The Impact of Radiation to Epicardial Adipose Tissue on Prognosis of Esophageal Squamous Cell Carcinoma Receiving Neoadjuvant Chemoradiotherapy and Esophagectomy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Neoadjuvant Chemoradiotherapy and Surgery
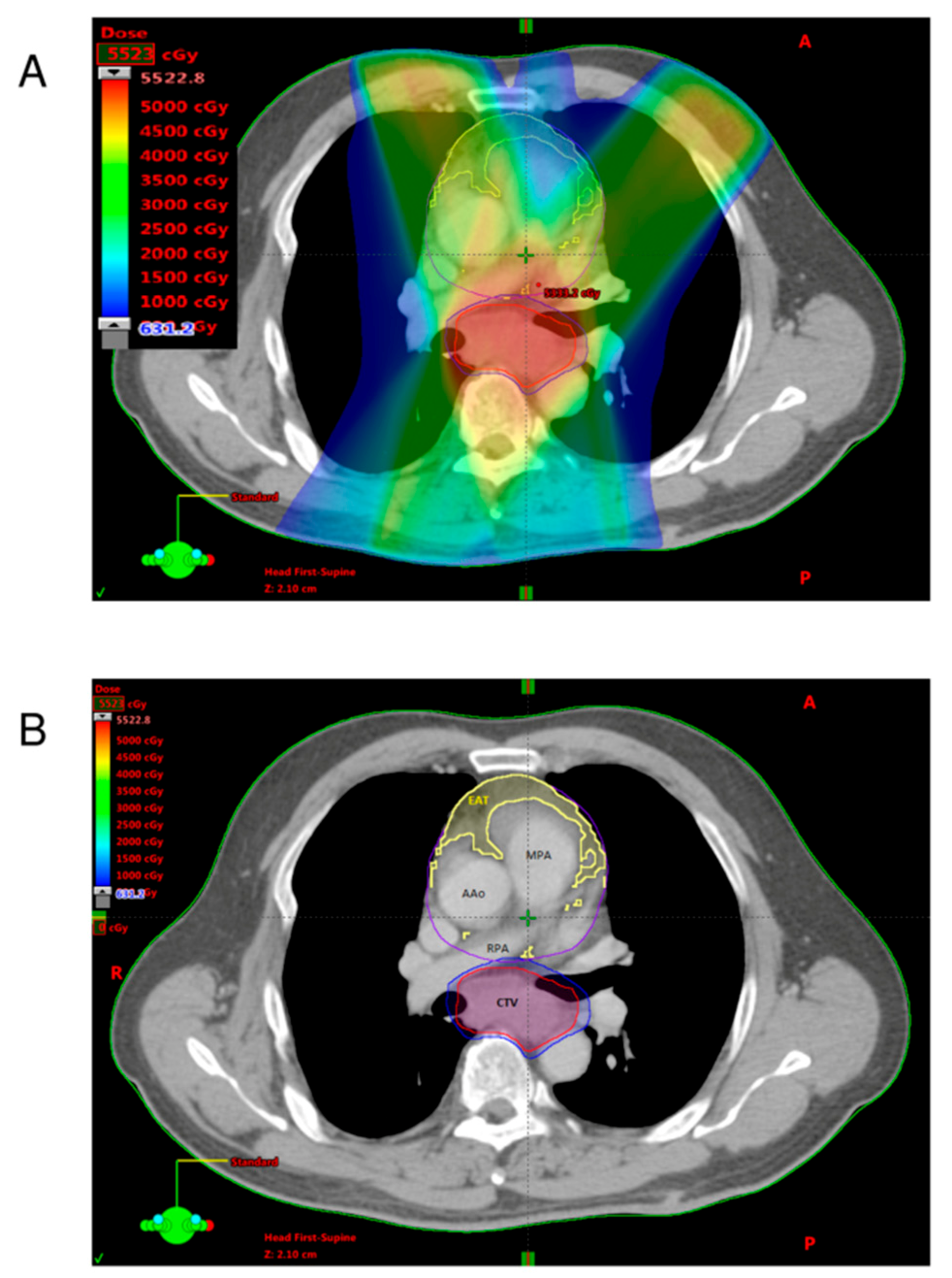
2.3. Quantification of Adipose Tissues
2.4. Definition of EAT-REI
2.5. Nutrition Status Evaluation
2.6. Surveillance and Recurrence Evaluation
2.7. Statistical Analysis
3. Results
3.1. Patients and Clinical Outcome
3.2. Analysis of ROC Curve
3.3. Analysis of Radiotherapy Dosimetric Parameters
3.4. Analysis of EAT-REI Ratio High and Low Group
3.5. Analysis of Nutrition Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapiro, J.; van Lanschot, J.J.B.; Hulshof, M.; van Hagen, P.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol. 2015, 16, 1090–1098. [Google Scholar] [CrossRef]
- Sjoquist, K.M.; Burmeister, B.H.; Smithers, B.M.; Zalcberg, J.R.; Simes, R.J.; Barbour, A.; Gebski, V. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: An updated meta-analysis. Lancet Oncol. 2011, 12, 681–692. [Google Scholar] [CrossRef]
- Tepper, J.; Krasna, M.J.; Niedzwiecki, D.; Hollis, D.; Reed, C.E.; Goldberg, R.; Kiel, K.; Willett, C.; Sugarbaker, D.; Mayer, R. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 1086–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muro, K.; Lordick, F.; Tsushima, T.; Pentheroudakis, G.; Baba, E.; Lu, Z.; Cho, B.C.; Nor, I.M.; Ng, M.; Chen, L.T.; et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic oesophageal cancer: A JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.D.; Paulus, R.; Komaki, R.; Masters, G.; Blumenschein, G.; Schild, S.; Bogart, J.; Hu, C.; Forster, K.; Magliocco, A.; et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015, 16, 187–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Bronnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Lin, J.B.; Sun, F.J.; Lu, K.W.; Lee, C.H.; Chen, Y.J.; Huang, W.C.; Liu, H.C.; Wu, M.H. Dosimetric predictors of acute haematological toxicity in oesophageal cancer patients treated with neoadjuvant chemoradiotherapy. Br. J. Radiol. 2016, 89, 20160350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef] [Green Version]
