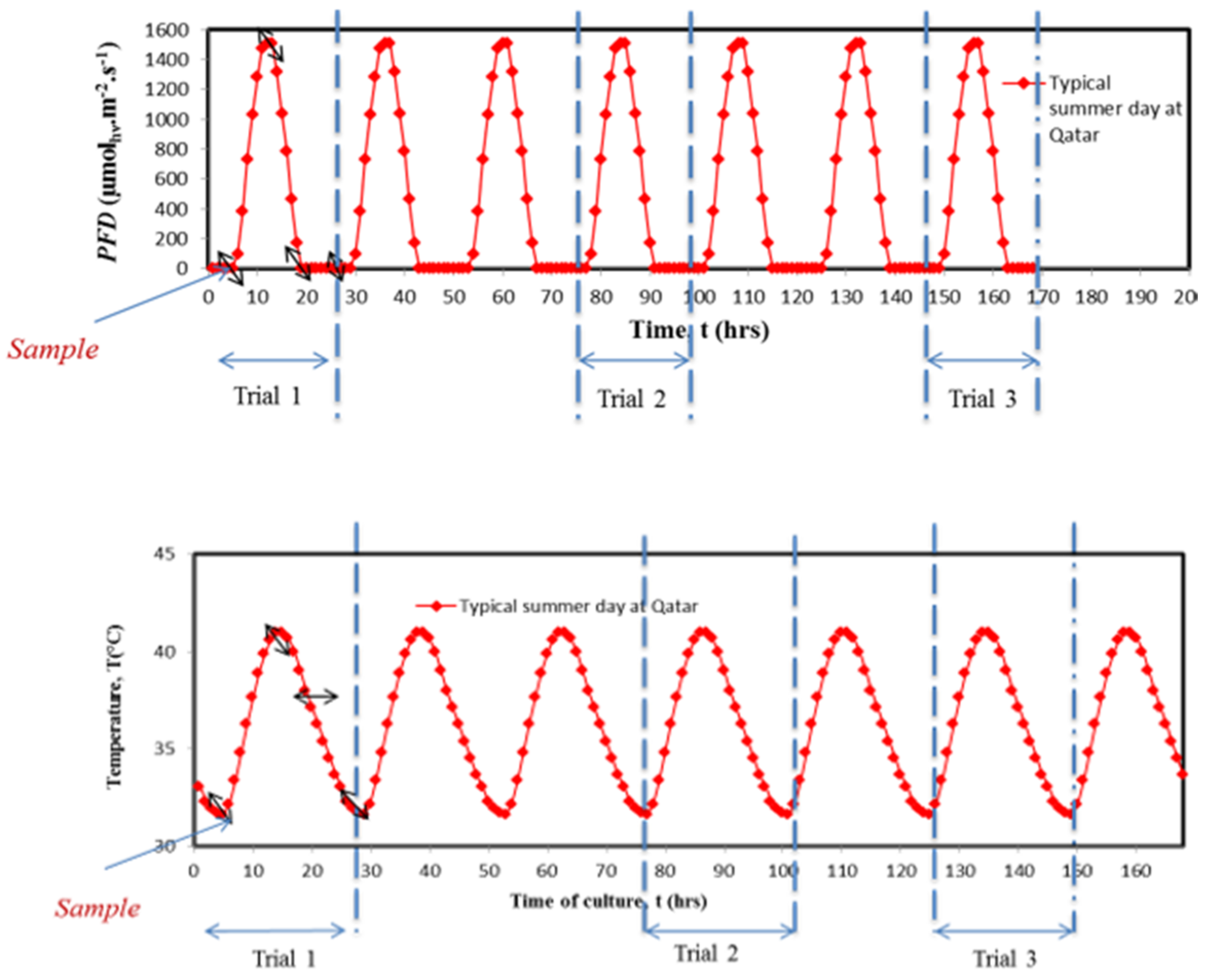
2.2.2. Algae Cultivation under Temperature Cycles (Regime B)
2.2.3. Algae Cultivation under Light Cycles (Regime C)
2.2.4. Algae Cultivation under Combined Day/Night and Temperature Cycles (Regime D)
2.3. Biomass Analysis
2.3.1. Biomass Concentration and Cell Size
2.3.2. Pigments Extraction and Quantification
2.3.3. Total Lipids Extraction
2.3.4. Proteins Extraction
2.3.5. Carbohydrates Extraction and Quantification
2.4. Statistical Analysis
