Fine Activated Carbon from Rubber Fruit Shell Prepared by Using ZnCl2 and KOH Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Characterization
3. Results
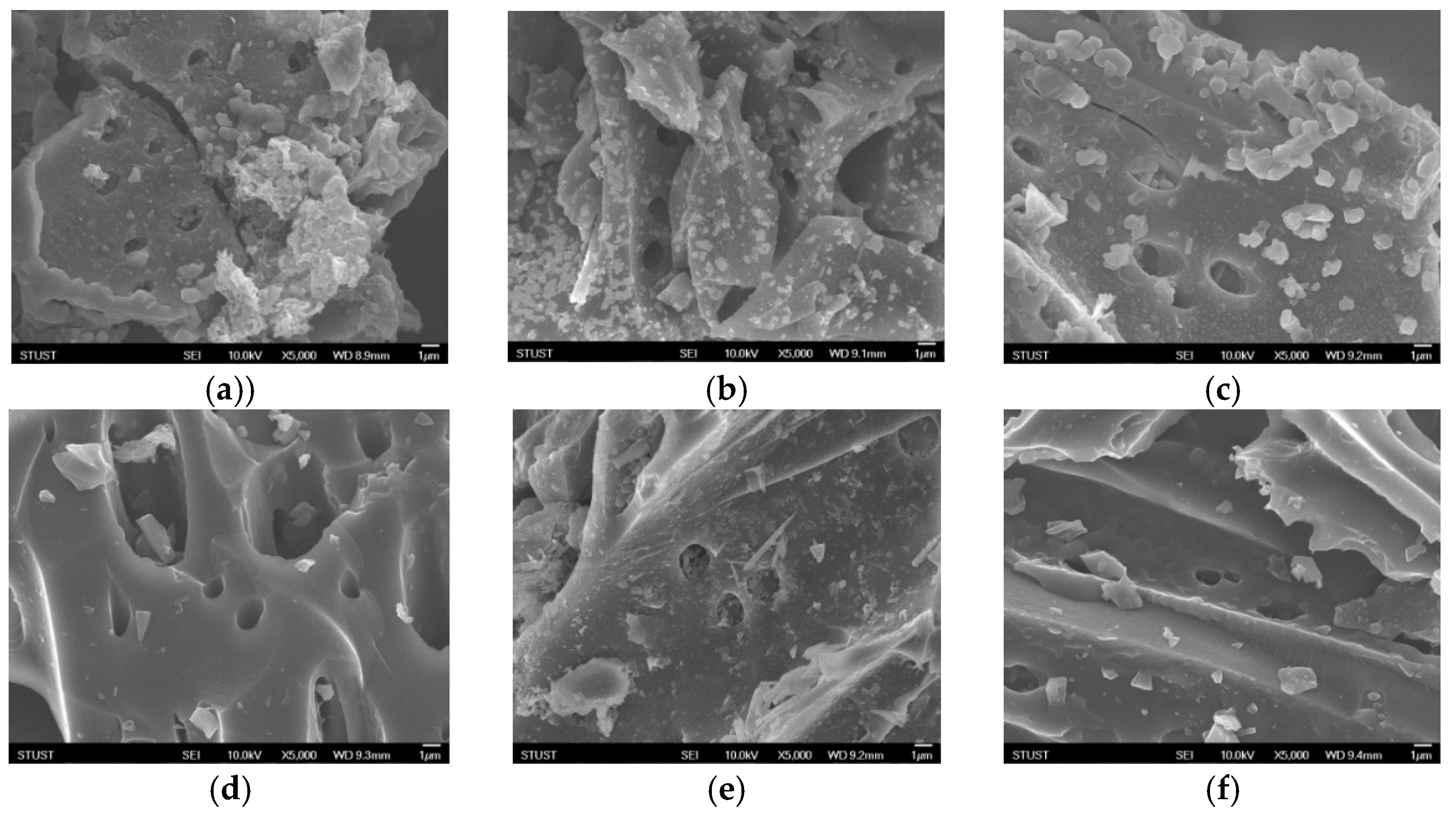
3.1. Characterization of RFSFACs
3.2. Performance Comparison of Present RFSFACs with Other Biomass Activated Carbons
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biloé, S.; Goetz, V.; Guillot, A. Optimal design of activated carbon for an adsorbed natural gas storage system. Carbon 2002, 40, 1295–1308. [Google Scholar] [CrossRef]
- Gundogdu, A.; Duran, C.; Senturk, H.B.; Soylak, M.; Ozdes, D.; Serencam, H.; Imamoglu, M. Adsorption of phenol from aqueous solution on a low-cost activated carbon produced from tea industry waste: Equilibrium, kinetic, and thermodynamic study. J. Chem. Eng. Data 2012, 57, 2733–2743. [Google Scholar] [CrossRef]
- Kårelid, V.; Larsson, G.; Björlenius, B. Pilot-scale removal of pharmaceuticals in municipal wastewater: Comparison of granular and powdered activated carbon treatment at three wastewater treatment plants. J. Environ. Manage. 2017, 193, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Triebskorn, R.; Blaha, L.; Gallert, C.; Giebner, S.; Hetzenauer, H.; Köhler, H.-R.; Kuch, B.; Lüddeke, F.; Oehlmann, J.; Peschke, K.; et al. Freshwater ecosystems profit from activated carbon-based wastewater treatment across various levels of biological organisation in a short timeframe. Environ. Sci. Eur. 2019, 31, 85. [Google Scholar] [CrossRef]
- Habila, M.A.; ALOthman, Z.A.; Al-Tamrah, S.A.; Ghafar, A.A.; Soylak, M. Activated carbon from waste as an efficient adsorbent for malathion for detection and removal purposes. J. Ind. Eng. Chem. 2015, 32, 336–344. [Google Scholar] [CrossRef]
- Patel, M.A.; Luo, F.; Savaram, K.; Kucheryavy, P.; Xie, Q.; Flach, C.; Mendelsohn, R.; Garfunkel, E.; Lockard, J.V.; He, H. P and S dual-doped graphitic porous carbon for aerobic oxidation reactions: Enhanced catalytic activity and catalytic sites. Carbon 2017, 114, 383–392. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.S. Photocatalytic oxidation technology for indoor environment air purification: The state-of-the-art. Appl. Catal. B Environ. 2017, 203, 247–269. [Google Scholar] [CrossRef]
- Yi, J.; Qing, Y.; Wu, C.T.; Zeng, Y.; Wu, Y.; Lu, X.; Tong, Y. Lignocellulose-derived porous phosphorus-doped carbon as advanced electrode for supercapacitors. J. Power Sources 2017, 351, 130–137. [Google Scholar] [CrossRef]
- Li, S.; Han, K.; Si, P.; Li, J.; Lu, C. High-performance activated carbons prepared by KOH activation of gulfweed for supercapacitors. Int. J. Electrochem. Sci. 2018, 13, 1728–1743. [Google Scholar] [CrossRef]
- Mahanim, S.M.A.; Asma, I.W.; Rafidah, J. Production of Activated Carbon from Indrustrial Bamboo Wastes. J. Trop. For. Sci. 2011, 23, 417–424. [Google Scholar]
- Rashidi, N.A.; Yusup, S. A review on recent technological advancement in the activated carbon production from oil palm wastes. Chem. Eng. J. 2017, 314, 277–290. [Google Scholar] [CrossRef]
