Extending Cooling Rate Performance of Fast Scanning Chip Calorimetry by Liquid Droplet Cooling
Abstract
:1. Introduction
2. Experimental
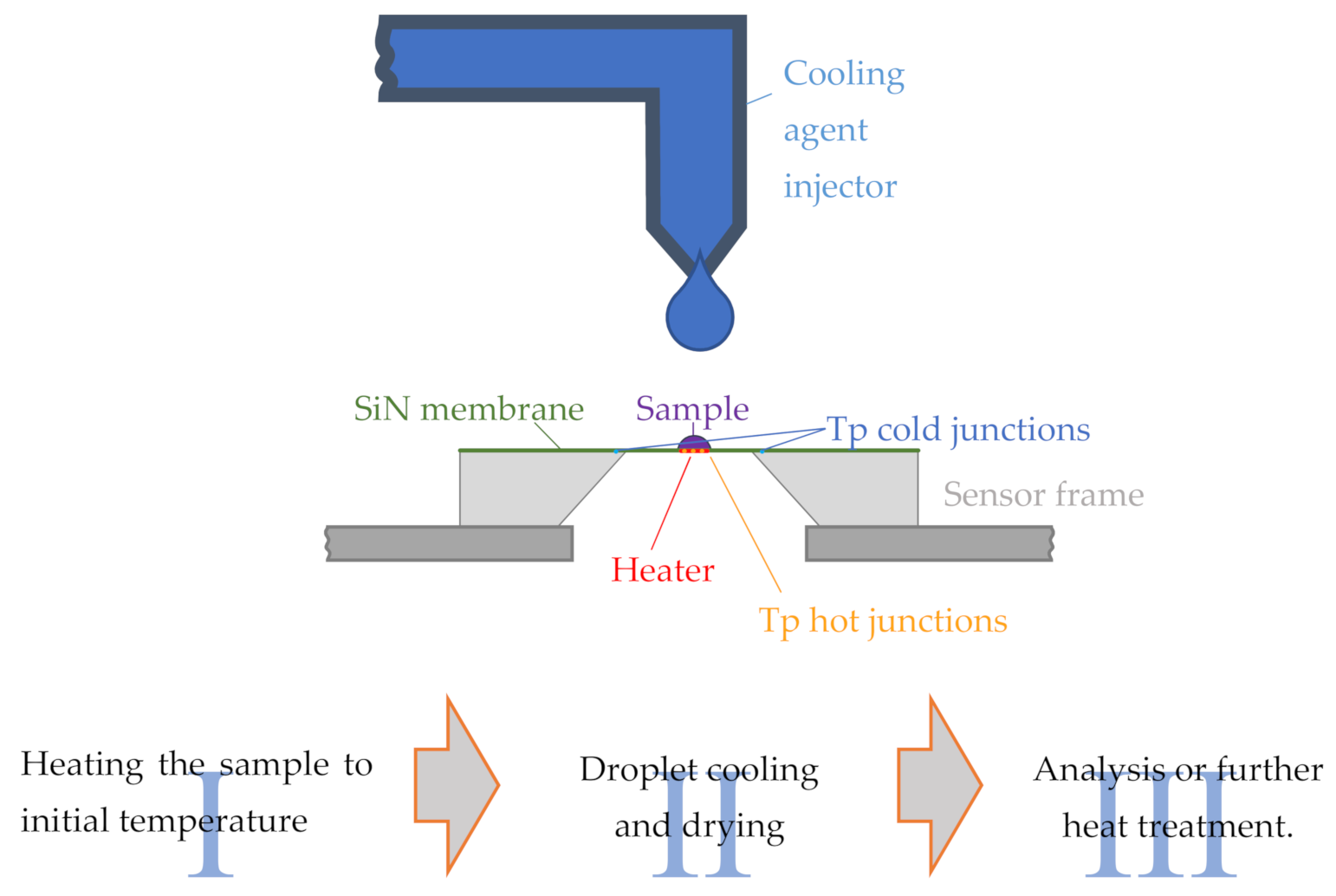
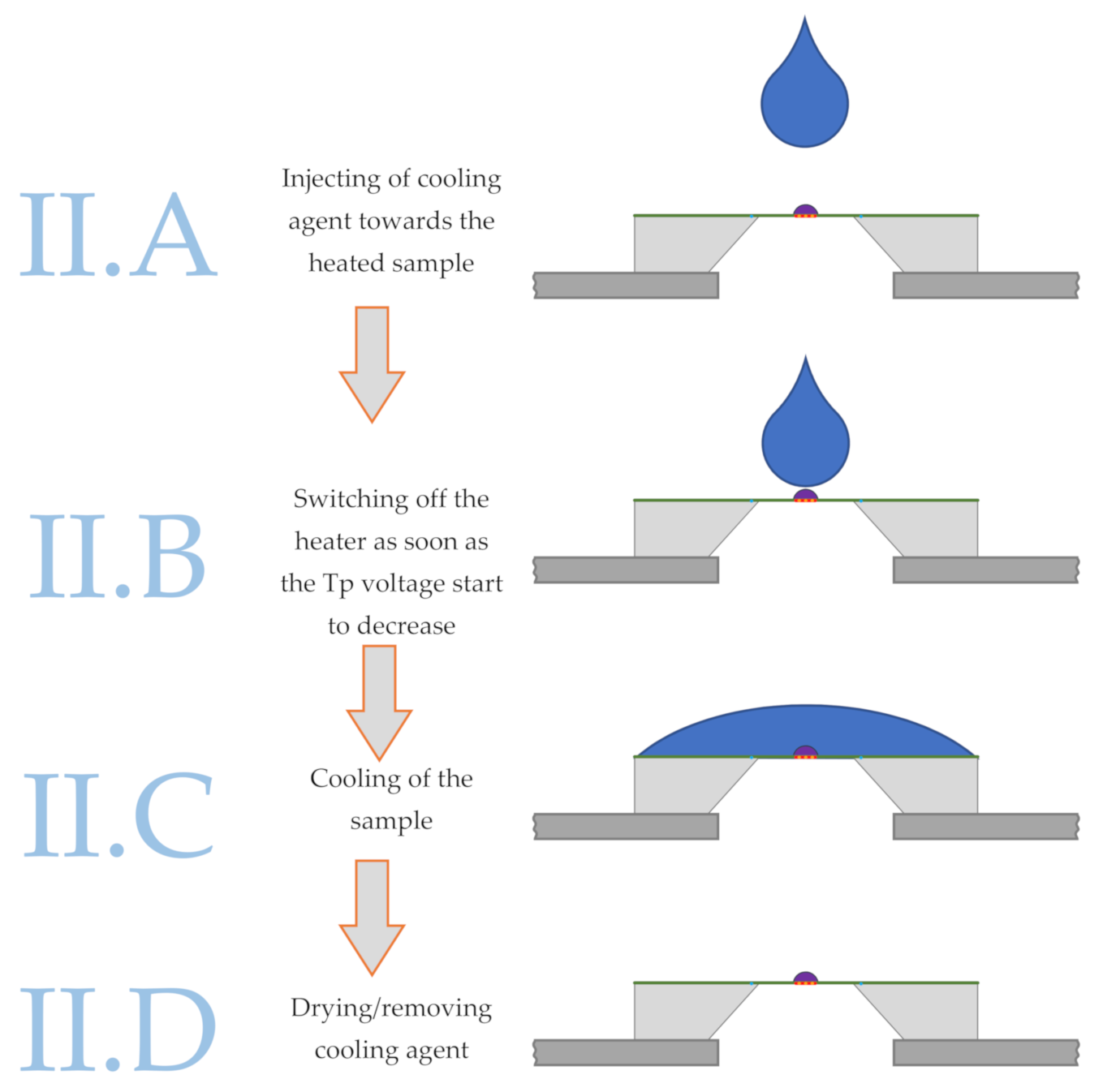
2.1. Liquid Droplet Cooling
- Heating the sample by the internal sensor heater to the start temperature, commonly above the melting temperature.
- Cooling the sample by synchronous deposition of a liquid droplet of the coolant and switching off the internal heater.
- Analyzing the obtained structure by a heating scan or other combined experimental techniques or performing a further heat treatment.