- Demir, E.; Harmankaya, N.O.; Kirac Utku, I.; Aciksari, G.; Uygun, T.; Ozkan, H.; Demir, B. The Relationship between Epicardial Adipose Tissue Thickness and Serum Interleukin-17a Level in Patients with Isolated Metabolic Syndrome. Biomolecules 2019, 9, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacks, H.S.; Fain, J.N.; Holman, B.; Cheema, P.; Chary, A.; Parks, F.; Karas, J.; Optican, R.; Bahouth, S.W.; Garrett, E.; et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: Epicardial fat functioning as brown fat. J. Clin. Endocrinol. Metab. 2009, 94, 3611–3615. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.C.; Dai, K.Y.; Wu, M.C.; Hua, K.L.; Tai, H.C.; Huang, W.C.; Chen, Y.J. Bio-physic constraint model using spatial registration of delta 18F-fluorodeoxyglucose positron emission tomography/computed tomography images for predicting radiation pneumonitis in esophageal squamous cell carcinoma patients receiving neoadjuvant chemoradiation. OncoTargets Ther. 2019, 12, 6439–6451. [Google Scholar] [CrossRef] [Green Version]
- Yun, C.H.; Lin, T.Y.; Wu, Y.J.; Liu, C.C.; Kuo, J.Y.; Yeh, H.I.; Yang, F.S.; Chen, S.C.; Hou, C.J.; Bezerra, H.G.; et al. Pericardial and thoracic peri-aortic adipose tissues contribute to systemic inflammation and calcified coronary atherosclerosis independent of body fat composition, anthropometric measures and traditional cardiovascular risks. Eur. J. Radiol. 2012, 81, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Mahabadi, A.A.; Massaro, J.M.; Rosito, G.A.; Levy, D.; Murabito, J.M.; Wolf, P.A.; O’Donnell, C.J.; Fox, C.S.; Hoffmann, U. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: The Framingham Heart Study. Eur. Heart J. 2009, 30, 850–856. [Google Scholar] [CrossRef] [Green Version]
- Lehman, S.J.; Massaro, J.M.; Schlett, C.L.; O’Donnell, C.J.; Hoffmann, U.; Fox, C.S. Peri-aortic fat, cardiovascular disease risk factors, and aortic calcification: The Framingham Heart Study. Atherosclerosis 2010, 210, 656–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, C.H.; Bezerra, H.G.; Wu, T.H.; Yang, F.S.; Liu, C.C.; Wu, Y.J.; Kuo, J.Y.; Hung, C.L.; Lee, J.J.; Hou, C.J.; et al. The normal limits, subclinical significance, related metabolic derangements and distinct biological effects of body site-specific adiposity in relatively healthy population. PLoS ONE 2013, 8, e61997. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.H.; Yun, C.H.; Yang, F.S.; Liu, C.C.; Wu, Y.J.; Kuo, J.Y.; Yeh, H.I.; Lin, T.Y.; Bezerra, H.G.; Shih, S.C.; et al. Epicardial adipose tissue relating to anthropometrics, metabolic derangements and fatty liver disease independently contributes to serum high-sensitivity C-reactive protein beyond body fat composition: A study validated with computed tomography. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2012, 25, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.H.; Hou, C.J.; Yun, C.H.; Sung, K.T.; Su, C.H.; Wu, T.H.; Yang, F.S.; Hung, T.C.; Hung, C.L.; Bezerra, H.G.; et al. The association among MDCT-derived three-dimensional visceral adiposities on cardiac diastology and dyssynchrony in asymptomatic population. BMC Cardiovasc. Disord. 2015, 15, 142. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.B.; Hung, L.C.; Cheng, C.Y.; Chien, Y.A.; Lee, C.H.; Huang, C.C.; Chou, T.W.; Ko, M.H.; Lai, Y.C.; Liu, M.T.; et al. Prognostic significance of lung radiation dose in patients with esophageal cancer treated with neoadjuvant chemoradiotherapy. Radiat. Oncol. 2019, 14, 85. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.Z.; Chou, K.J.; Huang, Y.L.; Wu, M.T. The relation of location-specific epicardial adipose tissue thickness and obstructive coronary artery disease: Systemic review and meta-analysis of observational studies. BMC Cardiovasc. Disord. 2014, 14, 62. [Google Scholar] [CrossRef]
- Wang, C.P.; Hsu, H.L.; Hung, W.C.; Yu, T.H.; Chen, Y.H.; Chiu, C.A.; Lu, L.F.; Chung, F.M.; Shin, S.J.; Lee, Y.J. Increased epicardial adipose tissue (EAT) volume in type 2 diabetes mellitus and association with metabolic syndrome and severity of coronary atherosclerosis. Clin. Endocrinol. 2009, 70, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Rengo, G.; Pagano, G.; D’Esposito, V.; Passaretti, F.; Caruso, A.; Grimaldi, M.G.; Lonobile, T.; Baldascino, F.; De Bellis, A.; et al. Epicardial adipose tissue has an increased thickness and is a source of inflammatory mediators in patients with calcific aortic stenosis. Int. J. Cardiol. 2015, 186, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Poglio, S.; Galvani, S.; Bour, S.; André, M.; Prunet-Marcassus, B.; Pénicaud, L.; Casteilla, L.; Cousin, B. Adipose tissue sensitivity to radiation exposure. Am. J. Pathol. 2009, 174, 44–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Y.; Mo, W.; Jia, H.; Yu, D.; Qiu, Y.; Jiao, Y.; Zhu, W.; Koide, H.; Cao, J.; Zhang, S. Ionizing radiation induces cutaneous lipid remolding and skin adipocytes confer protection against radiation-induced skin injury. J. Dermatol. Sci. 2020, 97, 152–160. [Google Scholar] [CrossRef]
