A Novel Bacterial-Mediated Method for the Differentiation of Aedes spp. Larvae in Mixed-Nursery Assays and Its Application in the Determination of Vegetative Lysinibacillus sphaericus Biocontrol Efficiency in Invasive Vector Eradication
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Lysinibacillus sphaericus, Aedes aegypti and Aedes albopictus Strains
2.2. Aedes Mosquito Maintenance
2.3. Bioassays of L. sphaericus against Individual Aedes Species Nurseries
2.4. Preparation, Toxicity Assessment and Coloration of Ae. aegypti Using mRFP-Modified E. coli
2.5. Bioassays of L. sphaericus against Mixed Aedes Nurseries
2.6. Statistical Analysis
3. Results
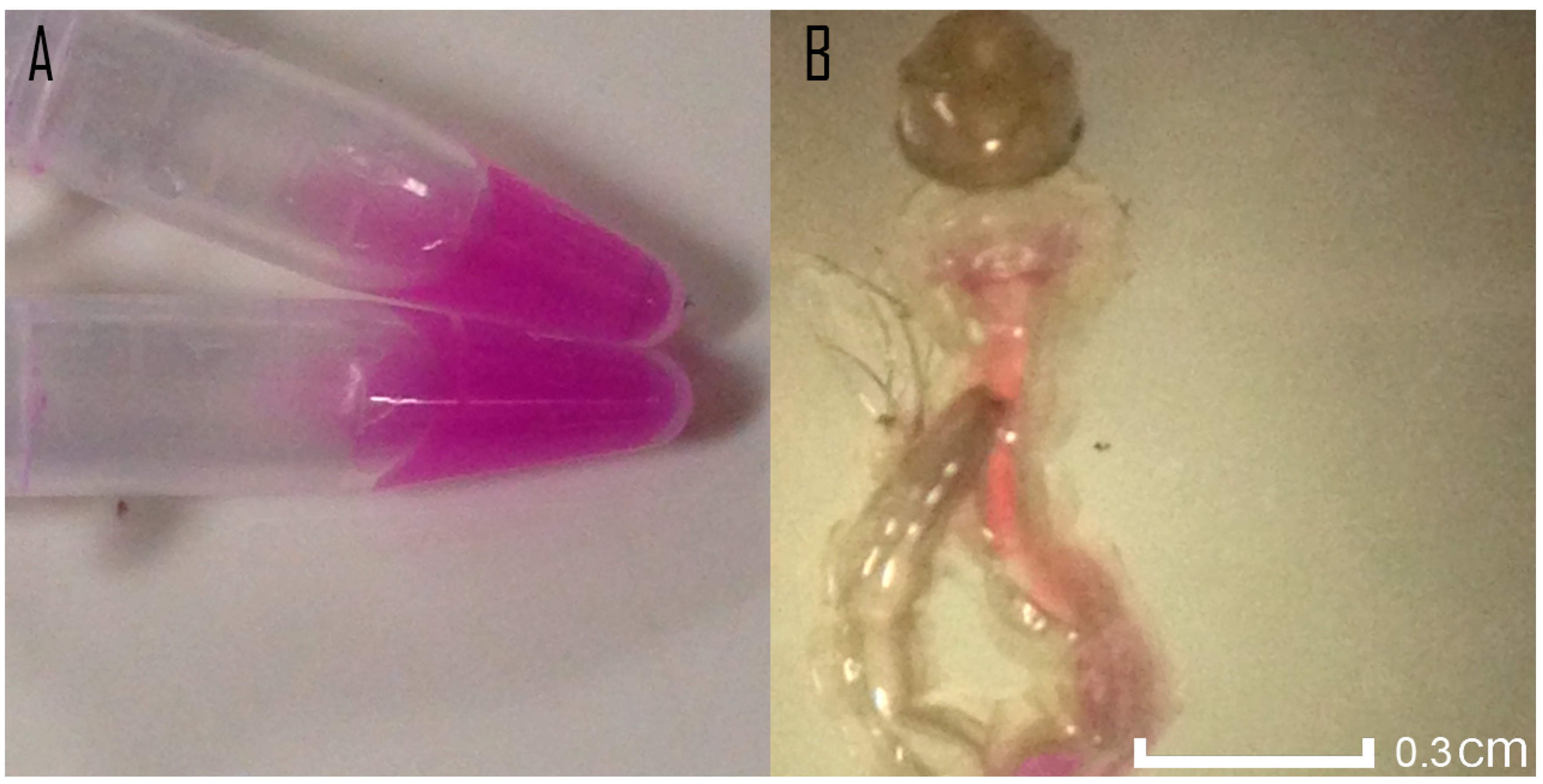
3.1. Toxicity Assessment and Coloration of Ae. aegypti Using mRFP-Modified E. coli
3.2. Lethal Doses for and among Different Aedes Nurseries
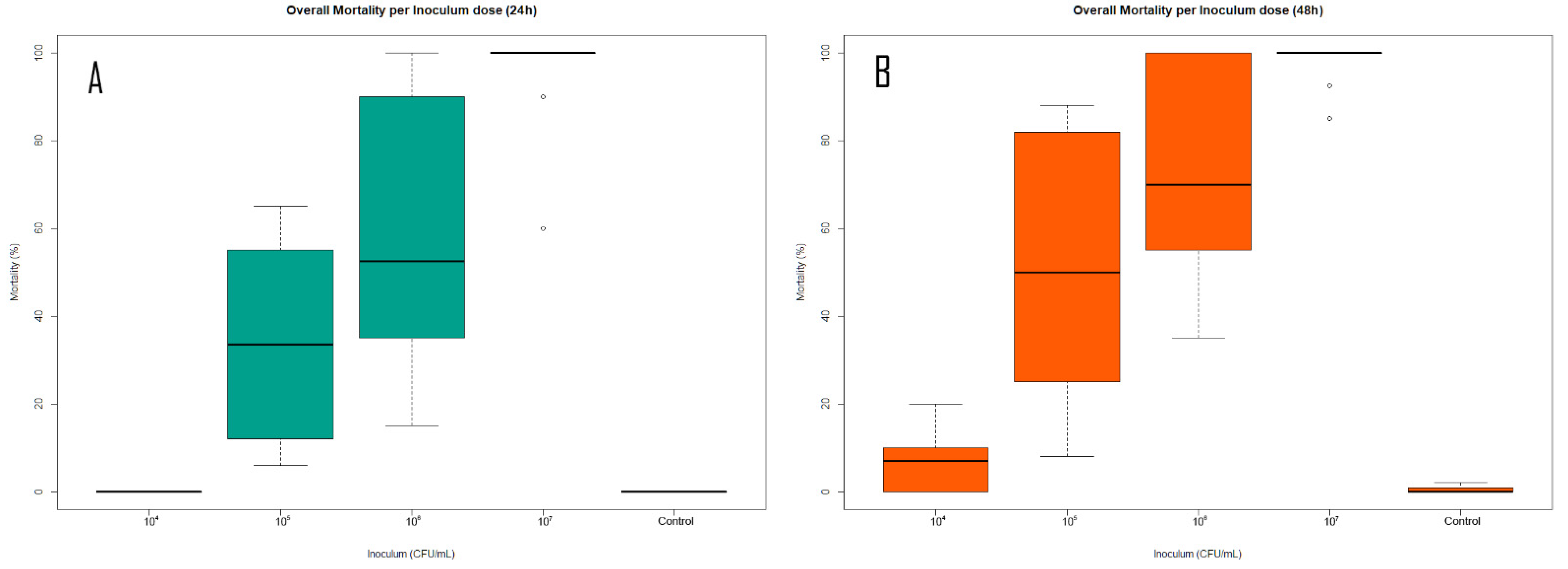
3.3. Bioassays against Different Bacterial Inoculum Concentrations
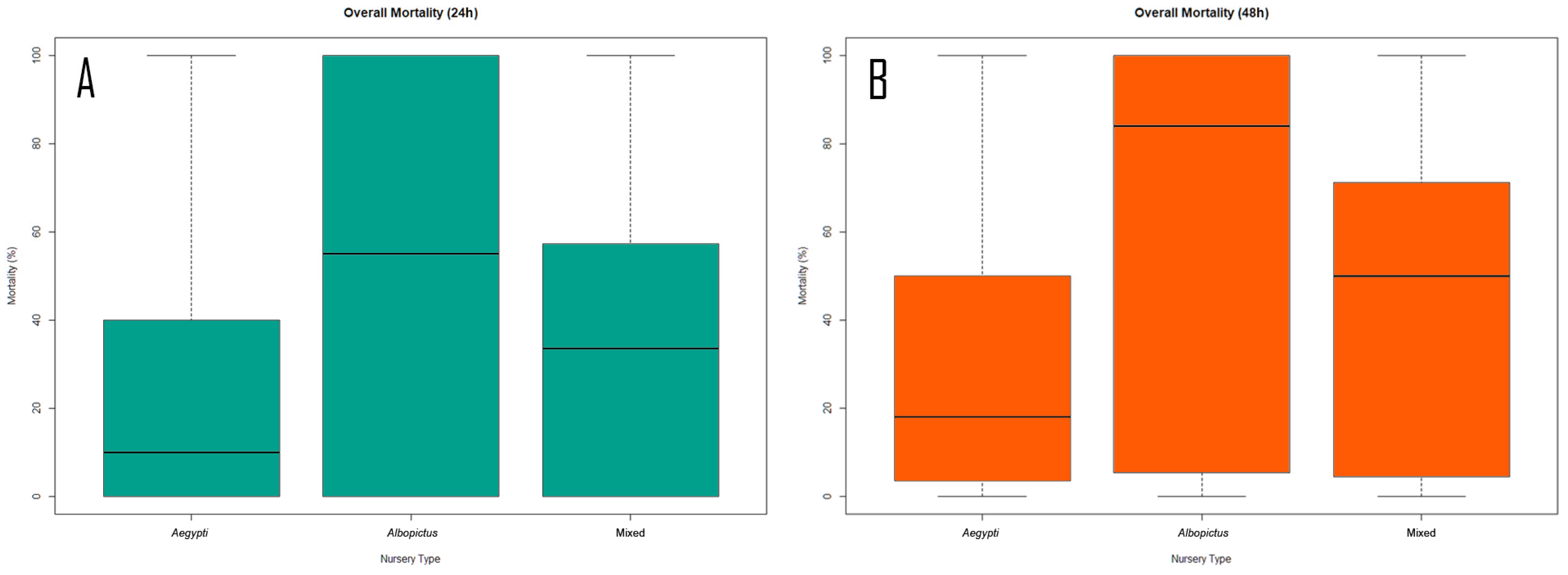
3.4. Nursery Response to Biocontrol Agent
4. Discussion
4.1. Toxicity Assessment and Coloration of Ae. aegypti Using mRFP-Modified E. coli
4.2. Lethal Doses for and among Different Aedes Nurseries
4.3. Bioassays against Different Bacterial Inoculum Concentrations
4.4. Nursery Response to Biocontrol Agent
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The Global Distribution and Burden of Dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Laserna, A.; Barahona-Correa, J.; Baquero, L.; Castañeda-Cardona, C.; Rosselli, D. Economic Impact of Dengue Fever in Latin America and the Caribbean: A Systematic Review. Rev. Panam. Salud Publica 2018, 42, e111. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef]
- Heintze, C.; Garrido, M.V.; Kroeger, A. What Do Community-Based Dengue Control Programmes Achieve? A Systematic Review of Published Evaluations. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 317–325. [Google Scholar] [CrossRef]
- Bäck, A.T.; Lundkvist, A. Dengue Viruses—An Overview. Infect. Ecol. Epidemiol. 2013, 3, 19839. [Google Scholar]
- Powell, J.R. Mosquito-Borne Human Viral Diseases: Why Aedes aegypti? Am. J. Trop. Med. Hyg. 2018, 98, 1563–1565. [Google Scholar] [CrossRef]