2.2. Implementation of the Liquid Droplet Cooling into the FSC Electronics
2.3. Materials and Sensors
3. Results
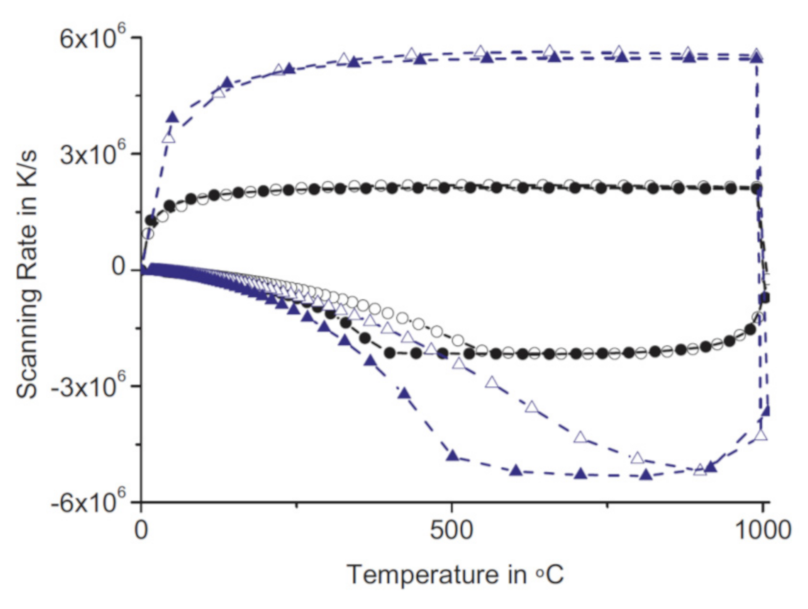
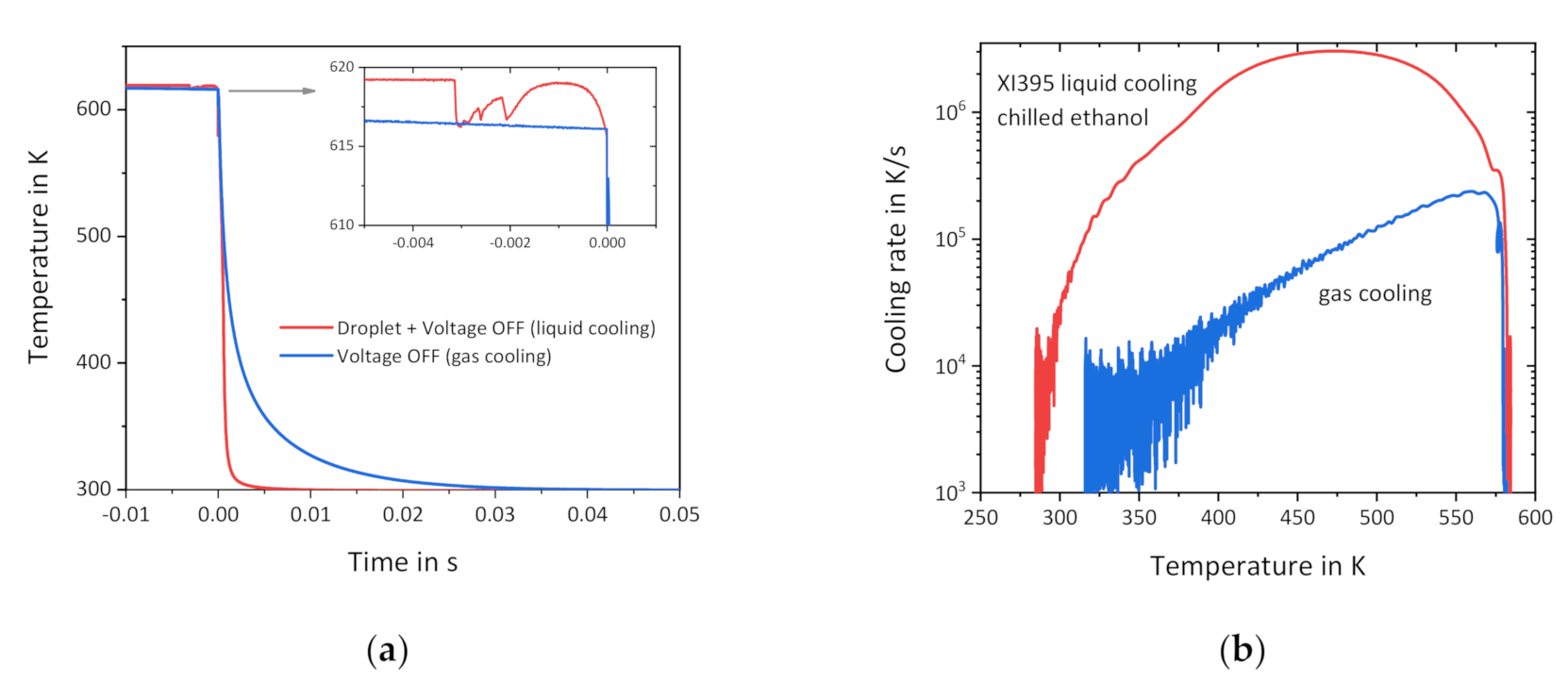
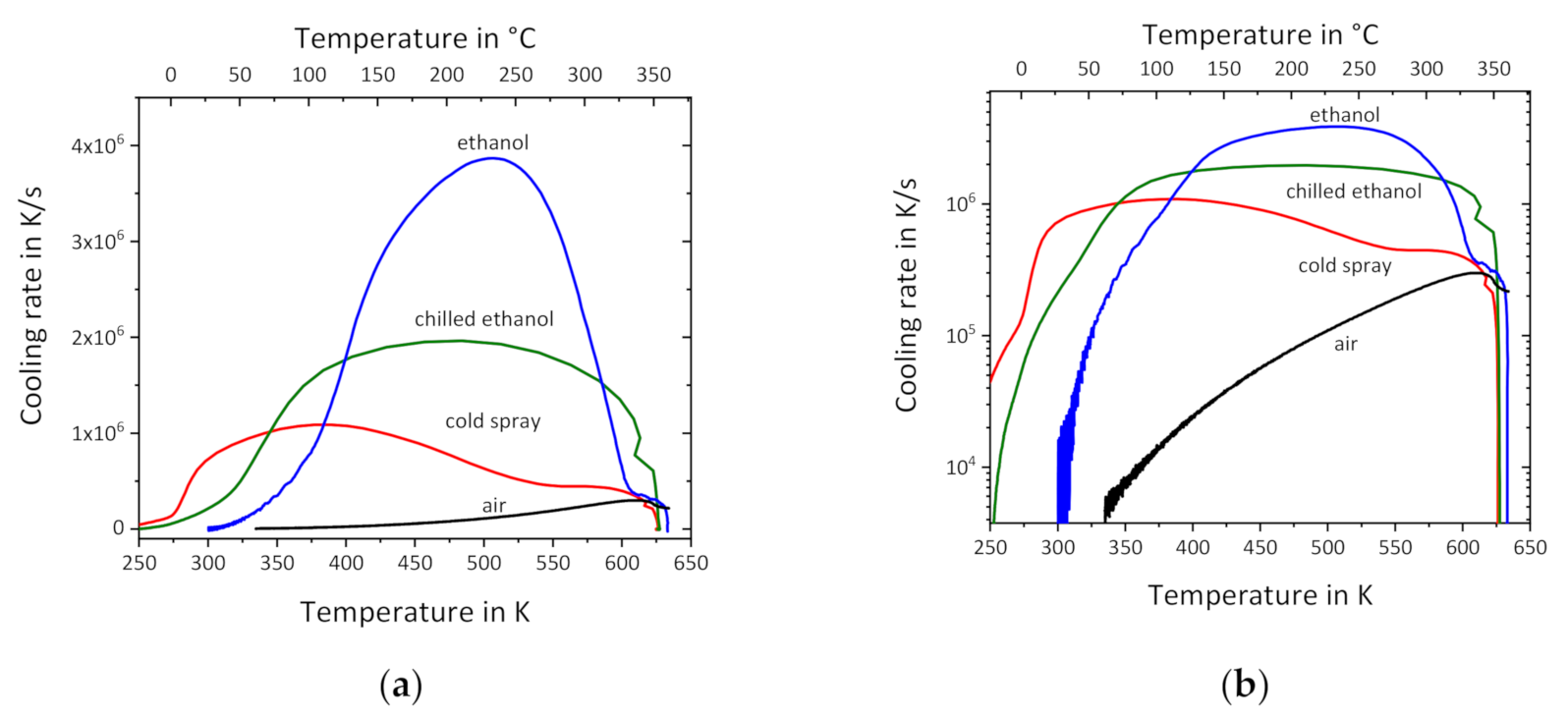
3.1. Liquid Cooling of Empty Sensors
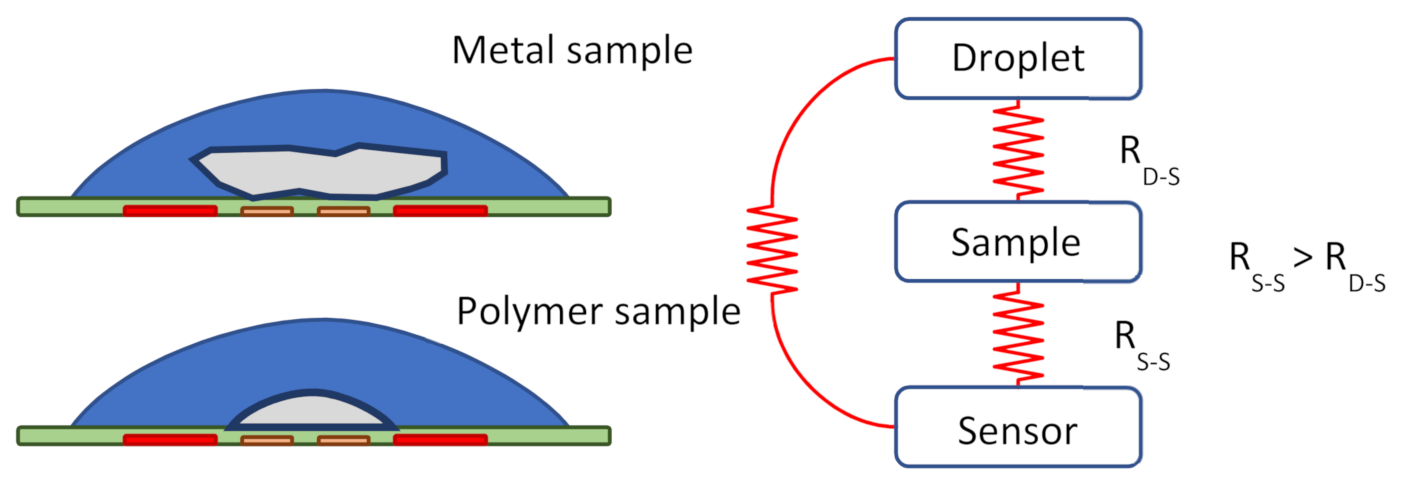
3.2. Liquid Cooling of Sensors with Samples
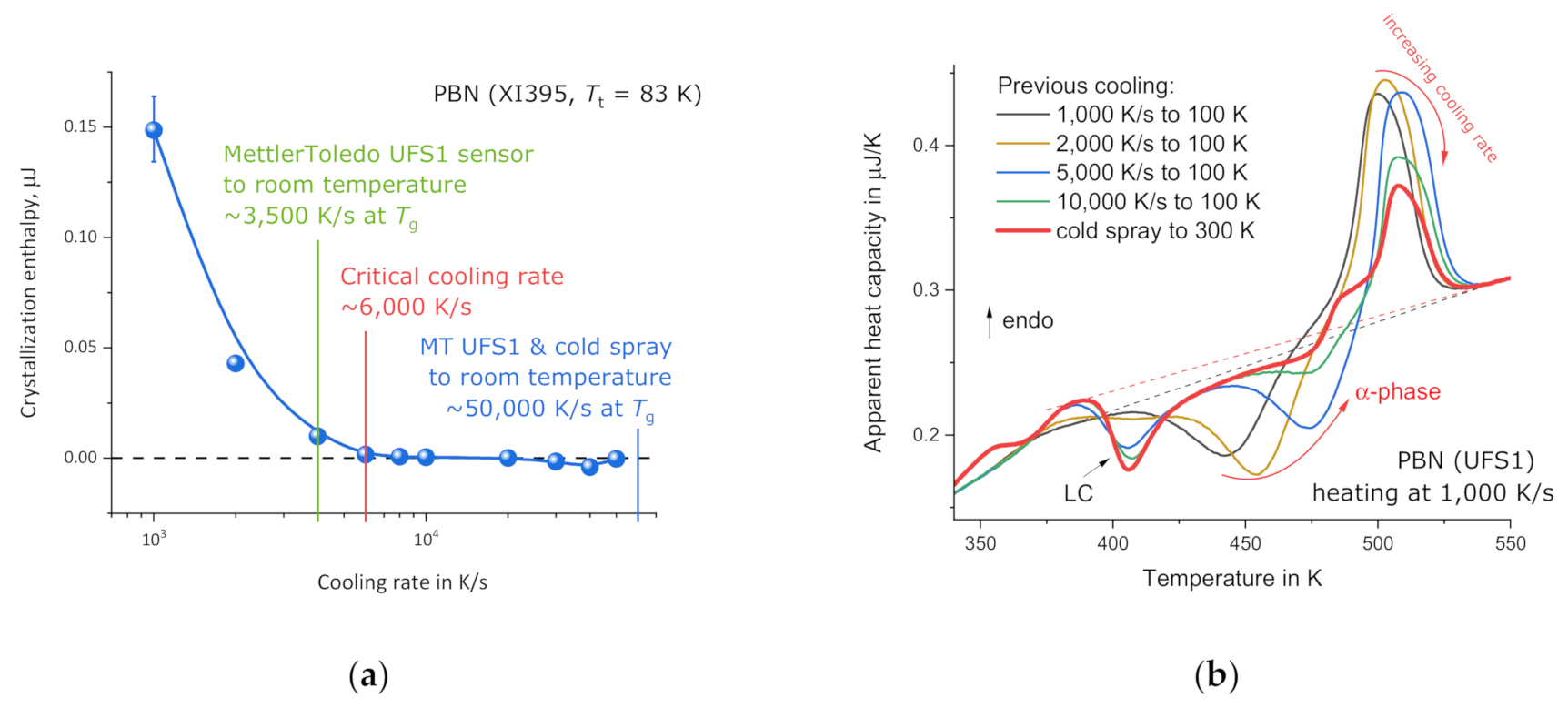
3.3. PBN Vitrification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yi, F.; LaVan, D.A. Nanocalorimetry: Exploring materials faster and smaller. Appl. Phys. Rev. 2019, 6, 031302. [Google Scholar] [CrossRef]
- Milkereit, B.; Reich, M.; Kessler, O. Detection of Quench Induced Precipitation in Al Alloys by Dilatometry. Mater. Sci. Forum 2016, 877, 147–152. [Google Scholar] [CrossRef]
- Zohrabyan, D.; Milkereit, B.; Shick, C.; Kessler, O. Continuous cooling precipitation diagram of high alloyed Al-Zn-Mg-Cu 7049A alloy. In Proceedings of the First Asian Conference on Aluminum Alloys, Beijing, China, 13–17 October 2013. [Google Scholar]
- Syazwan, M.; Sasaki, T. Rapid crystallization and mesophase formation of poly(L-lactic acid) during precipitation from a solution. e-Polymers 2018, 18, 331–337. [Google Scholar] [CrossRef]
- De Santis, F.; Adamovsky, S.; Titomanlio, A.G.; Schick, C. Scanning Nanocalorimetry at High Cooling Rate of Isotactic Polypropylene. Macromolecules 2006, 39, 2562–2567. [Google Scholar] [CrossRef]
- Minakov, A.A.; Schick, C. Ultrafast thermal processing and nanocalorimetry at heating and cooling rates up to 1MK/s. Rev. Sci. Instrum. 2007, 78, 073902. [Google Scholar] [CrossRef]