3. Results
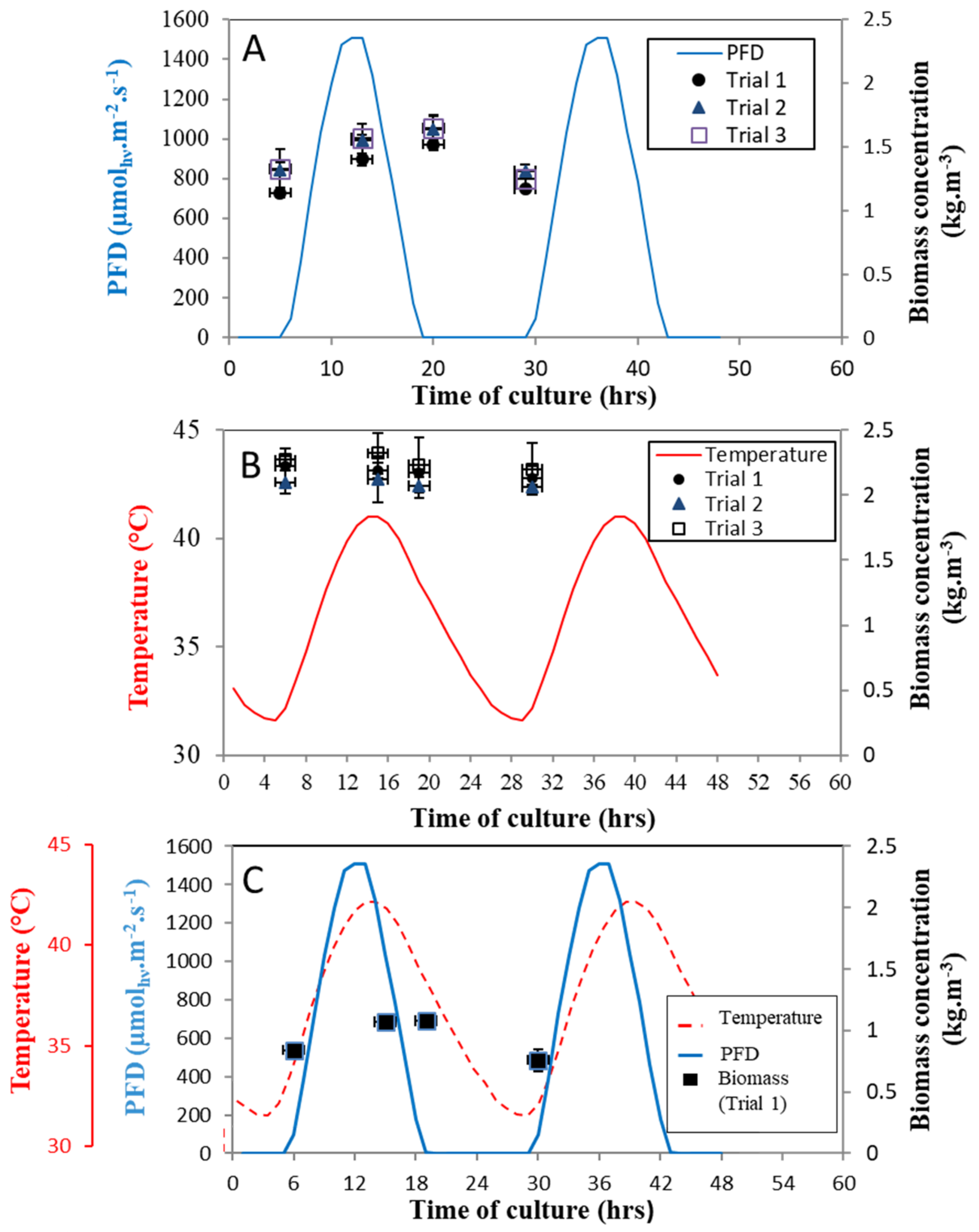
3.1. Assessment of the Light and Temperature Fluctuations on the Algae Growth
3.2. Assessment of Light and Temperature Fluctuations on the Number and Morphology of Algal Cells
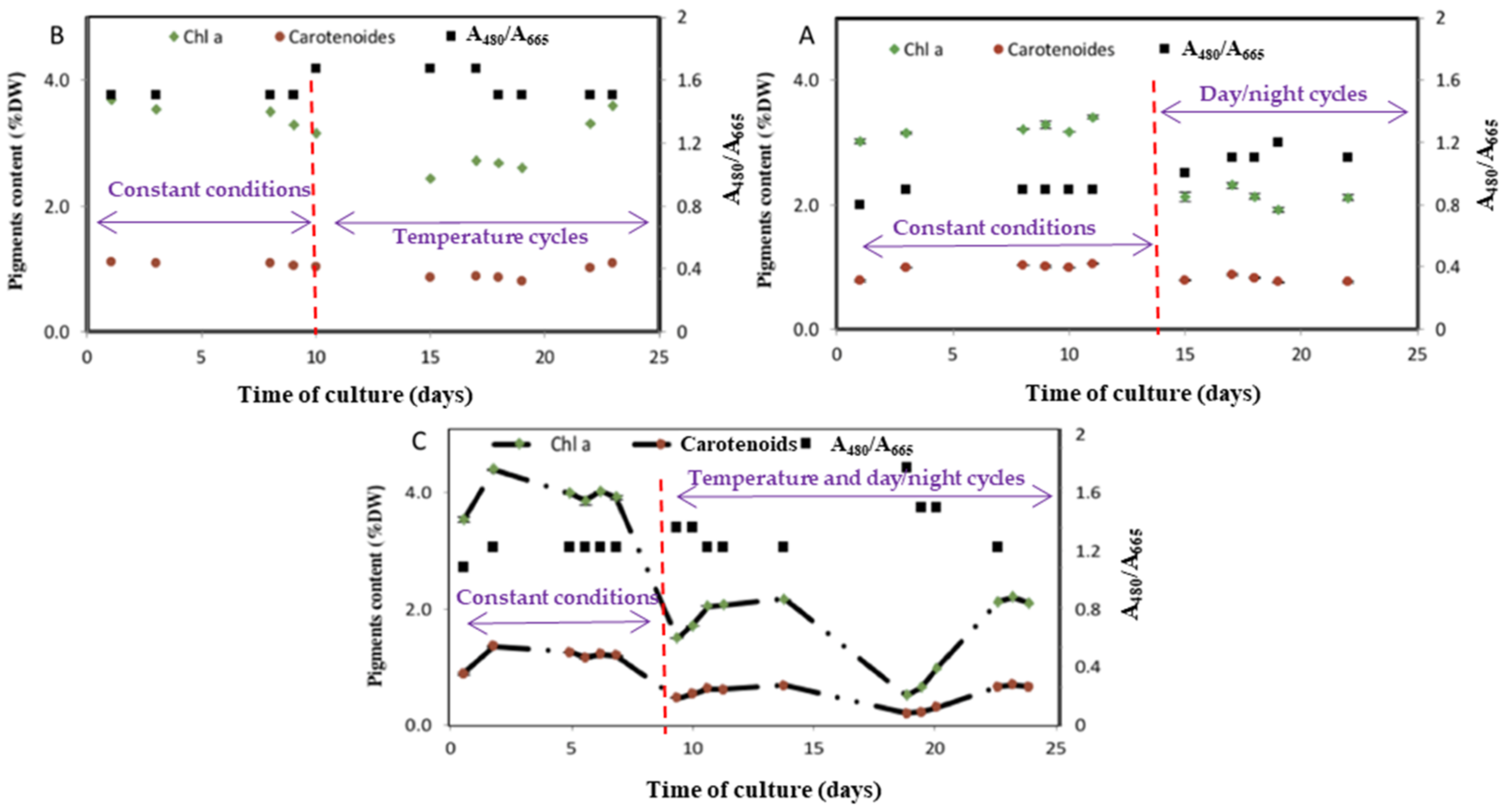
3.3. Assessment of the Light and Temperature Fluctuation on the Photosynthetic Pigments Synthesis
3.4. Metabolism Alterations in Response to Day/Night Cycle and Temperature Fluctuations
4. Conclusions
- -
- Light cycles with constant temperature demonstrated a sharp 50% decrease in biomass concentration when compared to steady-state culture at constant continuous light and temperature (1.9 kg.m−3 to 0.96 kg.m−3), following an acclimation time of around 5 days. This resulted in a biomass productivity of 0.67 kg.m−3.day−1 (i.e., 20.1 g.m−2.day−1), compared to constant light and optimal temperature, which resulted in a maximal volumetric biomass productivity of 1.1 kg.m−3d−1 (i.e., 32 g.m−2.d−1).