- Farajollahi, A.; Price, D.C. A Rapid Identification Guide for Larvae of the Most Common North American Container-Inhabiting Aedes Species of Medical Importance. J. Am. Mosq. Control Assoc. 2013, 29, 203–221. [Google Scholar] [CrossRef]
- Bargielowski, I.E.; Lounibos, L.P.; Shin, D.; Smartt, C.T.; Carrasquilla, M.C.; Henry, A.; Navarro, J.C.; Paupy, C.; Dennett, J.A. Widespread Evidence for Interspecific Mating between Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Nature. Infect. Genet. Evol. 2015, 36, 456–461. [Google Scholar] [CrossRef] [Green Version]
- Reiter, P.; Fontenille, D.; Paupy, C. Aedes albopictus as an Epidemic Vector of Chikungunya Virus: Another Emerging Problem? Lancet Infect. Dis. 2006, 6, 463–464. [Google Scholar] [CrossRef]
- Smith, C.G. The history of dengue in tropical Asia and its probable relationship to the mosquito Aedes aegypti. J. Trop. Med. Hyg. 1956, 59, 243–251. [Google Scholar]
- Reiter, P.A. Aedes albopictus and the world trade in used tires, 1988–1995: The shape of things to come? J. Am. Mosq. Control Assoc. 1998, 14, 83–94. [Google Scholar] [PubMed]
- Gratz, N.G. Critical Review of the Vector Status of Aedes albopictus. Med. Vet. Entomol. 2004, 18, 215–227. [Google Scholar] [CrossRef]
- Centers for Disease Control (CDC). Aedes albopictus introduction—Texas. MMWR. Morb. Mortal. Wkly. Rep. 1986, 35, 141–142. [Google Scholar]
- De Brito, M.; Marques, G.R.A.M.; Marques, C.C.A.; Tubaki, R.M. Primeiro Encontro de Aedes (Stegomyia) albopictus (Skuse) No Estado de São Paulo (Brasil). Rev. Saude Publica 1986, 20, 489. [Google Scholar] [CrossRef] [Green Version]
- Vélez, I.D.; Quiñones, M.L.; Suárez, M.; Olano, V.; Murcia, L.M.; Correa, E.; Arévalo, C.; Pérez, L.; Brochero, H.; Morales, A. Presencia de Aedes albopictus En Leticia, Amazonas, Colombia. Biomedica 1998, 18, 192. [Google Scholar] [CrossRef] [Green Version]
- Hawley, W.A.; Reiter, P.; Copeland, R.S.; Pumpuni, C.B.; Craig, G.B., Jr. Aedes albopictus in North America: Probable Introduction in Used Tires from Northern Asia. Science 1987, 236, 1114–1116. [Google Scholar] [CrossRef]
