Development of Candelilla Wax Oleogels as a Medium of Controlled Release of Phosphorus in an In Vitro Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Oleogel Design and Preparation
2.3. Thermomechanical Characterization of the Oleogels
2.3.1. Differential Scanning Calorimetry
2.3.2. Rheological Measurements
2.4. Determination of the Liquid Phase Release Capacity in Oleogels
2.5. Evaluation of the Release of Phosphorus from Oleogels in an Aqueous Medium
2.6. Evaluation of the Phosphorus Release Rate in Ruminal Fluid
2.7. Quantification of Phosphorus in Oleogels in Ruminal Fluid
2.8. Statistical Analysis
3. Results
3.1. Thermal and Mechanical Properties of Oleogels
3.2. Liquid Phase Release Capacity of Oleogels
3.3. Evaluation of the Release of Phosphorus in an Aqueous Medium
3.4. Evaluation of Phosphorus Release in Ruminal Fluid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Heffelfinger, J. Deer of the Southwest: A Complete Guide to the Natural History, Biology, and Management of Southwestern Mule Deer and White-Tailed Deer, 1st ed.; Texas A&M University Press: Austin, TX, USA, 2006; ISBN 1585445150. [Google Scholar]
- Gobierno de México. Producción Agrícola. Servicio de Información Agroalimentaria y Pesquera. Available online: https://www.gob.mx/siap/acciones-y-programas/produccion-agricola-33119 (accessed on 21 February 2021).
- McDowell, L.R.; Arthington, J.D. Minerals for Grazing Ruminants in Tropical Regions; McDowell, L.R., Arthington, J.D., Eds.; Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 2005. [Google Scholar]
- Moradi, H.; Musapuor, A.; Afsharmanesh, M.; Moradi Shahrbabak, H. Use of Microbial Phytase for Decrease of Pollutant Due to Environmental Poultry Excreta Phosphorus. Int. J. Agric. Biol. 2014, 8, 35–37. [Google Scholar]
- NRC (National Research Council). Nutrient Requirements of Dairy Cattle, 7th ed.; National Academies Press: Washington, DC, USA, 2001; p. 381. [Google Scholar]
- Coneglian, S.M.; Ferriani Branco, A.; Guimarães, K.C.; Mano, D.S.; Barreto, J.C.; Fávaro, V.R. Replacement of dicalcium phosphate by rock phosphate in cattle diets: Nutrients digestibility, plasma parameters, ruminal fermentation and microbial synthesis efficiency. Rev. Bras. Zootec. 2010, 39, 815–823. [Google Scholar] [CrossRef]
- Olvera, C.G.; Kurt, A.; Suárez, S.; Rosiles Martínez, R.; Ducoing Watty, A.; Ortiz Hernández, A. Blood and fecal selenium in sheep with the use of inorganic intraruminal boluses. Vet. México 2005, 36, 313–324. [Google Scholar]
- Revilla-Vázquez, A.; Ramírez-Bribiesca, E.; López-Arellano, R.; Marina Hernández-Calva, L.; Tórtora-Pérez, J.; García-García, E.; Cruz Monterrosa, R.G. Supplement of selenium with intraruminal bolus of sodium selenite in sheep. Agrociencia 2008, 42, 629–635. [Google Scholar]
- Muñoz-Gonzalez, J.C.; Huerta-Bravo, M.; Ramírez-Valverde, R.; Gonzalez-Alcorta, M.J. Estado mineral y suplemento con alambre de óxido de cobre en cabras de San José Teacalco, Tlaxcala. Ecosistemas Recur. Agropecu. 2015, 2, 203–210. [Google Scholar]