- -
- When temperature cycle stress was applied to the steady-state culture under constant light and temperature, a 15% decrease in biomass concentration was first observed (1.75 kg.m−3 to 1.5 kg.m−3), followed by a quick recovery of algae growth indicating rapid acclimation to temperature, leading to a biomass concentration 50% higher than under optimal constant conditions (2.3 kg.m−3). This surprising result can be explained by the N. sp. QU130 strain adapting to the harsh environmental conditions of the Qatar desert from where the strain was isolated [17,18]. In terms of benefits, it also highlights the potentially transitory effect of high temperature on growth. Exposure for a few hours revealed that it benefited the growth rate, with biomass productivity higher than under constant conditions. Consequently, the temperature cycle with constant light recorded the highest volumetric biomass productivity, with a rate of 1.10 kg.m−3 day−1 (i.e., 33 g.m−2.day−1).
- -
- Under conditions representing summer in the Qatar desert, with combined light and temperature cycles, the clear ability of N. sp. QU130 to acclimate to these extreme conditions has been demonstrated. However, the effects of such stressful conditions are also emphasized. The sudden shift from constant conditions to temperature/light cycles requires an acclimation period of around 25 days, with a significant decrease in productivity for the first 2 weeks preceding a slight increase, achieving a stable regime once the culture is acclimated. This resulted in the lowest rate of volumetric biomass productivity of 0.62 kg.m−3.day−1 (i.e., 18.6 g.m−2.day−1).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Markou, G.; Nerantzis, E. Microalgae for high-value compounds and biofuels production: A review with focus on cultivation under stress conditions. Biotechnol. Adv. 2013, 31, 1532–1542. [Google Scholar] [CrossRef]
- Hu, Q. Environmental Effects on Cell Composition. In Handbook of Microalgal Culture; Wiley Online Library: New York, NY, USA, 2013; pp. 114–122. [Google Scholar] [CrossRef]
- Aleya, L.; Dauta, A.; Reynolds, C.S. Endogenous regulation of the growth-rate responses of a spring-dwelling strain of the freshwater alga, Chlorella minutissima, to light and temperature. Eur. J. Protistol. 2011, 47, 239–244. [Google Scholar] [CrossRef]
- Han, F.; Wang, W.; Li, Y.; Shen, G.; Wan, M.; Wang, J. Changes of biomass, lipid content and fatty acids composition under a light–dark cyclic culture of Chlorella pyrenoidosa in response to different temperature. Bioresour. Technol. 2013, 132, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-H.; Chen, C.-Y.; Chang, J.-S. Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour. Technol. 2012, 113, 244–252. [Google Scholar] [CrossRef]
- Tamburic, B.; Guruprasad, S.; Radford, D.T.; Szabó, M.; Lilley, R.M.; Larkum, A.W.D.; Franklin, J.B.; Kramer, D.M.; Blackburn, S.I.; Raven, J.A.; et al. The Effect of Diel Temperature and Light Cycles on the Growth of Nannochloropsis oculata in a Photobioreactor Matrix. PLoS ONE 2014, 9, e86047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruangsomboon, S. Effect of light, nutrient, cultivation time and salinity on lipid production of newly isolated strain of the green microalga, Botryococcus braunii KMITL. Bioresour. Technol. 2012, 109, 261–265. [Google Scholar] [CrossRef]