- Hawley, W.A. The biology of Aedes albopictus. J. Am. Mosq. Control Assoc. Suppl. 1988, 1, 1–39. [Google Scholar]
- Rúa-Uribe, G.; Suárez-Acosta, C.; Londoño, V.; Sánchez, J.; Rojo, R.; Bello-Novoa, B. Detección de Aedes albopictus (Skuse) (Diptera: Culicidae) en la ciudad de Medellín, Colombia. Biomédica 2011, 31, 243–244. [Google Scholar]
- Cuéllar-Jiménez, M.E.; Velásquez-Escobar, O.L.; González-Obando, R.; Morales-Reichmann, C.A. Detection of Aedes albopictus (Skuse) (Diptera: Culicidae) in the city of Cali, Valle del Cauca, Colombia. Biomédica 2007, 27, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Grisales, N.; Poupardin, R.; Gomez, S.; Fonseca-Gonzalez, I.; Ranson, H.; Lenhart, A. Temephos Resistance in Aedes aegypti in Colombia Compromises Dengue Vector Control. PLoS Negl. Trop. Dis. 2013, 7, e2438. [Google Scholar] [CrossRef] [Green Version]
- Ramasamy, R.; Surendran, S.N.; Jude, P.J.; Dharshini, S.; Vinobaba, M. Larval Development of Aedes aegypti and Aedes albopictus in Peri-Urban Brackish Water and Its Implications for Transmission of Arboviral Diseases. PLoS Negl. Trop. Dis. 2011, 5, e1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benelli, G. Research in Mosquito Control: Current Challenges for a Brighter Future. Parasitol. Res. 2015, 114, 2801–2805. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, R.L. Century of DDT. J. Agric. Food Chem. 1973, 21, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Wanjala, C.L.; Mbugi, J.P.; Ototo, E.; Gesuge, M.; Afrane, Y.A.; Atieli, H.E.; Zhou, G.; Githeko, A.K.; Yan, G. Pyrethroid and DDT Resistance and Organophosphate Susceptibility among Anopheles spp. Mosquitoes, Western Kenya. Emerg. Infect. Dis. 2015, 21, 2178–2181. [Google Scholar] [CrossRef] [Green Version]
- Harrison, G. Mosquitoes, Malaria and Man: A History of the Hostilities Since 1880; Dutton: Boston, MA, USA, 1978. [Google Scholar]
- Garrett, L. The Coming Plague: Newly Emerging Diseases in a World out of Balance; Farrar, Straus and Giroux: New York, NY, USA, 1994. [Google Scholar]