- Haenlein, G.F.W.; Anke, M. Mineral and trace element research in goats: A review. Small Rumin. Res. 2011, 95, 2–19. [Google Scholar] [CrossRef]
- Wilkie, I.W. Mineral Levels in Animal Health—Diagnostic Data. Can. Vet. J. 1989, 30, 190. [Google Scholar]
- Hart, S. Meat Goat Nutrition. In Proceedings of the 23rd Anniversary. Goat Field Day; Langston University: Langton, UK, 2008; pp. 58–83. [Google Scholar]
- Soares, J.H. Phosphorus bioavailability. In Bioavailability of Nutrients for Animals; Clarence, B., Ammerman, D.H., Baker, A.J.L., Eds.; Elsevier: San Diego, CA, USA, 1995; pp. 257–294. [Google Scholar]
- Avendaño-Vásquez, G.; De la Peña-Gil, A.; Charó-Alvarado, M.E.; Charó-Alonso, M.A.; Toro-Vazquez, J.F. Self-Assembly of Symmetrical and Asymmetrical Alkyl Esters in the Neat State and in Oleogels. Front. Sustain. Food Syst. 2020, 4, 1–21. [Google Scholar] [CrossRef]
- López-Martínez, A.; Morales-Rueda, J.A.; Dibildox-Alvarado, E.; Charó-Alonso, M.A.; Marangoni, A.G.; Toro-Vazquez, J.F. Comparing the crystallization and rheological behavior of organogels developed by pure and commercial monoglycerides in vegetable oil. Food Res. Int. 2014, 64, 946–957. [Google Scholar] [CrossRef]
- Lan, Y.; Rogers, M.A. 12-Hydroxystearic acid SAFiNs in aliphatic diols—A molecular oddity. CrystEngComm 2015, 17, 8031–8038. [Google Scholar] [CrossRef]
- De La Peña-Gil, A.; Álvarez-Mitre, F.M.; González-Chávez, M.M.; Charó-Alonso, M.A.; Toro-Vazquez, J.F. Combined effect of shearing and cooling rate on the rheology of organogels developed by selected gelators. Food Res. Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Lv, M.; Chen, Y.; Hou, T.; Zhang, Y.; Huang, Z.; Cao, Y.; Rogers, M.; Lan, Y. Engineering water-induced ceramide/lecithin oleogels: Understanding the influence of water added upon pre- and post-nucleation. Food Funct. 2020, 11, 2048–2057. [Google Scholar] [CrossRef]
- Martínez-Ávila, M.; De la Peña-Gil, A.; Álvarez-Mitre, F.M.; Charó-Alonso, M.A.; Toro-Vazquez, J.F. Self-Assembly of Saturated and Unsaturated Phosphatidylcholine in Mineral and Vegetable Oils. J. Am. Oil Chem Soc. 2019, 96, 273–289. [Google Scholar] [CrossRef]
- Aguilar-Zárate, M.; De La Peña-Gil, A.; de Álvarez-Mitre, F.M.; Charó-Alonso, M.A.; Toro-Vazquez, J.F. Vegetable and Mineral Oil Organogels Based on Monoglyceride and Lecithin Mixtures. Food Biophys. 2019, 14, 326–345. [Google Scholar] [CrossRef]
- Toro-Vazquez, J.F.; Morales-Rueda, J.A.; Dibildox-Alvarado, E.; Charó-Alonso, M.; Alonzo-Macias, M.; González-Chávez, M.M. Thermal and Textural Properties of Organogels Developed by Candelilla Wax in Safflower Oil. J. Am. Oil Chem. Soc. 2007, 84, 989–1000. [Google Scholar] [CrossRef]
- Alvarez-Mitre, F.M.; Toro-Vazquez, J.F.; Moscosa-Santillán, M. Shear rate and cooling modeling for the study of candelilla wax organogelsâ€TM rheological properties. J. Food Eng. 2013, 119, 611–618. [Google Scholar] [CrossRef]