- Klok, A.J.; Martens, D.E.; Wijffels, R.H.; Lamers, P.P. Simultaneous growth and neutral lipid accumulation in microalgae. Bioresour. Technol. 2013, 134, 233–243. [Google Scholar] [CrossRef]
- Schagerl, M.; Müller, B. Acclimation of chlorophyll a and carotenoid levels to different irradiances in four freshwater cyanobacteria. J. Plant Physiol. 2006, 163, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Demeter, S.; Janda, T.; Kovács, L.; Mende, D.; Wiessner, W. Effects of in vivo CO2-depletion on electron transport and photoinhibition in the green algae, Chlamydobotrys stellata and Chlamydomonas reinhardtii. Biochim. Biophys. Acta BBA Bioenerg. 1995, 1229, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.J.; Lee, Y.K.; Chao, T.M. Effects of temperature and growth phase on lipid and biochemical composition of Isochrysis galbana TK. Environ. Biol. Fishes 1997, 9, 451–457. [Google Scholar] [CrossRef]
- Agrawal, S.C. Factors controlling induction of reproduction in algae—Review: The text. Folia Microbiol. 2012, 57, 387–407. [Google Scholar] [CrossRef]
- Maxwell, D.P.; Falk, S.; Trick, C.G.; Huner, N. Growth at Low Temperature Mimics High-Light Acclimation in Chlorella vulgaris. Plant Physiol. 1994, 105, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, C.; Krauss, R.W. Effects of Temperature & Illuminance on Chlorella Growth Uncoupled from Cell Division. Plant Physiol. 1962, 37, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Pruvost, J.; Goetz, V.; Artu, A.; Das, P.; Al Jabri, H. Thermal modeling and optimization of microalgal biomass production in the harsh desert conditions of State of Qatar. Algal Res. 2019, 38, 101381. [Google Scholar] [CrossRef]
- Pruvost, J.; Van Vooren, G.; Cogne, G.; Legrand, J. Investigation of biomass and lipids production with Neochloris oleoabundans in photobioreactor. Bioresour. Technol. 2009, 100, 5988–5995. [Google Scholar] [CrossRef] [Green Version]
- Saadaoui, I.; Al Emadi, M.; Bounnit, T.; Schipper, K.; Al Jabri, H. Cryopreservation of microalgae from desert environments of Qatar. Environ. Biol. Fishes 2015, 28, 2233–2240. [Google Scholar] [CrossRef]
- Saadaoui, I.; Al Ghazal, G.; Bounnit, T.; Al Khulaifi, F.; Al Jabri, H.; Potts, M. Evidence of thermo and halotolerant Nannochloris isolate suitable for biodiesel production in Qatar Culture Collection of Cyanobacteria and Microalgae. Algal Res. 2016, 14, 39–47. [Google Scholar] [CrossRef]
- Berges, J.A.; Franklin, D.J.; Harrison, P.J. Evolution of an Artificial Seawater Medium: Improvements In Enriched Seawater, Artificial Water over the Last Two Decades. J. Phycol. 2001, 37, 1138–1145. [Google Scholar] [CrossRef]
