Platelet-Rich Plasma in Anterior Cruciate Ligament Quadriceps Tendon Bone Reconstruction—Impact of PRP Administration on Pain, Range of Motion Restoration, Knee Stability, Tibial Tunnel Widening and Functional Results
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
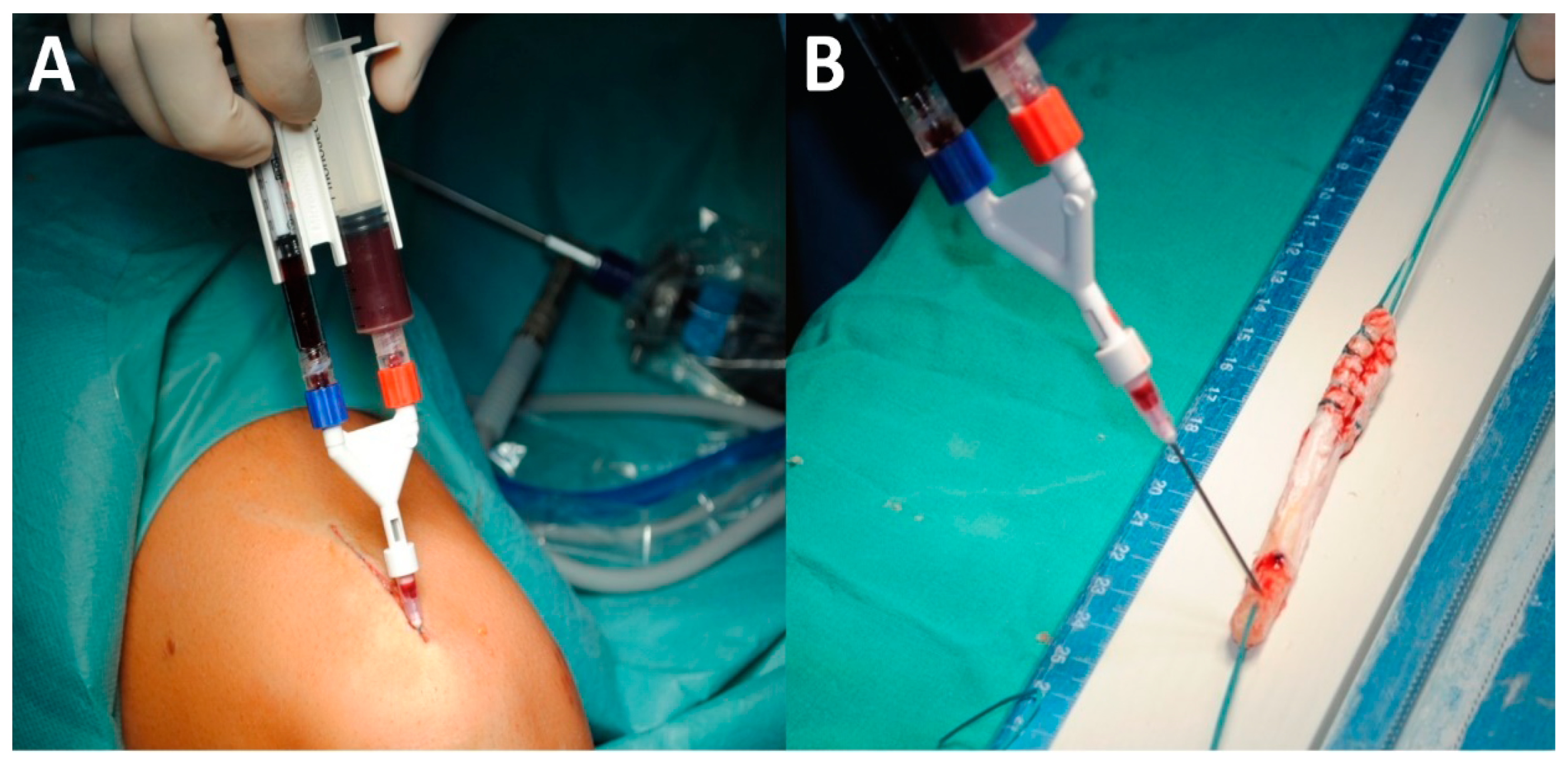
2.2. Procedure
2.3. Assessment of Outcomes
2.4. Statistical Analysis
3. Results
3.1. Early Postoperative Outcomes
3.2. Mid-Term Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lin, K.M.; Boyle, C.; Marom, N.; Marx, R.G. Graft Selection in Anterior Cruciate Ligament Reconstruction. Sports Med. Arthrosc. 2020, 28, 41–48. [Google Scholar] [CrossRef]
- Harris, N.L.; Smith, D.A.B.; Lamoreaux, L.; Purnell, M. Central Quadriceps Tendon for Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 1997, 25, 23–28. [Google Scholar] [CrossRef]
- Suzuki, T.; Shino, K.; Nakagawa, S.; Nakata, K.; Iwahashi, T.; Kinugasa, K.; Otsubo, H.; Yamashita, T. Early integration of a bone plug in the femoral tunnel in rectangular tunnel ACL reconstruction with a bone-patellar tendon-bone graft: A prospective computed tomography analysis. Knee Surg. Sport. Traumatol. Arthrosc. 2011, 19, S29–S35. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, M.; Soldati, F.; Szomolanyi, P.; Trattnig, S.; Bartolucci, F.; Fu, F.; Denti, M. Hamstring tendon autografts do not show complete graft maturity 6 months postoperatively after anterior cruciate ligament reconstruction. Knee Surg. Sport. Traumatol. Arthrosc. 2019, 27, 130–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouarbes, D.; Menetrey, J.; Marot, V.; Courtot, L.; Berard, E.; Cavaignac, E. Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-analysis of Outcomes for Quadriceps Tendon Autograft Versus Bone—Patellar Tendon—Bone and Hamstring-Tendon Autografts. Am. J. Sports Med. 2019, 47, 3531–3540. [Google Scholar] [CrossRef] [PubMed]
- Slone, H.S.; Romine, S.E.; Premkumar, A.; Xerogeanes, J.W. Quadriceps tendon autograft for anterior cruciate ligament reconstruction: A comprehensive review of current literature and systematic review of clinical results. Arthrosc. J. Arthrosc. Relat. Surg. 2015, 31, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Chahla, J.; Kennedy, M.I.; Aman, Z.S.; LaPrade, R.F. Ortho-Biologics for Ligament Repair and Reconstruction. Clin. Sports Med. 2019, 38, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.S.; Hurley, E.T.; Withers, D.; Moran, R.; Moran, C.J. Anterior Cruciate Ligament Reconstruction with Platelet-Rich Plasma: A Systematic Review of Randomized Control Trials. Arthrosc. J. Arthrosc. Relat. Surg. 2020, 36, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Dallo, I.; Chahla, J.; Mitchell, J.J.; Pascual-Garrido, C.; Feagin, J.A.; LaPrade, R.F. Biologic Approaches for the Treatment of Partial Tears of the Anterior Cruciate Ligament: A Current Concepts Review. Orthop. J. Sport. Med. 2017, 5. [Google Scholar] [CrossRef] [Green Version]