- Minakov, A.A.; Adamovsky, S.A.; Schick, C. Non-adiabatic thin-film (chip) nanocalorimetry. Thermochim. Acta 2005, 432, 177–185. [Google Scholar] [CrossRef]
- Xensor. Gas Nanocalorimeters. Available online: https://xensor.nl/images/pdf-files/nanogas3939.pdf (accessed on 15 April 2021).
- Minakov, A.A.; Van Herwaarden, A.; Wien, W.; Wurm, A.; Schick, C. Advanced nonadiabatic ultrafast nanocalorimetry and superheating phenomenon in linear polymers. Thermochim. Acta 2007, 461, 96–106. [Google Scholar] [CrossRef]
- Klement, W.; Willens, R.H.; Duwez, P.O.L. Non-crystalline Structure in Solidified Gold–Silicon Alloys. Nat. Cell Biol. 1960, 187, 869–870. [Google Scholar] [CrossRef]
- Liebermann, H.; Graham, C. Production of amorphous alloy ribbons and effects of apparatus parameters on ribbon dimensions. IEEE Trans. Magn. 1976, 12, 921–923. [Google Scholar] [CrossRef]
- Toda, A.; Taguchi, K.; Kono, G.; Nozaki, K. Crystallization and melting behaviors of poly(vinylidene fluoride) examined by fast-scan calorimetry: Hoffman-Weeks, Gibbs-Thomson and thermal Gibbs-Thomson plots. Polymer 2019, 169, 11–20. [Google Scholar] [CrossRef]
- Brucato, V.M.B.; De Santis, F.; Lamberti, G.; Titomanlio, G. A new method for on-line monitoring of non isothermal crystallization kinetics of polymers. Polym. Bull. 2002, 48, 207–212. [Google Scholar] [CrossRef]
- Zia, Q.; Androsch, R.; Radusch, H.-J.; Piccarolo, S. Morphology, reorganization and stability of mesomorphic nanocrystals in isotactic polypropylene. Polymer 2006, 47, 8163–8172. [Google Scholar] [CrossRef]
- Cavallo, D.; Gardella, L.; Alfonso, G.C.; Portale, G.; Balzano, L.; Androsch, R. Effect of cooling rate on the crystal/mesophase polymorphism of polyamide 6. Colloid Polym. Sci. 2011, 289, 1073–1079. [Google Scholar] [CrossRef]