- Onacik-Gür, S.; Żbikowska, A. Effect of high-oleic rapeseed oil oleogels on the quality of short-dough biscuits and fat migration. J. Food Sci. Technol. 2020, 57, 1609–1618. [Google Scholar] [CrossRef]
- Samuditha, L.; Dassanayake, K.; Kodali, D.R.; Ueno, S.; Sato, K. Physical Properties of Rice Bran Wax in Bulk and Organogels. J. Am. Oil Chem Soc. 2014, 86, 1163–1173. [Google Scholar] [CrossRef]
- Tang, S.; Liu, X.Y.; Strom, C.S. Producing supramolecular functional materials based on fiber network reconstruction. Adv. Funct. Mater. 2009, 19, 2252–2259. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.R.; Wang, S.-Q.; Wang, X. Nonlinearity in large amplitude oscillatory shear (LAOS) of different viscoelastic materials. J. Rheol. J. Rheol. 2009, 531. [Google Scholar] [CrossRef]
- Aguilar-Zárate, M.; Macias-Rodriguez, B.A.; Toro-Vazquez, J.F.; Marangoni, A.G. Engineering rheological properties of edible oleogels with ethylcellulose and lecithin. Carbohydr. Polym. 2019, 205, 98–105. [Google Scholar] [CrossRef]
- Alvarez-Mitre, F.M.; Morales-Rueda, J.A.; Dibildox-Alvarado, E.; Charó-Alonso, M.A.; Toro-Vazquez, J.F. Shearing as a variable to engineer the rheology of candelilla wax organogels. Food Res. Int. 2012, 49, 580–587. [Google Scholar] [CrossRef]
- Wang, F.C.; Gravelle, A.J.; Blake, A.I.; Marangoni, A.G. Novel trans fat replacement strategies. Curr. Opin. Food Sci. 2016, 7, 27–34. [Google Scholar] [CrossRef]
- Yılmaz, E.; Öğütcü, M. The texture, sensory properties and stability of cookies prepared with wax oleogels. Food Funct. 2015, 6, 1194–1204. [Google Scholar] [CrossRef]
- Yılmaz, E.; Öğütcü, M. Oleogels as spreadable fat and butter alternatives: Sensory description and consumer perception. RSC Adv. 2015, 5, 50259–50267. [Google Scholar] [CrossRef]
- Manzocco, L.; Calligaris, S.; Da Pieve, S.; Marzona, S.; Nicoli, M.C. Effect of monoglyceride-oil-water gels on white bread properties. Food Res. Int. 2012, 49, 778–782. [Google Scholar] [CrossRef]
- Plamen, K.; Cong, L.E.; Khanh, A.N.H.; Alice, D.; Halima, R.; Silvia, R.U.M.; Carla, V.; Marek, H.; Fabrice, P. Organogels for cosmetic and dermo-cosmetic applications. Classification, preparation and characterization of organogel formulations. Part 1. Househ. Pers. Care Today 2015, 10, 15–19. [Google Scholar]
- Beri, A.; Pichot, R.; Norton, I.T. Physical and material properties of an emulsion-based lipstick produced via a continuous process. Int. J. Cosmet. Sci. 2014, 36, 148–158. [Google Scholar] [CrossRef]
- Vintiloiu, A.; Leroux, J.-C. Organogels and their use in drug delivery—A review. J. Control. Release 2008, 125, 179–192. [Google Scholar] [CrossRef]
- Lo Nostro, P.; Ambrosi, M.; Ninham, B.W.; Baglioni, P. Effect of Headgroup Chirality in Nanoassemblies. 2. Thermal Behavior of Vitamin C-Based Surfactants. J. Phys. Chem. B 2009, 113, 8324–8331. [Google Scholar] [CrossRef]
- Davidovich-Pinhas, M.; Gravelle, A.J.; Barbut, S.; Marangoni, A.G. Temperature effects on the gelation of ethylcellulose oleogels. Food Hydrocoll. 2015, 46, 76–83. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm (accessed on 31 January 2021).