- Jurewicz, K.; Babeł, K. Efficient capacitor materials from active carbons based on coconut shell/melamine precursors. Energy Fuels 2010, 24, 3429–3435. [Google Scholar] [CrossRef]
- Tangjuank, S.; Insuk, N.; Udeye, V.; Tontrakoon, J. Chromium (III) sorption from aqueous solutions using activated carbon prepared from cashew nut shells. Int. J. Phys. Sci. 2009, 4, 412–417. [Google Scholar]
- Latiff, M.F.P.M.; Abustan, I.; Ahmad, M.A.; Yahaya, N.K.E.M.; Khalid, A.M. Effect of preparation conditions of activated carbon prepared from corncob by CO2 activation for removal of Cu (II) from aqueous solution. AIP Conf. Proc. 2016, 1774, 45–49. [Google Scholar]
- Chen, H.; Wang, H.; Yang, L.; Xiao, Y.; Zheng, M.; Liu, Y.; Fu, H. High specific surface area rice hull based porous carbon prepared for EDLCs. Int. J. Electrochem. Sci. 2012, 7, 4889–4897. [Google Scholar]
- Jun, T.Y.; Arumugam, S.D.; Latip, N.H.A.; Abdullah, A.M.; Latif, P.A. Effect of activation temperature and heating duration on physical characteristics of activated carbon prepared from agriculture waste. Environ. Asia 2010, 3, 143–148. [Google Scholar]
- Jadhav, A.S.; Salwe, S.; Tambe, M.; Shinde, N.H. Chemical Characterisation of Biomass Waste by Proximate Analysis Method Using Catalyst. Int. J. Adv. Eng. Technol. 2011, 2, 1–19. [Google Scholar]
- Balathanigaimani, M.S.; Shim, W.G.; Lee, M.J.; Kim, C.; Lee, J.W.; Moon, H. Highly porous electrodes from novel corn grains-based activated carbons for electrical double layer capacitors. Electrochem. Commun. 2008, 10, 868–871. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Yang, K.; Lu, W.; Yu, J.; Gao, J.; Liao, G.; Qu, Y.; Wang, X.; Li, X.; et al. Hierarchical porous carbon microspheres derived from biomass-corncob as ultra-high performance supercapacitor electrode. Int. J. Electrochem. Sci. 2017, 12, 5604–5617. [Google Scholar] [CrossRef]
- Li, Q.; Liu, F.; Zhang, L.; Nelson, B.J.; Zhang, S.; Ma, C.; Tao, X.; Cheng, J.; Zhang, X. In situ construction of potato starch based carbon nanofiber/activated carbon hybrid structure for high-performance electrical double layer capacitor. J. Power Sources 2012, 207, 199–204. [Google Scholar] [CrossRef]
- Lv, Y.; Gan, L.; Liu, M.; Xiong, W.; Xu, Z.; Zhu, D.; Wright, D.S. A self-template synthesis of hierarchical porous carbon foams based on banana peel for supercapacitor electrodes. J. Power Sources 2012, 209, 152–157. [Google Scholar] [CrossRef]
- Ukanwa, K.S.; Patchigolla, K.; Sakrabani, R.; Anthony, E.; Mandavgane, S. A review of chemicals to produce activated carbon from agricultural waste biomass. Sustainability 2019, 11, 6204. [Google Scholar] [CrossRef]
- Direktorat Jenderal Perkebunan Kementerian Pertanian Republik Indonesia. Pembangunan Perkebunan 2019. Available online: http://ditjenbun.pertanian.go.id/2019/ (accessed on 14 September 2020).