- Mollova, A.; Androsch, R.; Mileva, D.; Gahleitner, M.; Funari, S.S. Crystallization of isotactic polypropylene containing beta-phase nucleating agent at rapid cooling. Eur. Polym. J. 2013, 49, 1057–1065. [Google Scholar] [CrossRef] [Green Version]
- Mileva, D.; Cavallo, D.; Gardella, L.; Alfonso, G.C.; Portale, G.; Balzano, L.; Androsch, R. In situ X-ray analysis of mesophase formation in random copolymers of propylene and 1-butene. Polym. Bull. 2011, 67, 497–510. [Google Scholar] [CrossRef]
- Toda, A.; Kojima, I.; Hikosaka, M. Melting Kinetics of Polymer Crystals with an Entropic Barrier. Macromolecules 2008, 41, 120–127. [Google Scholar] [CrossRef]
- Greer, A.L. Metallic Glasses. Science 1995, 267, 1947–1953. [Google Scholar] [CrossRef]
- Zhuravlev, E.; Schick, C. Fast scanning power compensated differential scanning nano-calorimeter: 2. Heat capacity analysis. Thermochim. Acta 2010, 505, 14–21. [Google Scholar] [CrossRef]
- Quick, C.; Schawe, J.; Uggowitzer, P.; Pogatscher, S. Measurement of specific heat capacity via fast scanning calorimetry—Accuracy and loss corrections. Thermochim. Acta 2019, 677, 12–20. [Google Scholar] [CrossRef]
- Cebe, P.; Partlow, B.P.; Kaplan, D.L.; Wurm, A.; Zhuravlev, E.; Schick, C. Using flash DSC for determining the liquid state heat capacity of silk fibroin. Thermochim. Acta 2015, 615, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Bolmatenkov, D.N.; Yagofarov, M.I.; Mukhametzyanov, T.A.; Ziganshin, M.A.; Schick, C.; Solomonov, B.N. A new method for heat capacity determination in supercooled liquid state using fast scanning calorimetry: Thermochemical study of 9,9′-bifluorenyl. Thermochim. Acta 2020, 694, 178805. [Google Scholar] [CrossRef]