- Giudice, A.; Barone, S.; Giudice, C.; Bennardo, F.; Fortunato, L. Can platelet-rich fibrin improve healing after surgical treatment of medication-related osteonecrosis of the jaw? A pilot study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 390–403. [Google Scholar] [CrossRef]
- Fortunato, L.; Bennardo, F.; Buffone, C.; Giudice, A. Is the application of platelet concentrates effective in the prevention and treatment of medication-related osteonecrosis of the jaw? A systematic review. J. Cranio-Maxillo-Facial Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-Facial Surg. 2020, 48, 268–285. [Google Scholar] [CrossRef] [PubMed]
- Kwapisz, A.; Prabhakar, S.; Compagnoni, R.; Sibilska, A.; Randelli, P. Platelet-Rich Plasma for Elbow Pathologies: A Descriptive Review of Current Literature. Curr. Rev. Musculoskelet. Med. 2018, 11, 598–606. [Google Scholar] [CrossRef] [Green Version]
- Bennardo, F.; Liborio, F.; Barone, S.; Antonelli, A.; Buffone, C.; Fortunato, L.; Giudice, A. Efficacy of platelet-rich fibrin compared with triamcinolone acetonide as injective therapy in the treatment of symptomatic oral lichen planus: A pilot study. Clin. Oral Investig. 2021. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, K.; Paszkowski, J.; Mostowy, M.; Góralczyk, A.; LaPrade, R.F.; Hermanowicz, K. Quadriceps Tendon-Bone Full-Thickness Autograft: Reproducible and Easy Harvesting Technique Using Simple Surgical Tools. Arthrosc. Tech. 2021. [Google Scholar] [CrossRef]
- Zhang, H.; Bai, Y.; Liu, C.; Jin, S.; Su, K.; Liu, Y.; Lü, Z. Effect of intra-articular injection of platelet-rich plasma on interleukin-17 expression in synovial fluid and venous plasma of knee osteoarthritis patients. Zhongguo Xiufu Chongjian Waike Zazhi = Chin. J. Reparative Reconstr. Surg. 2017, 31, 918–921. [Google Scholar] [CrossRef]
- Seijas, R.; Ares, O.; Catala, J.; Alvarez-Diaz, P.; Cusco, X.; Cugat, R. Magnetic resonance imaging evaluation of patellar tendon graft remodelling after anterior cruciate ligament reconstruction with or without platelet-rich plasma. J. Orthop. Surg. (Hong Kong) 2013, 21, 10–14. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, A.M.; Demange, M.K.; Sobrado, M.F.; Rodrigues, M.B.; Pedrinelli, A.; Hernandez, A.J. Patellar tendon healing with platelet-rich plasma: A prospective randomized controlled trial. Am. J. Sports Med. 2012, 40, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Azcárate, A.V.; Lamo-Espinosa, J.; Beola, J.D.A.; Gonzalez, M.H.; Gasque, G.M.; Nin, J.R.V. Comparison between two different platelet-rich plasma preparations and control applied during anterior cruciate ligament reconstruction. Is there any evidence to support their use? Injury 2014, 45, S36–S41. [Google Scholar] [CrossRef]