- Eka, H.D.; Tajul Aris, Y.; Wan Nadiah, W.A. Potential use of Malaysian rubber (Hevea brasiliensis) seed as food, feed and biofuel. Int. Food Res. J. 2010, 17, 527–534. [Google Scholar]
- Mulyadi, E. Proses Produksi Biodiesel Berbasis Biji Karet. J. Rekayasa Proses 2011, 5, 40–44. [Google Scholar]
- Grycova, B.; Pryszcz, A.; Matejova, L.; Lestinsky, P. Influence of activating reagents on the porous structure of activated carbon. Chem. Eng. Trans. 2018, 70, 1897–1902. [Google Scholar]
- Ozdemir, I.; Şahin, M.; Orhan, R.; Erdem, M. Preparation and characterization of activated carbon from grape stalk by zinc chloride activation. Fuel Process. Technol. 2014, 125, 200–206. [Google Scholar] [CrossRef]
- Basta, A.H.; Fierro, V.; El-Saied, H.; Celzard, A. 2-Steps KOH activation of rice straw: An efficient method for preparing high-performance activated carbons. Bioresour. Technol. 2009, 100, 3941–3947. [Google Scholar] [CrossRef] [PubMed]
- Hirunpraditkoon, S.; Tunthong, N.; Ruangchai, A.; Nuithitikul, K. Adsorption Capacities of Activated Carbons Prepared from Bamboo by KOH Activation. World Acad. Sci. Eng. Technol. Int. J. Chem. Mol. Eng. 2011, 5, 477–481. [Google Scholar]
- Cruz, G. Production of Activated Carbon from Cocoa (Theobroma cacao) Pod Husk. J. Civ. Environ. Eng. 2012, 2, 2–7. [Google Scholar] [CrossRef]
- Abechi, S.E.; Gimba, C.E.; Uzairu, A.; Dallatu, Y.A. Preparation and Characterization of Activated Carbon from Palm Kernel Shell by Chemical Activation. Res. J. Chem. Sci. 2013, 3, 54–61. [Google Scholar]
- Gao, J.J.; Qin, Y.B.; Zhou, T.; Cao, D.D.; Xu, P.; Hochstetter, D.; Wang, Y.F. Adsorption of methylene blue onto activated carbon produced from tea (Camellia sinensis L.) seed shells: Kinetics, equilibrium, and thermodynamics studies. J. Zhejiang Univ. Sci. B 2013, 14, 650–658. [Google Scholar] [CrossRef]
- Theydan, S.K.; Ahmed, M.J. Optimization of preparation conditions for activated carbons from date stones using response surface methodology. Powder Technol. 2012, 224, 101–108. [Google Scholar] [CrossRef]