- Minakov, A.A.; Schick, C. Heat conduction in ultrafast thin-film nanocalorimetry. Thermochim. Acta 2016, 640, 42–51. [Google Scholar] [CrossRef]
- Baeten, D.; Mathot, V.B.F.; Pijpers, T.F.J.; Verkinderen, O.; Portale, G.; Van Puyvelde, P.; Goderis, B. Simultaneous Synchrotron WAXD and Fast Scanning (Chip) Calorimetry: On the (Isothermal) Crystallization of HDPE and PA11 at High Supercoolings and Cooling Rates up to 200 °C s−1. Macromol. Rapid Commun. 2015, 36, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhuravlev, E.; Androsch, R.; Schick, C. Visualization of Polymer Crystallization by In Situ Combination of Atomic Force Microscopy and Fast Scanning Calorimetry. Polymers 2019, 11, 890. [Google Scholar] [CrossRef] [Green Version]
- Androsch, R.; Di Lorenzo, M.L.; Schick, C. Optical Microscopy to Study Crystal Nucleation in Polymers Using a Fast Scanning Chip Calorimeter for Precise Control of the Nucleation Pathway. Macromol. Chem. Phys. 2017, 219, 1700479. [Google Scholar] [CrossRef]
- Minakov, A.A.; Schick, C. Dynamics of the temperature distribution in ultra-fast thin-film calorimeter sensors. Thermochim. Acta 2015, 603, 205–217. [Google Scholar] [CrossRef]
- Schröter, C. Fast Droplet Cooling of Bulk Tellurium on a Chip Calorimeter. Master’s Thesis, Institute of Physics, Rostock University, Rostock, Germany, 2015. [Google Scholar]
- Zhuravlev, E.; Schick, C. Fast scanning power compensated differential scanning nano-calorimeter: 1. The device. Thermochim. Acta 2010, 505, 1–13. [Google Scholar] [CrossRef]