- Cervellin, M.; de Girolamo, L.; Bait, C.; Denti, M.; Volpi, P. Autologous platelet-rich plasma gel to reduce donor-site morbidity after patellar tendon graft harvesting for anterior cruciate ligament reconstruction: A randomized, controlled clinical study. Knee Surg. Sport. Traumatol. Arthrosc. 2012, 20, 114–120. [Google Scholar] [CrossRef]
- Walters, B.L.; Porter, D.A.; Hobart, S.J.; Bedford, B.B.; Hogan, D.E.; McHugh, M.M.; Klein, D.A.; Harousseau, K.; Nicholas, S.J. Effect of Intraoperative Platelet-Rich Plasma Treatment on Postoperative Donor Site Knee Pain in Patellar Tendon Autograft Anterior Cruciate Ligament Reconstruction: A Double-Blind Randomized Controlled Trial. Am. J. Sports Med. 2018, 46, 1827–1835. [Google Scholar] [CrossRef]
- Di Matteo, B.; Andriolo, L.; Filardo, G.; Loibl, M.; Zellner, J.; Koch, M.; Angele, P. Biologic agents for anterior cruciate ligament healing: A systematic review. World J. Orthop. 2016, 7, 592–603. [Google Scholar] [CrossRef]
- Mirzatolooei, F.; Alamdari, M.T.; Khalkhali, H.R. The impact of platelet-rich plasma on the prevention of tunnel widening in anterior cruciate ligament reconstruction using quadrupled autologous hamstring tendon: A randomised clinical trial. Bone Jt. 2013, 95B, 65–69. [Google Scholar] [CrossRef]
- Colombet, P.; Graveleau, N.; Jambou, S. Incorporation of Hamstring Grafts Within the Tibial Tunnel After Anterior Cruciate Ligament Reconstruction: Magnetic Resonance Imaging of Suspensory Fixation Versus Interference Screws. Am. J. Sports Med. 2016, 44, 2838–2845. [Google Scholar] [CrossRef] [PubMed]
- Lui, P.P.Y.; Ho, G.; Shum, W.T.; Lee, Y.W.; Ho, P.Y.; Lo, W.N.; Lo, C.K. Inferior tendon graft to bone tunnel healing at the tibia compared to that at the femur after anterior cruciate ligament reconstruction. J. Orthop. Sci. 2010, 15, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Rupreht, M.; Vogrin, M.; Hussein, M. MRI evaluation of tibial tunnel wall cortical bone formation after platelet-rich plasma applied during anterior cruciate ligament reconstruction. Radiol. Oncol. 2013, 47, 119–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventura, A.; Terzaghi, C.; Borgo, E.; Verdoia, C.; Gallazzi, M.; Failoni, S. Use of growth factors in ACL surgery: Preliminary study. J. Orthop. Traumatol. 2005, 6, 76–79. [Google Scholar] [CrossRef]



| Outcome | PRP Group, Mean | No-PRP Group, Mean |
|---|---|---|
| Time of presence of fever | 3.5 days | 3 days |