- Köseoʇlu, E.; Akmil-Başar, C. Preparation, structural evaluation and adsorptive properties of activated carbon from agricultural waste biomass. Adv. Powder Technol. 2015, 26, 811–818. [Google Scholar] [CrossRef]
- Chang, B.; Wang, Y.; Pei, K.; Yang, S.; Dong, X. ZnCl2-activated porous carbon spheres with high surface area and superior mesoporous structure as an efficient supercapacitor electrode. RSC Adv. 2014, 4, 40546–40552. [Google Scholar] [CrossRef]
- Yang, H.M.; Zhang, D.H.; Chen, Y.; Ran, M.J.; Gu, J.C. Study on the application of KOH to produce activated carbon to realize the utilization of distiller’s grains. IOP Conf. Ser. Earth Environ. Sci. 2017, 69, 012051. [Google Scholar] [CrossRef]
- Mozammel, H.M.; Masahiro, O.; Bhattacharya, S.C. Activated charcoal from coconut shell using ZnCl2 activation. Biomass Bioenergy 2002, 22, 397–400. [Google Scholar] [CrossRef]
- Baseri, J.R.; Palanisamy, P.N.; Sivakumar, P. Preparation and characterization of activated carbon from Thevetia peruviana for the removal of dyes from textile waste water. Adv. Appl. Sci. Res. 2012, 3, 377–383. [Google Scholar]
- García, R.; Pizarro, C.; Lavín, A.G.; Bueno, J.L. Biomass proximate analysis using thermogravimetry. Bioresour. Technol. 2013, 139, 1–4. [Google Scholar] [CrossRef]
- Zulfadhli, M. Iriany Pembuatan Karbon Dari Cangkang Buah karet (Hevea brasilliensis) dengan Aktivator H3PO4 dan Aplikasinya Sebagai Penyerap Cr (VI). J. Tek. Kim. USU 2017, 6, 23–28. [Google Scholar] [CrossRef]
- Maulina, S.; Iriansyah, M. Characteristics of activated carbon resulted from pyrolysis of the oil palm fronds powder. IOP Conf. Ser. Mater. Sci. Eng. 2018, 309, 012072. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, J.; Chen, Y.; Hao, X.; Jin, X. Characterization, preparation, and reaction mechanism of hemp stem based activated carbon. Results Phys. 2017, 7, 1628–1633. [Google Scholar] [CrossRef]