- Taleb, A.; Kandilian, R.; Touchard, R.; Montalescot, V.; Rinaldi, T.; Taha, S.; Takache, H.; Marchal, L.; Legrand, J.; Pruvost, J. Screening of freshwater and seawater microalgae strains in fully controlled photobioreactors for biodiesel production. Bioresour. Technol. 2016, 218, 480–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porra, R.; Thompson, W.; Kriedemann, P. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta BBA Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Arora, N.; Patel, A.; Pruthi, P.A.; Pruthi, V. Synergistic dynamics of nitrogen and phosphorous influences lipid productivity in Chlorella minutissima for biodiesel production. Bioresour. Technol. 2016, 213, 79–87. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-Dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Dubois, M.Y.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F.G. A Colorimetric Method for the Determination of Sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Merchuk, J.C. A model integrating fluid dynamics in photosynthesis and photoinhibition processes. Chem. Eng. Sci. 2001, 56, 3527–3538. [Google Scholar] [CrossRef]
- Taleb, A.; Legrand, J.; Takache, H.; Taha, S.; Pruvost, J. Investigation of lipid production by nitrogen-starved Parachlorella kessleri under continuous illumination and day/night cycles for biodiesel application. Environ. Biol. Fishes 2017, 30, 761–772. [Google Scholar] [CrossRef]
- Singh, S.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Salvucci, M.E.; Crafts-Brandner, S.J. Inhibition of photosynthesis by heat stress: The activation state of Rubisco as a limiting factor in photosynthesis. Physiol. Plant. 2004, 120, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B Biol. 2014, 137, 116–126. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J. Effects of heat stress on photosynthetic electron transport in a marine cyanobacterium Arthrospira sp. Environ. Biol. Fishes 2016, 28, 757–763. [Google Scholar] [CrossRef]
- Huertas, I.E.; Rouco, M.; López-Rodas, V.; Costas, E. Warming will affect phytoplankton differently: Evidence through a mechanistic approach. Proc. R. Soc. B Biol. Sci. 2011, 278, 3534–3543. [Google Scholar] [CrossRef] [Green Version]
- Rhee, G.-Y.; Gotham, I.J. The effect of environmental factors on phytoplankton growth: Temperature and the interactions of temperature with nutrient limitation. Limnol. Oceanogr. 1981, 26, 635–648. [Google Scholar] [CrossRef]
- Loera-Quezada, M.M.; Angeles, G.; Olguín, E.J. Effect of irradiance on the cell density, size and lipid accumulation of Neochloris oleoabundans. Rev. Latinoam. Biotecnol. Ambient. Algal 2011, 2, 8. [Google Scholar]
- Bišová, K.; Zachleder, V. Cell-cycle regulation in green algae dividing by multiple fission. J. Exp. Bot. 2014, 65, 2585–2602. [Google Scholar] [CrossRef]
- Masamoto, K.; Furukawa, K.-I. Accumulation of zeaxanthin in cells of the cyanobacterium, Synechococcus sp. Strain PCC 7942 grown under high irradiance. J. Plant Physiol. 1997, 151, 257–261. [Google Scholar] [CrossRef]
- Govender, T.; Ramanna, L.; Rawat, I.; Bux, F. BODIPY staining, an alternative to the Nile Red fluorescence method for the evaluation of intracellular lipids in microalgae. Bioresour. Technol. 2012, 114, 507–511. [Google Scholar] [CrossRef]