- Kuznesof, P.M.; Whitehouse, B. Candelilla wax. In Japan’s Specifications and Standards for Food Additives; Ministry of Health and Welfare: Tokyo, Japan, 2003; Volume 7. [Google Scholar]
- Morales-Rueda, J.A.; Dibildox-Alvarado, E.; Charó-Alonso, M.A.; Weiss, R.G.; Toro-Vazquez, J.F. Thermo-mechanical properties of candelilla wax and dotriacontane organogels in safflower oil. Eur. J. Lipid Sci. Technol. 2009, 111, 207–215. [Google Scholar] [CrossRef]
- Castro-Costa, A.; Salama, A.A.K.; Moll, X.; Aguiló, J.; Caja, G. Using wireless rumen sensors for evaluating the effects of diet and ambient temperature in nonlactating dairy goats. J. Dairy Sci. 2015, 98, 4646–4658. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, T.L.T.; Barrera Arellano, D.; Martini, S. Interactions between candelilla wax and saturated triacylglycerols in oleogels. Food Res. Int. 2019, 121, 900–909. [Google Scholar] [CrossRef]
- León-Cruz, M.; Ramírez-Bribiesca, E.; López-Arellano, R.; Miranda-Jiménez, L.; Rodríguez-Patiño, G.; Díaz-Sánchez, V.M.; Revilla-Vázquez, A.L. Trace mineral controlled-release intraruminal boluses. Revision. Rev. Mex. Ciencias Pecu. 2020, 11, 498–516. [Google Scholar] [CrossRef]