- Zhuravlev, E.; Madhavi, V.; Lustiger, A.; Androsch, R.; Schick, C. Crystallization of Polyethylene at Large Undercooling. ACS Macro Lett. 2016, 5, 365–370. [Google Scholar] [CrossRef]
- Bourque, A.J.; Locker, C.R.; Tsou, A.H.; Vadlamudi, M. Nucleation and mechanical enhancements in polyethylene-graphene nanoplate composites. Polymer 2016, 99, 263–272. [Google Scholar] [CrossRef]
- Minakov, A.; Morikawa, J.; Ryu, M.; Zhuravlev, E.; Schick, C. Variations of interfacial thermal conductance at melting and crystallization of an indium micro-particle in contact with a solid. Mater. Des. 2021, 201, 109475. [Google Scholar] [CrossRef]
- Beck, M.; Schmidt, C.; Ahrenberg, M.; Schick, C.; Kragl, U.; Keßler, O. The Ideal Quenching Medium?—Characterisation of Ionic Liquids for Heat Treatment of Metallic Components. HTM J. Heat Treat. Mater. 2013, 68, 214–223. [Google Scholar] [CrossRef]
- Ding, Q.; Soccio, M.; Lotti, N.; Cavallo, D.; Androsch, R. Melt Crystallization of Poly(butylene 2,6-naphthalate). Chin. J. Polym. Sci. 2019, 38, 311–322. [Google Scholar] [CrossRef]
- Konishi, T.; Nishida, K.; Matsuba, G.; Kanaya, T. Mesomorphic Phase of Poly(butylene-2,6-naphthalate). Macromolecules 2008, 41, 3157–3161. [Google Scholar] [CrossRef]
- Detailed Technical Information about FSC Chips Are Available by Xensor Integration®. 2015. Available online: https://xensor.nl/images/pdf-files/Calorimeter_chips/nanogas-opto-xen-39476.pdf (accessed on 15 April 2021).