- Hsu, L.Y.; Teng, H. Influence of different chemical reagents on the preparation of activated carbons from bituminous coal. Fuel Process. Technol. 2000, 64, 155–166. [Google Scholar] [CrossRef]
- Tseng, R.L.; Tseng, S.K.; Wu, F.C.; Hu, C.C.; Wang, C.C. Effects of micropore development on the physicochemical properties of KOH-activated carbons. J. Chin. Inst. Chem. Eng. 2008, 39, 37–47. [Google Scholar] [CrossRef]
- Mopoung, S.; Moonsri, P.; Palas, W.; Khumpai, S. Characterization and Properties of Activated Carbon Prepared from Tamarind Seeds by KOH Activation for Fe(III) Adsorption from Aqueous Solution. Sci. World J. 2015, 2015. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, E.; Zhao, G. Thermal and structure analysis on reaction mechanisms during the preparation of activated carbon fibers by KOH activation from liquefied wood-based fibers. Ind. Crops Prod. 2015, 69, 447–455. [Google Scholar] [CrossRef]
- Chen, Y.D.; Huang, M.J.; Huang, B.; Chen, X.R. Mesoporous activated carbon from inherently potassium-rich pokeweed by in situ self-activation and its use for phenol removal. J. Anal. Appl. Pyrolysis 2012, 98, 159–165. [Google Scholar] [CrossRef]
- He, X.J.; Wang, T.; Qiu, J.S.; Zhang, X.Y.; Wang, X.T.; Zheng, M.D. Effect of microwave-treatment time on the properties of activated carbons for electrochemical capacitors. Xinxing Tan Cailiao/New Carbon Mater. 2011, 26, 313–319. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Li, L.; Shi, X.; Wang, Z. Preparation and analysis of activated carbon from sewage sludge and corn stalk. Adv. Powder Technol. 2016, 27, 684–691. [Google Scholar] [CrossRef]
- Meilianti, M. Karakteristik Karbon Aktif Dari Cangkang Buah Karet Menggunakan Aktivator H3Po4. J. Distilasi 2018, 2, 1. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Arami-Niya, A.; Daud, W.M.A.W.; Mjalli, F.S. Comparative study of the textural characteristics of oil palm shell activated carbon produced by chemical and physical activation for methane adsorption. Chem. Eng. Res. Des. 2011, 89, 657–664. [Google Scholar] [CrossRef]