- Mezger, T.G. The Rheology Handbook, 4th ed.; Vincentz Network: Hanover, Germany, 2014; ISBN 978-3-86630-842-8. [Google Scholar]
- Blake, A.I.; Co, E.D.; Marangoni, A.G. Structure and Physical Properties of Plant Wax Crystal Networks and Their Relationship to Oil Binding Capacity. J. Am. Oil Chem Soc. 2014, 91, 885–903. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Tong, B.X.; Wu, Z.G.; Ma, W.Q.; Ma, L. Dietary manipulation to reduce nitrogen and phosphorus excretion by dairy cows. Livest. Sci. 2019, 228, 61–66. [Google Scholar] [CrossRef]
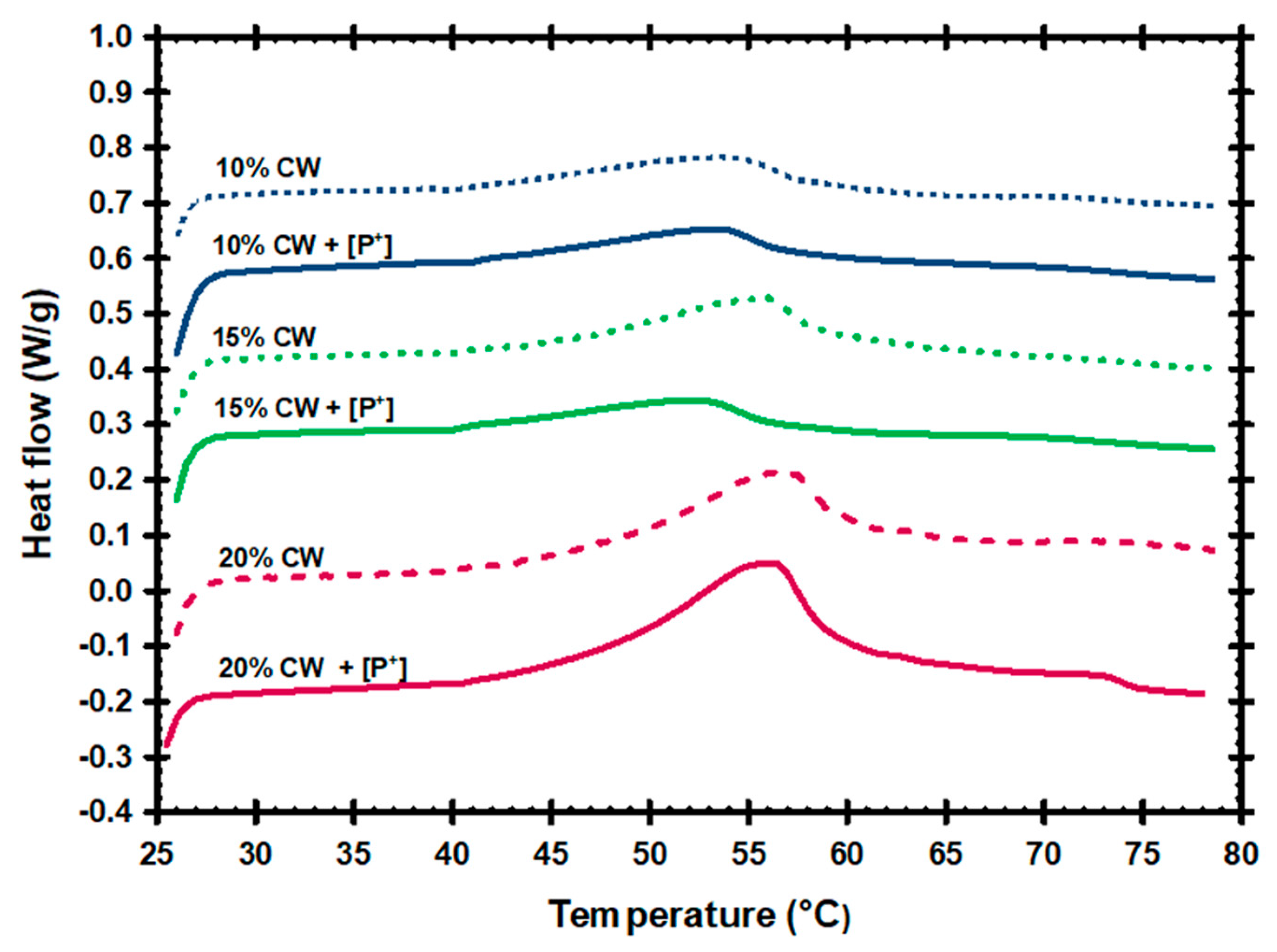
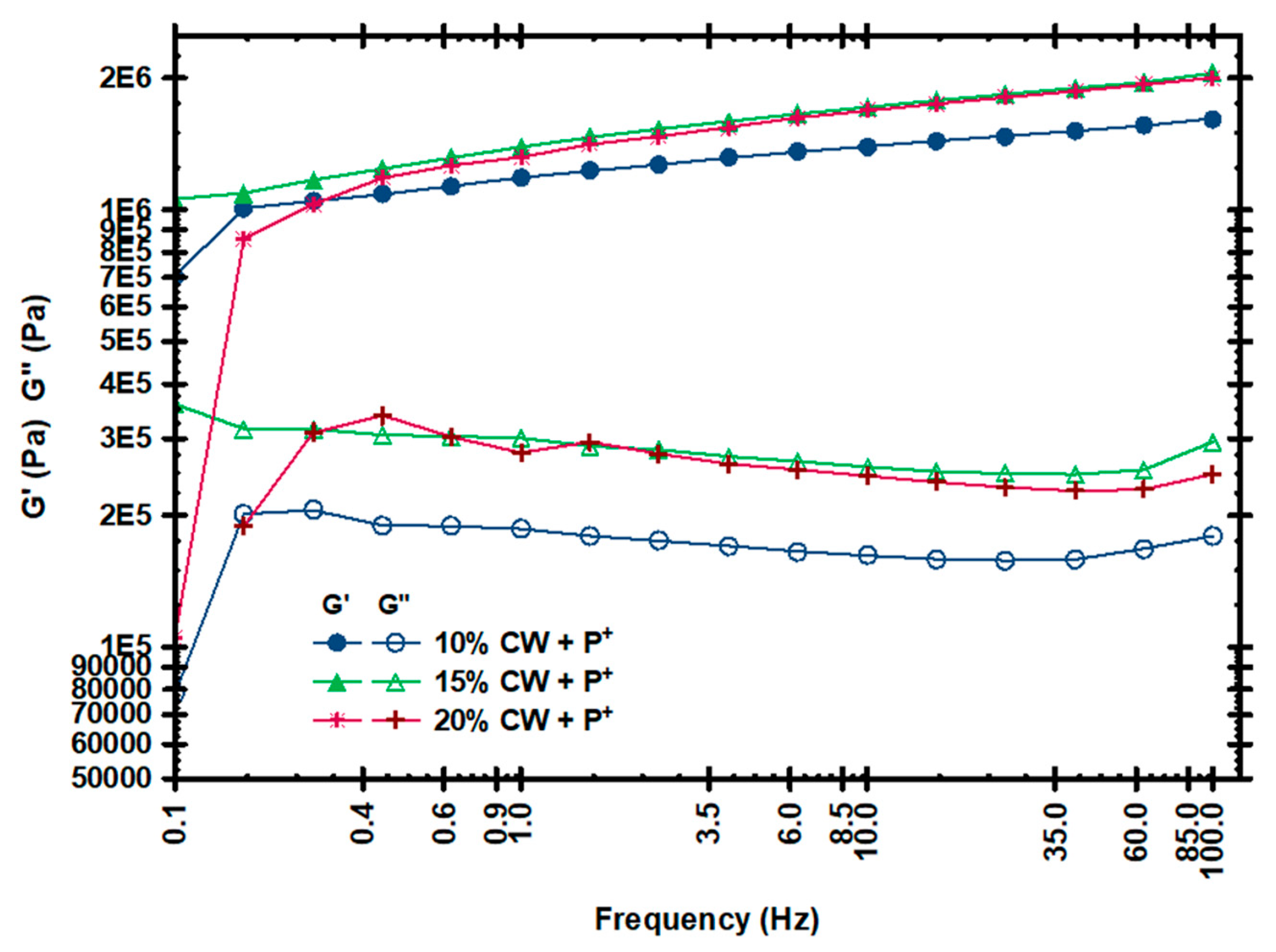

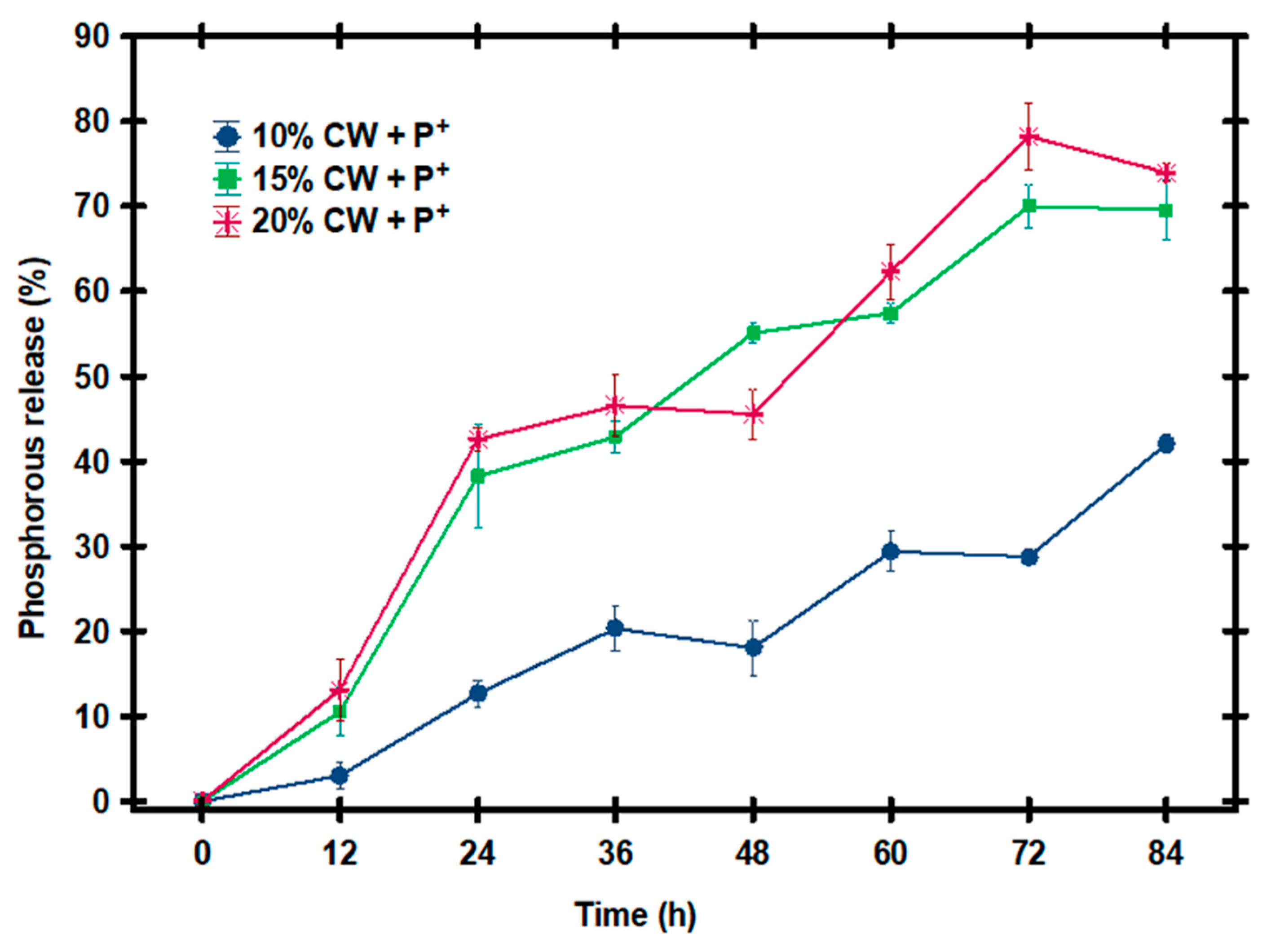
| CW Concentration | G′ (Pascals, Pa) |
|---|---|
| 10% | 1.9 × 106 ± 9.2 × 105 a |
| 10% + [P+] | 1.8 × 106 ± 7.8 × 105 a |
| 15% | 2.4 × 106 ± 2.5 × 105 a |
| 15% + [P+] | 2.1 × 106 ± 1.8 × 105 a |
| 20% | 1.1 × 106 ± 5.2 × 105 b |