- Geisz, H.N.; Dickhut, R.M.; Cochran, M.A.; Fraser, W.R.; Ducklow, H.W. Melting Glaciers: A Probable Source of DDT to the Antarctic Marine Ecosystem. Environ. Sci. Technol. 2008, 42, 3958–3962. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, D.S. Pesticides: White House Advisory Body Issues Report Recommending Steps to Reduce Hazard to Public. Science 1963, 140, 878–879. [Google Scholar] [CrossRef] [PubMed]
- El-Temsah, Y.S.; Sevcu, A.; Bobcikova, K.; Cernik, M.; Joner, E.J. DDT Degradation Efficiency and Ecotoxicological Effects of Two Types of Nano-Sized Zero-Valent Iron (NZVI) in Water and Soil. Chemosphere 2016, 144, 2221–2228. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Sun, Q.; Yue, Y.; Yoon, K.S.; Whang, K.-Y.; Clark, J.M.; Park, Y. 4,4′-Dichlorodiphenyltrichloroethane (DDT) and 4,4′-Dichlorodiphenyldichloroethylene (DDE) Promote Adipogenesis in 3T3-L1 Adipocyte Cell Culture. Pestic. Biochem. Physiol. 2016, 131, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Eskenazi, B.; Chevrier, J.; Rosas, L.G.; Anderson, H.A.; Bornman, M.S.; Bouwman, H.; Chen, A.; Cohn, B.A.; de Jager, C.; Henshel, D.S.; et al. The Pine River Statement: Human Health Consequences of DDT Use. Environ. Health Perspect. 2009, 117, 1359–1367. [Google Scholar] [CrossRef] [Green Version]
- Connell, D.W.; Lam, P.; Richardson, B.; Wu, R. Introduction to Ecotoxicology, 1st ed.; Blackwell Science: Philadelphia, PA, USA, 2009. [Google Scholar]
- Sharma, V.P. Current scenario of malaria in India. Parassitologia 1999, 41, 349–353. [Google Scholar]
- Wacker, M.; Linton, D.; Hitchen, P.G.; Nita-Lazar, M.; Haslam, S.M.; North, S.J.; Aebi, M. N-linked glycosylation in Campylobacter jejuni and its functional transfer into E. coli. Science 2002, 298, 1790–1793. [Google Scholar] [CrossRef]
- Gong, Z.; Wan, H.; Tay, T.L.; Wang, H.; Chen, M.; Yan, T. Development of Transgenic Fish for Ornamental and Bioreactor by Strong Expression of Fluorescent Proteins in the Skeletal Muscle. Biochem. Biophys. Res. Commun. 2003, 308, 58–63. [Google Scholar] [CrossRef]