- Sudaryanto, Y.; Hartono, S.B.; Irawaty, W.; Hindarso, H.; Ismadji, S. High surface area activated carbon prepared from cassava peel by chemical activation. Bioresour. Technol. 2006, 97, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Subha, R.; Namasivayam, C. ZnCl2-modified activated carbon from biomass coir pith for the removal of 2-chlorophenol by adsorption process. Bioremediat. J. 2010, 14, 1–9. [Google Scholar] [CrossRef]
- Hamzah, Y.; Umar, L. Preparation of creating active carbon from cigarette filter waste using microwave-induced KOH activation. J. Phys. Conf. Ser. 2017, 853, 012027. [Google Scholar] [CrossRef]
- Hidayu, A.R.; Muda, N. Preparation and Characterization of Impregnated Activated Carbon from Palm Kernel Shell and Coconut Shell for CO2 Capture. Procedia Eng. 2016, 148, 106–113. [Google Scholar] [CrossRef]
- Ahmed, S.; Mada, V.V.; Kamath, V.; Jeppu, G.P. Characterization Of Activated Carbon Prepared From Coconut Shell Using Various Reagents For A Low Cost Water- Filter. Int. J. Eng. Technol. 2017, 9, 180–188. [Google Scholar] [CrossRef]
- Gawande, P.R.; Kaware, J. Characterization and activation of coconut shell activated carbon Research Paper. Int. J. Eng. Sci. Invent. 2017, 6, 43–49. [Google Scholar]
- Alhamed, Y. Activated Carbon from Dates’ Stone by ZnCl2 Activation. J. King Abdulaziz Univ. Sci. 2006, 17, 75–98. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, Y.; Zhao, G. Preparation and characterization of activated carbon fibers from liquefied wood by ZnCl2 activation. BioResources 2016, 11, 3178–3190. [Google Scholar] [CrossRef]
- Anis, D.; Zaidi, A.G.; Khudzir, I.; Iqbaldin, M.N.; Osman, U.M.; Nawawi, W.I. Production of Rubber Seed Pericarp Based Activated Carbon Using Microwave-Induced Different Chemical Activating Agent. Int. J. Sci. Res. Publ. 2014, 4, 1–8. [Google Scholar]