| Body temperature | 37.7 °C | 37.4 °C |
| Time of presence of palpable hematoma/swelling | 22 days | 21 days |
| Time of need for on-demand analgesia | 8 days | 11 days |
| Time to walking without crutches | 21 days | 25 days |
| Time of restoring full knee extension | 40 days | 53 days |
| Time of restoring 90° of knee flexion | 21 days | 25 days |
| Outcome | PRP Group | No-PRP Group | p Value Availability |
|---|---|---|---|
| Harvest site pain, number of patients | 3/54 patients | 3/52 patients | NA |
| Harvest site pain, intensity scale 0—3 | mean 1.3 | mean 2.3 | NA |
| Dynamic test score scale 0—2 | mean 1.98 | mean 1.8 | NA |
| KOOS Symptoms | mean 92 | mean 90 | NA |
| KOOS Pain | mean 93 | mean 89 | NA |
| KOOS Function daily living | mean 94 | mean 92 | NA |
| KOOS Function sport activities | mean 90 | mean 88 | NA |
| KOOS Quality of live | mean 83 | mean 85 | NA |
| IKDC grade A | 17 patients | 16 patients | A |
| IKDC grade B | 36 patients | 32 patients | A |
| IKDC grade C | 1 patients | 2 patients | A |
| IKDC grade D | 0 patients | 2 patients | A |
| Anterior tibial translation SSD | 1.3 mm | 2.7 mm | NA |
| Presence of tibial widening | 23/54 patients | 28/52 patients | A |
| Mean tibial widening | 1.4 mm | 2.1 mm | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malinowski, K.; Ebisz, M.; LaPrade, R.F.; Mostowy, M. Platelet-Rich Plasma in Anterior Cruciate Ligament Quadriceps Tendon Bone Reconstruction—Impact of PRP Administration on Pain, Range of Motion Restoration, Knee Stability, Tibial Tunnel Widening and Functional Results. Appl. Sci. 2021, 11, 3993. https://doi.org/10.3390/app11093993
Malinowski K, Ebisz M, LaPrade RF, Mostowy M. Platelet-Rich Plasma in Anterior Cruciate Ligament Quadriceps Tendon Bone Reconstruction—Impact of PRP Administration on Pain, Range of Motion Restoration, Knee Stability, Tibial Tunnel Widening and Functional Results. Applied Sciences. 2021; 11(9):3993. https://doi.org/10.3390/app11093993
Chicago/Turabian StyleMalinowski, Konrad, Michał Ebisz, Robert F LaPrade, and Marcin Mostowy. 2021. "Platelet-Rich Plasma in Anterior Cruciate Ligament Quadriceps Tendon Bone Reconstruction—Impact of PRP Administration on Pain, Range of Motion Restoration, Knee Stability, Tibial Tunnel Widening and Functional Results" Applied Sciences 11, no. 9: 3993. https://doi.org/10.3390/app11093993
APA StyleMalinowski, K., Ebisz, M., LaPrade, R. F., & Mostowy, M. (2021). Platelet-Rich Plasma in Anterior Cruciate Ligament Quadriceps Tendon Bone Reconstruction—Impact of PRP Administration on Pain, Range of Motion Restoration, Knee Stability, Tibial Tunnel Widening and Functional Results. Applied Sciences, 11(9), 3993. https://doi.org/10.3390/app11093993