- Molina, T.L.; Patel, R.; Molina, D.D.; Persans, M.W.; Lowe, K.L. Isolation of a Naturally-Occurring Nickel Resistance Plasmid from a Rare Hypersaline Estuary (Laguna Madre, Texas, USA) for Potential Use as a Bio-Indicator of Metal Contamination. World J. Microbiol. Biotechnol. 2011, 27, 2163–2171. [Google Scholar] [CrossRef]
- Berry, C. The Bacterium, Lysinibacillus sphaericus, as an Insect Pathogen. J. Invertebr. Pathol. 2012, 109, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gould, G.W.; Russell, A.D.; Stewart-Tull, D.E.S. (Eds.) Fundamental and Applied Aspects of Bacterial Spores; Blackwell Science: Philadelphia, PA, USA, 1994. [Google Scholar]
- Yap, H.-H. Field Trials of Bacillus sphaericus for Mosquito Control. In Bacterial Control of Mosquitoes and Black Flies; Springer: Dordrecht, The Netherlands, 1990; pp. 307–320. [Google Scholar]
- Rojas-Pinzón, P.A.; Dussán, J. Efficacy of the Vegetative Cells of Lysinibacillus sphaericus for Biological Control of Insecticide-Resistant Aedes aegypti. Parasit. Vectors 2017, 10, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peña-Montenegro, T.; Lozano, L.; Dussán, J. Genome Sequence and Description of the Mosquitocidal and Heavy Metal Tolerant Strain Lysinibacillus sphaericus CBAM5. Stand. Genom. Sci. 2015, 10, 2. [Google Scholar]
- Lozano, L.C.; Ayala, J.A.; Dussán, J. Lysinibacillus sphaericus S-Layer Protein Toxicity against Culex quinquefasciatus. Biotechnol. Lett. 2011, 33, 2037–2041. [Google Scholar] [CrossRef] [PubMed]
- Nielsen-Leroux, C.; Charles, J.-F. Binding of Bacillus sphaericus Binary Toxin to a Specific Receptor on Midgut Brush-Border Membranes from Mosquito Larvae. Eur. J. Biochem. 1992, 210, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.M.; Romão, T.P.; Pompílio de-Melo-Neto, O.; Silva-Filha, M.H.N.L. The Orthologue to the Cpm1/Cqm1 Receptor in Aedes aegypti Is Expressed as a Midgut GPI-Anchored α-Glucosidase, Which Does Not Bind to the Insecticidal Binary Toxin. Insect Biochem. Mol. Biol. 2010, 40, 604–610. [Google Scholar] [CrossRef]
- Dániel-Gómez, M.; Dussán, J. Assessment of the Synergic Effect between Lysinibacillus sphaericus S-Layer Protein and Glyphosate in the Lethality of the Invasive Arboviral Vector Aedes albopictus. Insects 2020, 11, 793. [Google Scholar] [CrossRef]