- Koehlert, K. Activated carbon: Fundamentals and new applications. Chem. Eng. 2017, 124, 32–40. [Google Scholar]
- Sujita, R.S.; Siswanto, E.; Widodo, T.D. Influence of Reheating in Pack Carburizing Process with Bamboo Charcoal and Cow Bone Powder Media for Hardness Number and Impact Strength Low Carbon Steel. Int. J. Appl. Eng. Res. 2018, 13, 2078–2083. [Google Scholar]

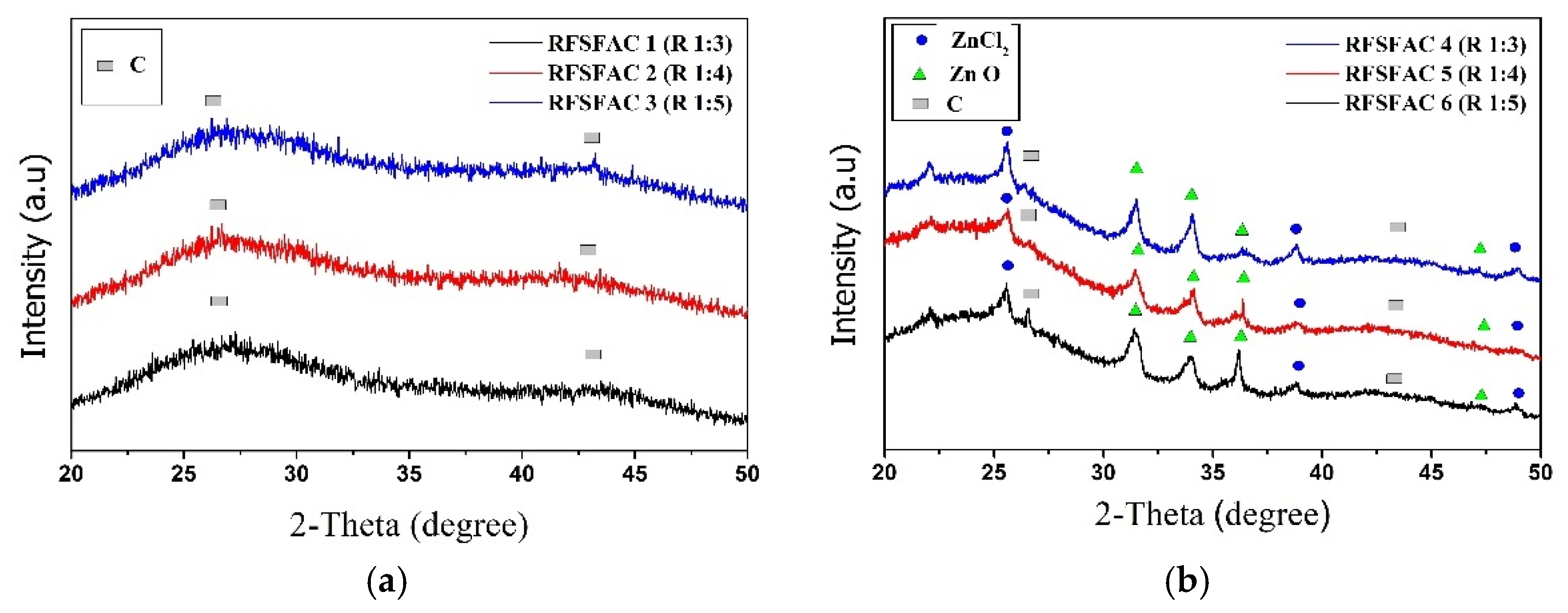
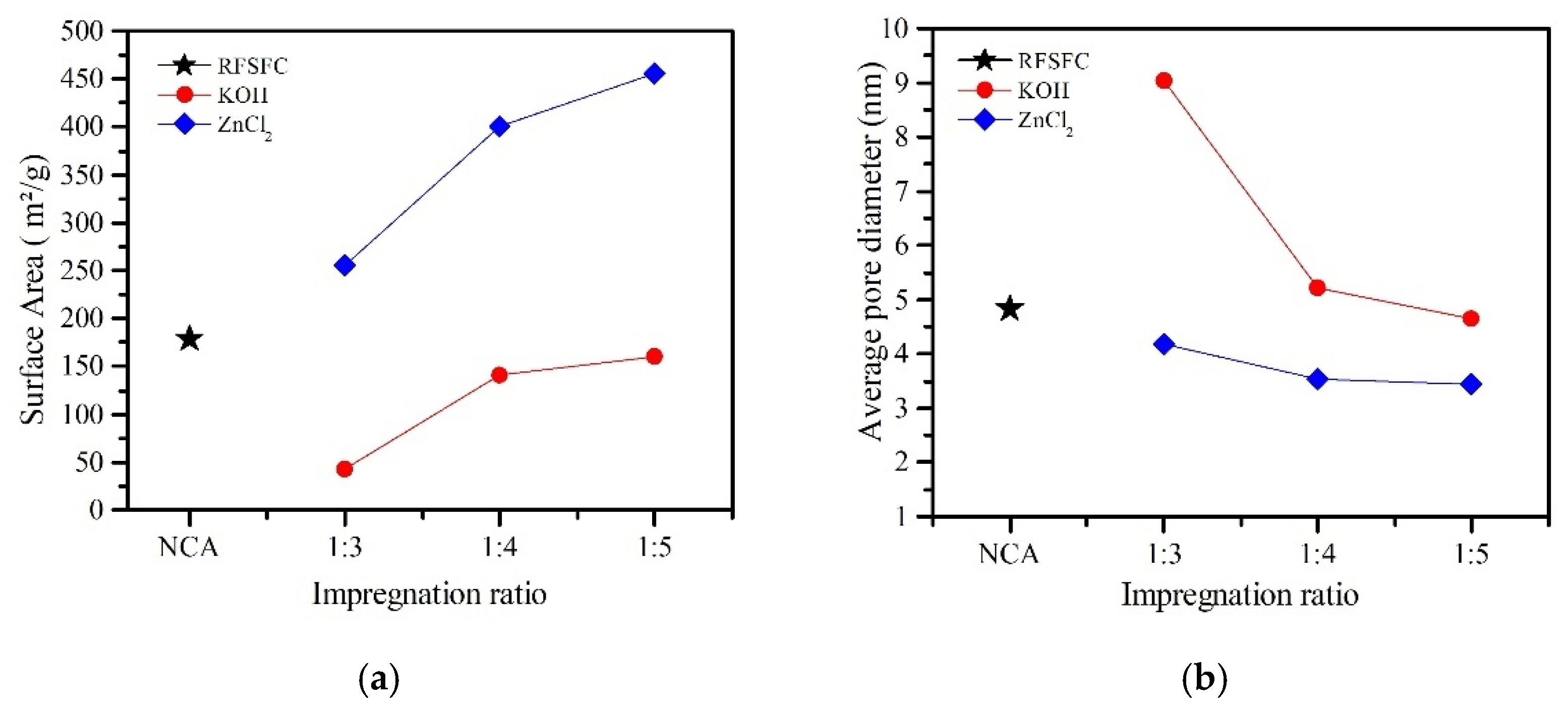
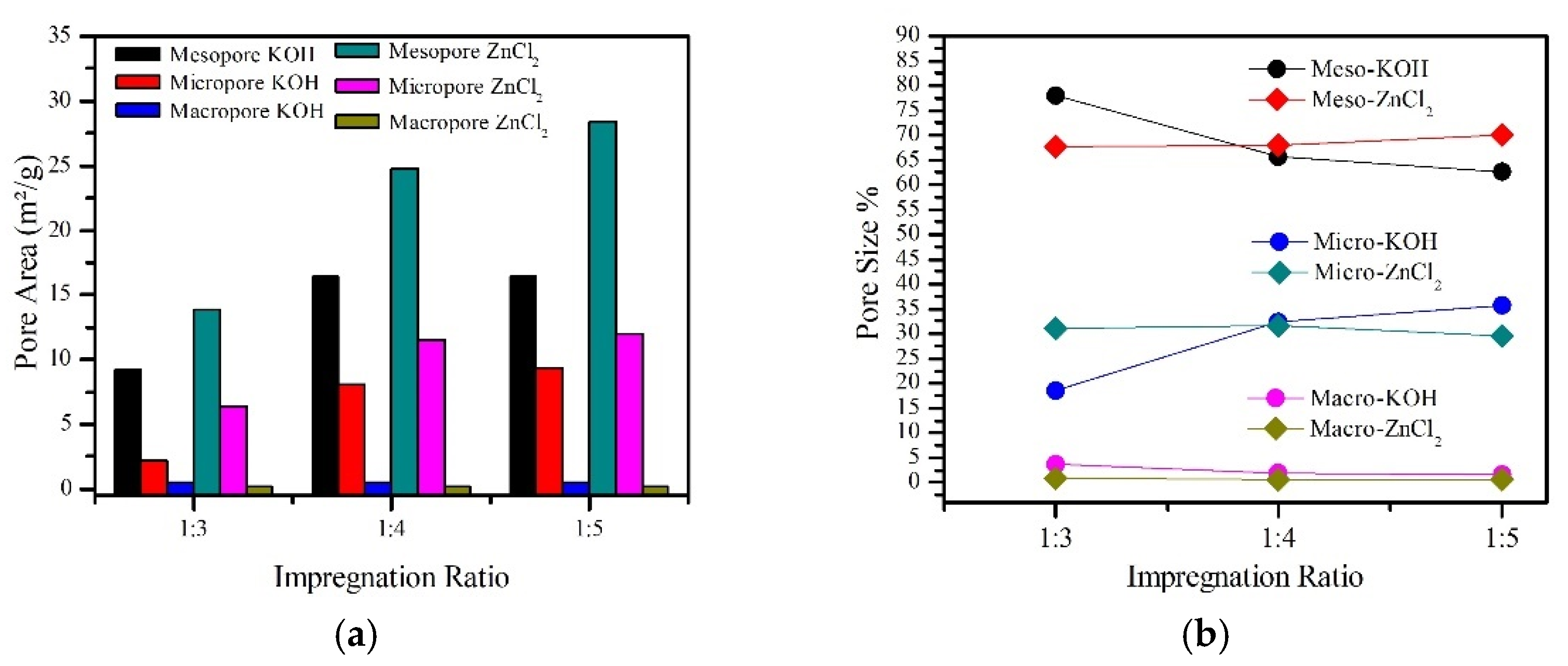
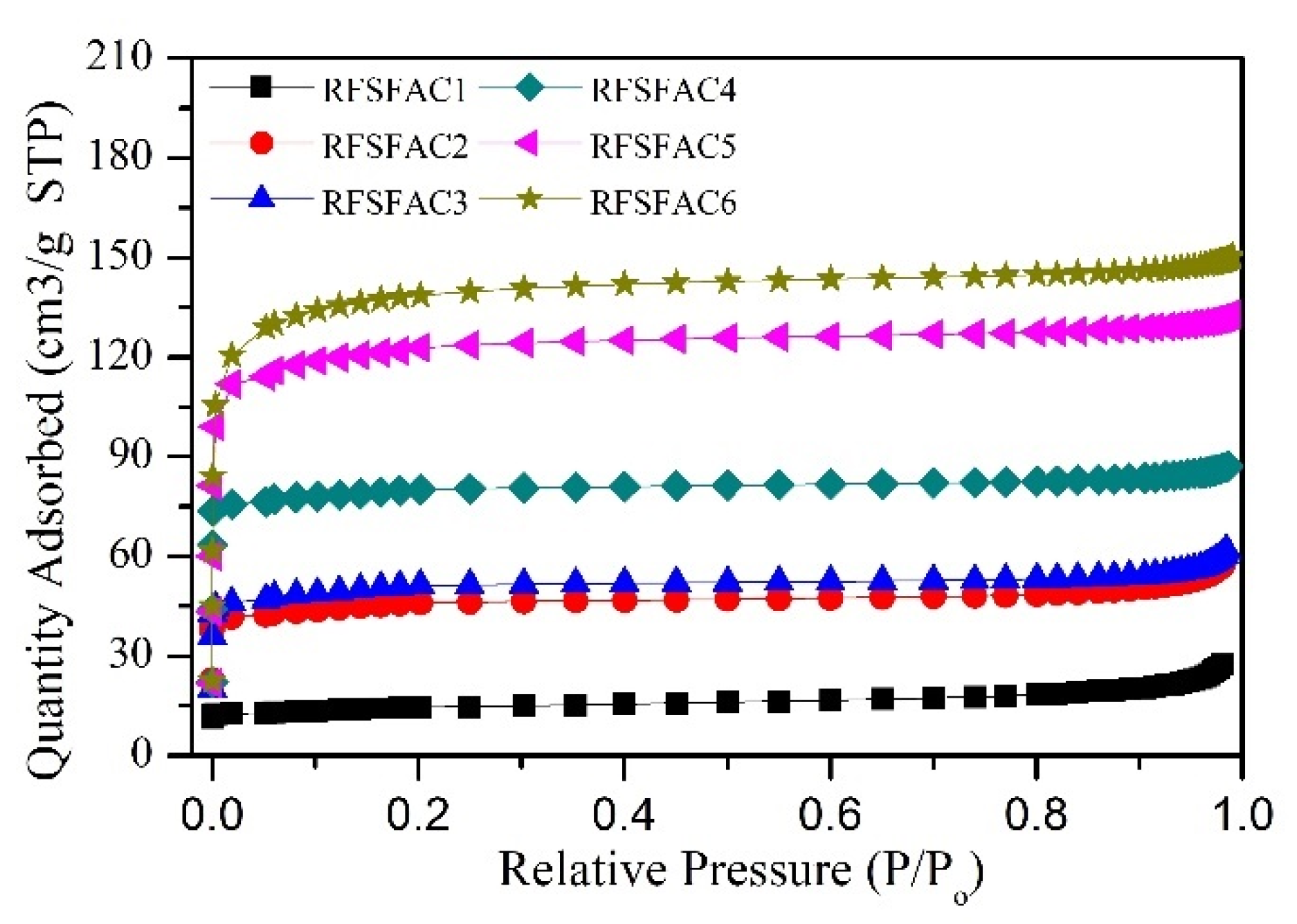
| Sample | Impregnation Ratio (wt.%) | Chemical Solution | Temperature (°C) |
|---|---|---|---|
| RFSFC | - | - | - |
| RFSFAC1 | 1:3 | KOH | 800 |
| RFSFAC2 | 1:4 | KOH | 800 |
| RFSFAC3 | 1:5 | KOH | 800 |
| RFSFAC4 | 1:3 | ZnCl2 | 500 |
| RFSFAC5 | 1:4 | ZnCl2 | 500 |
| RFSFAC6 | 1:5 | ZnCl2 | 500 |
| Proximate Analysis (Weight %) | RFSFC Powder | KOH | ZnCl2 | ||||
|---|---|---|---|---|---|---|---|
| RFSFAC | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Moisture | 0.43 | 16.76 | 20.61 | 23.51 | 13.31 | 13.76 | 14.98 |
| Volatile matter | 97.06 | 56.38 | 47.37 | 42.24 | 48.24 | 34.90 | 33.44 |
| Fixed carbon | 1.05 | 16.26 | 21.25 | 23.84 | 23.26 | 36.97 | 38.09 |
| Ash | 1.46 | 10.59 | 10.77 | 10.41 | 15.19 | 14.38 | 13.50 |
| Elemental Contents (Atomic %) | |||||||
| Carbon | 74.11 | 72.29 | 77.63 | 78.40 | 65.94 | 81.83 | 80.05 |
| Oxygen | 11.06 | 27.65 | 19.92 | 18.73 | 23.77 | 12.67 | 17.42 |
| Nitrogen | 13.66 | - | - | - | - | - | - |
| Silicon | 0.16 | 0.05 | 0.61 | 0.37 | 0.93 | 0.03 | 0.10 |
| Potassium | 0.86 | - | 1.84 | 2.5 | 0.08 | 0.02 | 0.07 |
| Chlorine | - | - | - | - | 4.41 | 3.45 | 1.25 |
| Zinc | - | - | - | - | 4.86 | 2.00 | 1.11 |
| Sample | BET (m2/g) | Adsorption Quantity (cm3/g) | Average Pore Diameter (nm) | Pore Area | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mesopores | Micropores | Macropores | |||||||
| (m2/g) | (%) | (m2/g) | (%) | (m2/g) | (%) | ||||
| RFSFC | 178 | 55.91 | 4.83 | 11.41 | 68 | 5.18 | 31 | 0.24 | 1 |