- Anton, A.M.; Zhuravlev, E.; Kossack, W.; Andrianov, R.; Schick, C.; Kremer, F. Fingerprints of homogeneous nucleation and crystal growth in polyamide 66 as studied by combined infrared spectroscopy and fast scanning chip calorimetry. Colloid Polym. Sci. 2020, 298, 697–706. [Google Scholar] [CrossRef]
- Androsch, R.; Soccio, M.; Lotti, N.; Cavallo, D.; Schick, C. Cold-crystallization of poly(butylene 2,6-naphthalate) following Ostwald’s rule of stages. Thermochim. Acta 2018, 670, 71–75. [Google Scholar] [CrossRef]
- Zhuravlev, E.; Schmelzer, J.W.P.; Abyzov, A.S.; Fokin, V.M.; Androsch, R.; Schick, C.; Schmelzer, J.W.P. Experimental Test of Tammann’s Nuclei Development Approach in Crystallization of Macromolecules. Cryst. Growth Des. 2015, 15, 786–798. [Google Scholar] [CrossRef]
- Androsch, R.; Zhuravlev, E.; Schick, C. Solid-state reorganization, melting and melt-recrystallization of conformationally disordered crystals (α′-phase) of poly (l-lactic acid). Polymer 2014, 55, 4932–4941. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuravlev, E.; Jiang, J.; Zhou, D.; Androsch, R.; Schick, C. Extending Cooling Rate Performance of Fast Scanning Chip Calorimetry by Liquid Droplet Cooling. Appl. Sci. 2021, 11, 3813. https://doi.org/10.3390/app11093813
Zhuravlev E, Jiang J, Zhou D, Androsch R, Schick C. Extending Cooling Rate Performance of Fast Scanning Chip Calorimetry by Liquid Droplet Cooling. Applied Sciences. 2021; 11(9):3813. https://doi.org/10.3390/app11093813
Chicago/Turabian StyleZhuravlev, Evgeny, Jing Jiang, Dongshan Zhou, René Androsch, and Christoph Schick. 2021. "Extending Cooling Rate Performance of Fast Scanning Chip Calorimetry by Liquid Droplet Cooling" Applied Sciences 11, no. 9: 3813. https://doi.org/10.3390/app11093813