- Penña, G.; Miranda-Rios, J.; de la Riva, G.; Pardo-Loópez, L.; Soberoón, M.; Bravo, A. A Bacillus thuringiensis S-Layer Protein Involved in Toxicity against Epilachna varivestis (Coleoptera: Coccinellidae). Appl. Environ. Microbiol. 2006, 72, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Allievi, M.C.; Palomino, M.M.; Prado Acosta, M.; Lanati, L.; Ruzal, S.M.; Sánchez-Rivas, C. Contribution of S-Layer Proteins to the Mosquitocidal Activity of Lysinibacillus sphaericus. PLoS ONE 2014, 9, e111114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanabalu, T.; Porter, A.G. Efficient expression of a 100-kilodalton mosquitocidal toxin in protease-deficient recombinant Bacillus sphaericus. Appl. Environ. Microbiol. 1995, 61, 4031–4036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garzón, J.D.; Linares, D.R.A.; Ardila, L.C.L.; Moyano, S.D.P.V. Caracterización Fisiológica y Genética de Cepas Nativas de Bacillus sphaericus. Rev. Colomb. Biotecnol. 2002, 4, 89–99. [Google Scholar]
- Weiser, J. A Mosquito-Virulent Bacillus sphaericus in Adult Simulium damnosum from Northern Nigeria. Zentralbl. Mikrobiol. 1984, 139, 57–60. [Google Scholar] [CrossRef]
- Rojas-Pinzón, P.A.; Silva-Fernández, J.J.; Dussán, J. Laboratory and Simulated-Field Bioassays for Assessing Mixed Cultures of Lysinibacillus sphaericus against Aedes aegypti (Diptera: Culicidae) Larvae Resistant to Temephos. Appl. Entomol. Zool. 2018, 53, 183–191. [Google Scholar] [CrossRef]
- Bernal, L.; Dussán, J. Synergistic Effect of Lysinibacillus sphaericus and Glyphosate on Temephos-Resistant Larvae of Aedes aegypti. Parasit. Vectors 2020, 13, 68. [Google Scholar] [CrossRef] [Green Version]
- Aldrich, J.; Nelson, F.D. Linear Probability, Logit, and Probit Models; SAGE Publications: Thousand Oaks, CA, USA, 1985. [Google Scholar]
- Microsoft Corporation. Microsoft Excel. Available online: https://office.microsoft.com/excel (accessed on 20 December 2019).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; ISBN 3-900051-07-0. Available online: http://www.Rproject.org/ (accessed on 10 May 2020).