| RFSFAC1 | 42 | 27.35 | 9.03 | 9.25 | 78 | 2.19 | 18 | 0.44 | 4 |
| RFSFAC2 | 141 | 58.03 | 5.21 | 16.38 | 66 | 8.08 | 32 | 0.46 | 2 |
| RFSFAC3 | 160 | 61.83 | 4.64 | 16.39 | 63 | 9.34 | 36 | 0.44 | 2 |
| RFSFAC4 | 256 | 87.20 | 4.19 | 13.88 | 68 | 6.39 | 31 | 0.20 | 1 |
| RFSFAC5 | 400 | 132.92 | 3.54 | 24.74 | 68 | 11.50 | 32 | 0.20 | 1 |
| RFSFAC6 | 456 | 150.38 | 3.44 | 28.46 | 70.1 | 11.95 | 29.4 | 0.20 | 0.5 |
| Precursor | Chemical Activation Agent | BET (m2/g) | Adsorption Quantity (cm3/g) | Average Pore Diameter (nm) | Carbon Content (at.%) | Ref. |
|---|---|---|---|---|---|---|
| Oil palm shell | ZnCl2 | 1118 | 330 | 2.2 | 50 | [52] |
| Tamarind seeds | KOH | 2.72 | Na | Na | 23 | [45] |
| Coir pith | ZnCl2 | 910 | Na | Na | 70 | [54] |
| Bamboo wastes | - | 719 | Na | Na | 77 | [10] |
| Cassava peel | KOH | 1605 | Na | Na | 59 | [53] |
| Tea industry waste | ZnCl2 | 1066 | Na | 2.2 | 79 | [2] |
| Cigarette filter waste | KOH | 328 | 74 | 3.01 | 69 | [55] |
| Coconut shell | ZnCl2 | 953 | Na | 18.2 Å | 75 | [56] |
| ZnCl2 | 251 | Na | Na | Na | [57] | |
| H2SO4 | 435 | Na | Na | Na | [58] | |
| RFSFAC | ZnCl2 | 456 | 151 | 3.4 | 80.05 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suhdi; Wang, S.-C. Fine Activated Carbon from Rubber Fruit Shell Prepared by Using ZnCl2 and KOH Activation. Appl. Sci. 2021, 11, 3994. https://doi.org/10.3390/app11093994
Suhdi, Wang S-C. Fine Activated Carbon from Rubber Fruit Shell Prepared by Using ZnCl2 and KOH Activation. Applied Sciences. 2021; 11(9):3994. https://doi.org/10.3390/app11093994
Chicago/Turabian StyleSuhdi, and Sheng-Chang Wang. 2021. "Fine Activated Carbon from Rubber Fruit Shell Prepared by Using ZnCl2 and KOH Activation" Applied Sciences 11, no. 9: 3994. https://doi.org/10.3390/app11093994
APA StyleSuhdi, & Wang, S.-C. (2021). Fine Activated Carbon from Rubber Fruit Shell Prepared by Using ZnCl2 and KOH Activation. Applied Sciences, 11(9), 3994. https://doi.org/10.3390/app11093994