| 20% + [P+] | 1.6 × 106 ± 6.0 × 104 b |
| Liquid Phase Loss (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 h | 12 h | 24 h | 36 h | 48 h | 60 h | 72 h | 84 h | |
| 10% CW | 0 ± 0.00 A,a | 2.82 ± 0.10 B,a | 2.92 ± 0.04 B,a | 3.08 ± 0.05 C,a | 3.27 ± 0.17 C,a | 4.56 ± 0.09 D,b | 5.72 ± 0.08 E,b | 6.76 ± 0.08 F,a |
| 15% CW | 0 ± 0.00 A,a | 2.76 ± 0.15 B,a | 3.09 ± 0.12 B,a | 3.33 ± 0.06 C,c | 3.40 ± 0.06 C,a | 4.69 ± 0.04 D,b | 5.80 ± 0.20 E,b | 6.93 ± 0.11 F,a |
| 20% CW | 0 ± 0.00 A,a | 3.28 ± 0.15 B,b | 5.37 ± 0.31 C,b | 5.94 ± 0.06 D,d | 6.75 ± 0.11 E,c | 6.77 ± 0.14 E,c | 8.79 ± 0.04 F,c | 10.87 ± 0.3 G,b |
| 10% CW + P+ | 0 ± 0.00 A,a | 2.80 ± 0.26 B,a | 2.95 ± 0.09 B,a | 3.19 ± 0.04 C,b | 3.20 ± 0.04 C,a | 4.28 ± 0.15 D,a | 4.48 ± 0.4 D,a | 6.66 ± 0.34 E,a |
| 15% CW + P+ | 0 ± 0.00 A,a | 2.94 ± 0.10 B,a | 3.08 ± 0.15 B,a | 3.36 ± 0.03 C,c | 3.42 ± 0.19 C,a | 4.48 ± 0.04 D,ab | 4.74 ± 0.13 E,b | 6.87 ± 0.09 F,a |
| 20% CW + P+ | 0 ± 0.00 A,a | 3.12 ± 0.02 B,b | 5.34 ± 0.01 C,b | 6.05 ± 0.11 D,d | 6.46 ± 0.06 E,b | 6.74 ± 0.29 E,c | 8.47 ± 0.30 F,c | 10.95 ± 0.3 G,b |