- Tchioffo, M.T.; Abate, L.; Boissière, A.; Nsango, S.E.; Gimonneau, G.; Berry, A.; Oswald, E.; Dubois, D.; Morlais, I. An Epidemiologically Successful Escherichia coli Sequence Type Modulates Plasmodium falciparum Infection in the Mosquito Midgut. Infect. Genet. Evol. 2016, 43, 22–30. [Google Scholar] [CrossRef]
- Valzania, L.; Martinson, V.G.; Harrison, R.E.; Boyd, B.M.; Coon, K.L.; Brown, M.R.; Strand, M.R. Both Living Bacteria and Eukaryotes in the Mosquito Gut Promote Growth of Larvae. PLoS Negl. Trop. Dis. 2018, 12, e0006638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunze, S.; Kochmann, J.; Koch, L.K.; Klimpel, S. Niche Conservatism of Aedes albopictus and Aedes aegypti—Two Mosquito Species with Different Invasion Histories. Sci. Rep. 2018, 8, 7733. [Google Scholar] [CrossRef] [Green Version]
- Braks, M.A.H.; Honório, N.A.; Lounibos, L.P.; Lourenço-De-Oliveira, R.; Juliano, S.A. Interspecific Competition Between Two Invasive Species of Container Mosquitoes, Aedes aegypti and Aedes albopictus (Diptera: Culicidae), in Brazil. Ann. Entomol. Soc. Am. 2004, 97, 130–139. [Google Scholar] [CrossRef]
- Kress, A. Research into the Potential to Adapt and Establish of the invasive Asian Tiger Mosquito Aedes (Stegomyia) albopictus (SKUSE). arXiv 2016, arXiv:1611.10318. [Google Scholar]
- Bonizzoni, M.; Gasperi, G.; Chen, X.; James, A.A. The Invasive Mosquito Species Aedes albopictus: Current Knowledge and Future Perspectives. Trends Parasitol. 2013, 29, 460–468. [Google Scholar] [CrossRef] [Green Version]
- Ponnusamy, L.; Xu, N.; Stav, G.; Wesson, D.M.; Schal, C.; Apperson, C.S. Diversity of Bacterial Communities in Container Habitats of Mosquitoes. Microb. Ecol. 2008, 56, 593–603. [Google Scholar] [CrossRef] [Green Version]
- Echeverry-Cárdenas, E.; López-Castañeda, C.; Carvajal-Castro, J.D.; Aguirre-Obando, O.A. Potential Geographic Distribution of the Tiger Mosquito Aedes albopictus (Skuse, 1894) (Diptera: Culicidae) in Current and Future Conditions for Colombia. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Leyton-Ramos, L.M.; Aguirre-Obando, O.A.; Duque, J.E.; García-Merchán, V.H. Wing Metric Variation in Aedes aegypti Effect of Altitude on Wing Metric Variation of Aedes aegypti (Diptera: Culicidae) in a Region of the Colombian Central Andes. PLoS ONE 2020, 15, e0228975. [Google Scholar] [CrossRef] [PubMed]
- Bagny Beilhe, L.; Delatte, H.; Juliano, S.A.; Fontenille, D.; Quilici, S. Ecological Interactions In Aedes species on Reunion Island. Med. Vet. Entomol. 2013, 27, 387–397. [Google Scholar] [CrossRef]