- Morris, I.; Glover, H.E.; Yentsch, C.S. Products of photosynthesis by marine phytoplankton: The effect of environmental factors on the relative rates of protein synthesis. Mar. Biol. 1974, 27, 1–9. [Google Scholar] [CrossRef]
- Cuhel, R.L.; Ortner, P.B.; Lean, D.R.S. Night synthesis of protein by algae. Limnol. Oceanogr. 1984, 29, 731–744. [Google Scholar] [CrossRef]
- Iqbal, M.; Zafar, S.I. Effects of photon flux density, CO2, aeration rate, and inoculum density on growth and extracellular polysaccharide production byPorphyridium cruentum. Folia Microbiol. 1993, 38, 509–514. [Google Scholar] [CrossRef]
- Smith, R.; Cavaletto, J.; Eadie, B.; Gardner, W. Growth and lipid composition of high Arctic ice algae during the spring bloom at Resolute, Northwest Territories, Canada. Mar. Ecol. Prog. Ser. 1993, 97, 19–29. [Google Scholar] [CrossRef]
- Raven, J.A.; Beardall, J. Carbohydrate metabolism and respiration in algae. In Photosynthesis in Algae; Springer: Berlin/Heidelberg, Germany, 2003; pp. 205–224. [Google Scholar]
- Granum, E.; Myklestad, S.M. A simple combined method for determination of β-1,3-glucan and cell wall polysaccharides in diatoms. Hydrobiology 2002, 477, 155–161. [Google Scholar] [CrossRef]
- Pruvost, J.; Cornet, J.; Le Borgne, F.; Goetz, V.; Legrand, J. Theoretical investigation of microalgae culture in the light changing conditions of solar photobioreactor production and comparison with cyanobacteria. Algal Res. 2015, 10, 87–99. [Google Scholar] [CrossRef] [Green Version]



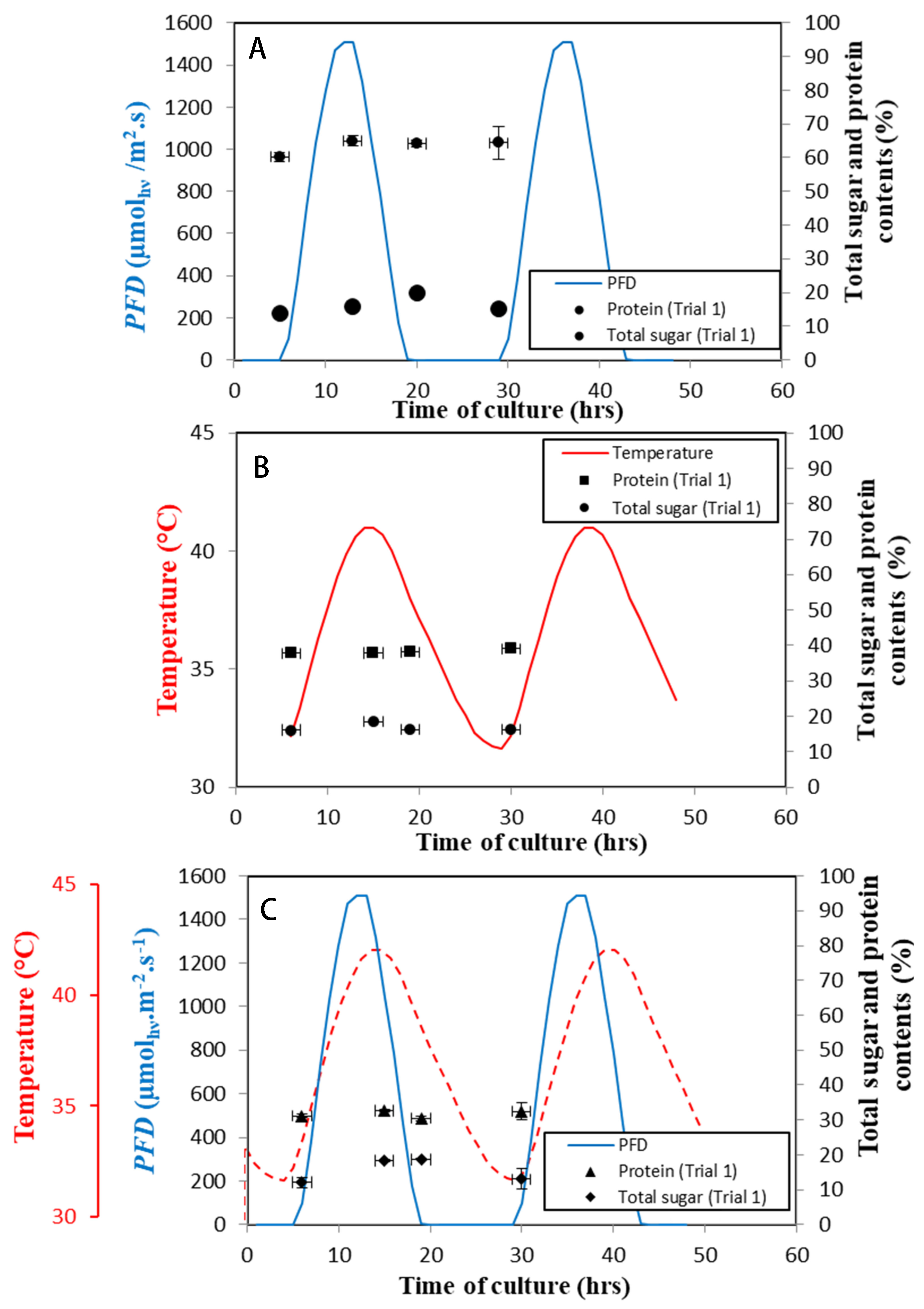
| Dawn | Midday | Dusk | End of the Day | |
|---|---|---|---|---|
| Regime B | 2.5 0.06 | 3.13 0.06 | 2.7 0.1 | 2.4 0.1 |
| Regime C | 2.31 0.03 | 2.7 0.18 | 2.68 0.09 | 2 0.04 |
| Regime D | 2.57 0.06 | 2.8 0.06 | 2.99 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Jabri, H.; Taleb, A.; Touchard, R.; Saadaoui, I.; Goetz, V.; Pruvost, J. Cultivating Microalgae in Desert Conditions: Evaluation of the Effect of Light-Temperature Summer Conditions on the Growth and Metabolism of Nannochloropsis QU130. Appl. Sci. 2021, 11, 3799. https://doi.org/10.3390/app11093799
Al Jabri H, Taleb A, Touchard R, Saadaoui I, Goetz V, Pruvost J. Cultivating Microalgae in Desert Conditions: Evaluation of the Effect of Light-Temperature Summer Conditions on the Growth and Metabolism of Nannochloropsis QU130. Applied Sciences. 2021; 11(9):3799. https://doi.org/10.3390/app11093799
Chicago/Turabian StyleAl Jabri, Hareb, Aumaya Taleb, Raphaelle Touchard, Imen Saadaoui, Vincent Goetz, and Jeremy Pruvost. 2021. "Cultivating Microalgae in Desert Conditions: Evaluation of the Effect of Light-Temperature Summer Conditions on the Growth and Metabolism of Nannochloropsis QU130" Applied Sciences 11, no. 9: 3799. https://doi.org/10.3390/app11093799
APA StyleAl Jabri, H., Taleb, A., Touchard, R., Saadaoui, I., Goetz, V., & Pruvost, J. (2021). Cultivating Microalgae in Desert Conditions: Evaluation of the Effect of Light-Temperature Summer Conditions on the Growth and Metabolism of Nannochloropsis QU130. Applied Sciences, 11(9), 3799. https://doi.org/10.3390/app11093799