| Concentration of Released Phosphorus (mg) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 h | 12 h | 24 h | 36 h | 48 h | 60 h | 72 h | 84 h | |
| 10% CW + P+ | 0 ± 0.00 A,a | 9.03 ± 1.50 B,a | 37.96 ± 1.52 C,a | 60.92 ± 2.61 D,a | 54.15 ± 3.19 E,a | 88.22 ± 2.3 F,a | 86.32 ± 0.66 F,a | 126.11 ± 1.03 G,a |
| 15% CW + P+ | 0 ± 0.00 A,a | 31.59 ± 2.81 B,b | 144.77 ± 6.1 D,c | 128.62 ± 1.8 C,b | 165.23 ± 1.1 E,b | 172.32 ± 1.2 F,b | 209.89 ± 2.6 F,b | 208.73 ± 3.56 F,b |
| 20% CW + P+ | 0 ± 0.00 A,a | 39.35 ± 3.65 B,c | 127.61 ± 1.4 D,b | 139.58 ± 3.6 E,c | 54.15 ± 3.68 C,a | 186.96 ± 3.2 F,c | 234.67 ± 3.84 H,c | 221.85 ± 1.13 G,c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olmos-Oropeza, G.; Aguilar-Zárate, M.; Saavedra-Leos, M.Z.; Martínez-Juárez, L.G.; Toro-Vazquez, J.F.; Sánchez-Macías, A.; López-Martínez, L.A. Development of Candelilla Wax Oleogels as a Medium of Controlled Release of Phosphorus in an In Vitro Model. Appl. Sci. 2021, 11, 3815. https://doi.org/10.3390/app11093815