- Noden, B.H.; O’neal, P.A.; Fader, J.E.; Juliano, S.A. Impact of Inter- and Intra-Specific Competition among Larvae on Larval, Adult, and Life-Table Traits of Aedes aegypti and Aedes albopictus Females: Impact of Larval Competition on Adult Aedes. Ecol. Entomol. 2016, 41, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Legros, M.; Lloyd, A.L.; Huang, Y.; Gould, F. Density-Dependent Intraspecific Competition in the Larval Stage Of Aedes aegypti (Diptera: Culicidae): Revisiting the Current Paradigm. J. Med. Entomol. 2009, 46, 409–419. [Google Scholar] [CrossRef] [Green Version]
- Alto, B.W.; Lounibos, L.P.; Mores, C.N.; Reiskind, M.H. Larval Competition Alters Susceptibility of Adult Aedes Mosquitoes to Dengue Infection. Proc. Biol. Sci. 2008, 275, 463–471. [Google Scholar] [CrossRef]
- McMeniman, C.J.; Lane, R.V.; Cass, B.N.; Fong, A.W.C.; Sidhu, M.; Wang, Y.-F.; O’Neill, S.L. Stable Introduction of a Life-Shortening Wolbachia Infection into the Mosquito Aedes aegypti. Science 2009, 323, 141–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Nursery Type | 107 | 106 | 105 | 104 |
|---|---|---|---|---|
| Ae. aegypti | 3.26 × 107 (± 5.89 × 105) | 1.91 × 106 (± 8.28 × 104) | 4.9 × 105 (± 1.5 × 104) | 7.87 × 104 (± 3.17 × 102) |
| Ae. albopictus | 6.39 × 107 (± 7.32 × 104) | 9.24 × 106 (± 2.71 × 104) | 3.76 × 105 (± 6.86 × 104) | 3.22 × 104 (± 9.27 × 103) |
| Mixed | 3.54 × 107 (± 9.51 × 105) | 6.12 × 106 (± 6.42 × 105) | 9.69 × 105 (± 2.91 × 104) | 4.57 × 104 (± 5.93 × 103) |
| Nursery Type | LD50 (CFU/mL) | LD95 (CFU/mL) |
|---|---|---|
| Ae. aegypti | 1.8 × 107 | 6.3 × 107 |
| Ae. albopictus | 5.9 × 105 | 1.23 × 106 |
| Mixed (both species) | 1.45 × 107 | 3.04 × 107 |
| Mixed (Ae. aegypti) | 7.23 × 106 | 3.25 × 107 |
| Mixed (Ae. albopictus) | 1.88 × 105 | 9.74 × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dániel-Gómez, M.; Dussán, J. A Novel Bacterial-Mediated Method for the Differentiation of Aedes spp. Larvae in Mixed-Nursery Assays and Its Application in the Determination of Vegetative Lysinibacillus sphaericus Biocontrol Efficiency in Invasive Vector Eradication. Appl. Sci. 2021, 11, 3970. https://doi.org/10.3390/app11093970
Dániel-Gómez M, Dussán J. A Novel Bacterial-Mediated Method for the Differentiation of Aedes spp. Larvae in Mixed-Nursery Assays and Its Application in the Determination of Vegetative Lysinibacillus sphaericus Biocontrol Efficiency in Invasive Vector Eradication. Applied Sciences. 2021; 11(9):3970. https://doi.org/10.3390/app11093970
Chicago/Turabian StyleDániel-Gómez, Mario, and Jenny Dussán. 2021. "A Novel Bacterial-Mediated Method for the Differentiation of Aedes spp. Larvae in Mixed-Nursery Assays and Its Application in the Determination of Vegetative Lysinibacillus sphaericus Biocontrol Efficiency in Invasive Vector Eradication" Applied Sciences 11, no. 9: 3970. https://doi.org/10.3390/app11093970