Olmos-Oropeza G, Aguilar-Zárate M, Saavedra-Leos MZ, Martínez-Juárez LG, Toro-Vazquez JF, Sánchez-Macías A, López-Martínez LA. Development of Candelilla Wax Oleogels as a Medium of Controlled Release of Phosphorus in an In Vitro Model. Applied Sciences. 2021; 11(9):3815. https://doi.org/10.3390/app11093815
Chicago/Turabian StyleOlmos-Oropeza, Genaro, Mayra Aguilar-Zárate, María Zenaida Saavedra-Leos, Luis Gerardo Martínez-Juárez, Jorge Fernando Toro-Vazquez, Armando Sánchez-Macías, and Laura Araceli López-Martínez. 2021. "Development of Candelilla Wax Oleogels as a Medium of Controlled Release of Phosphorus in an In Vitro Model" Applied Sciences 11, no. 9: 3815. https://doi.org/10.3390/app11093815
APA StyleOlmos-Oropeza, G., Aguilar-Zárate, M., Saavedra-Leos, M. Z., Martínez-Juárez, L. G., Toro-Vazquez, J. F., Sánchez-Macías, A., & López-Martínez, L. A. (2021). Development of Candelilla Wax Oleogels as a Medium of Controlled Release of Phosphorus in an In Vitro Model. Applied Sciences, 11(9), 3815. https://doi.org/10.3390/app11093815