- Lee, J.; Lin, J.B.; Wu, M.H.; Jan, Y.T.; Chang, C.L.; Huang, C.Y.; Sun, F.J.; Chen, Y.J. Muscle radiodensity loss during cancer therapy is predictive for poor survival in advanced endometrial cancer. J. Cachexia Sarcopenia Muscle 2019, 10, 814–826. [Google Scholar] [CrossRef] [Green Version]


| Characteristics | Overall (n = 36) |
|---|---|
| Age (years) | 58.8 ± 8.6 |
| Sex | |
| Man | 34 (94.4%) |
| Woman | 2 (5.6%) |
| BMI (kg/m2) | 22.1 ± 4.3 |
| Albumin (g/dL) | 4.0 ± 0.5 |
| Hb (g/dL) | 12.0 ± 1.4 |
| Lymphocyte (%) | 21.8 ± 9.0 |
| Cholesterol (mg/dL) | 181.5 ± 40.0 |
| Triglycerides (mg/dL) | 116.2 ± 57.2 |
| Metabolic diseases | 6 (16.7%) |
| Cardiac diseases | 9 (25.0%) |
| Clinical T stage | |
| cT1-2 | 9 (25.0%) |
| cT3-4 | 27 (75.0%) |
| Clinical N stage | |
| cN0-1 | 19 (52.8%) |
| cN2-3 | 17 (47.2%) |
| cTNM stage | |
| II | 10 (27.8%) |
| III | 26 (72.2%) |
| Target volume (mL) | 688.8 ± 271.2 |
| Heart volume (mL) | 621.4 ± 117.1 |
| Heart mean dose (Gy) | 25.9 ± 6.9 |
| Dose-volume of EAT | |
| Volume (mL) | 19.2 (8.4–29.4) |
| Mean dose (Gy) | 21.5 (16.2–26.4) |
| V5 * | 16.5 (8.2–26.6) |
| V10 | 14.0 (6.8–21.9) |
| V20 | 10.2 (3.5–16.5) |
| V30 | 4.6 (1.5–11.4) |
| V40 | 1.1 (0.5–3.5) |
| SAT volume (mL) | 1239.8 (682.8–1795.5) |
| SAT mean dose (Gy) | 6.7 (5.4–7.8) |
| VAT volume (mL) | 124.2 (76.3–251.3) |
| VAT mean dose (Gy) | 22.5 (17.7–26.6) |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Characteristics | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Age | 1.005 (0.953–1.060) | 0.859 | ||
| BMI (kg/m2) | 0.999 (0.907–1.099) | 0.979 | ||
| Albumin (g/dL) | 0.772 (0.351–1.702) | 0.522 | ||
| Hb (g/dL) | 0.986 (0.733–1.326) | 0.926 | ||
| Lymphocyte (%) | 0.976 (0.929–1.025) | 0.325 | ||
| Cholesterol (mg/dL) | 1.002 (0.987–1.017) | 0.785 | ||
| Triglycerides (mg/dL) | 1.000 (0.991–1.008) | 0.931 | ||
| Metabolic diseases | 1.392 (0.470–4.123) | 0.550 | ||
| Cardiac diseases | 0.558 (0.188–1.655) | 0.293 | ||
| Clinical T (T1–2 vs. T3–4) | 0.658 (0.303–1.432) | 0.292 | ||
| Clinical N (N0–1 vs. N2–3) | 1.235 (0.534–2.854) | 0.621 | ||
| cTNM stage (II vs. III) | 0.790 (0.321–1.943) | 0.608 | ||
| Pathological response (non-pCR vs. pCR) | 0.561 (0.218–1.445) | 0.231 | ||
| Target volume | 1.001 (1.000–1.003) | 0.125 | ||
| Heart mean dose | 1.000 (1.000–1.001) | 0.639 | ||
| Dose-volume of EAT * | ||||
| Volume (mL) | 0.981 (0.957–1.007) | 0.149 | ||
| Mean dose (Gy) | 1.000 (1.000–1.001) | 0.702 | ||
| V5 | 0.971 (0.943–1.000) | 0.051 | ||
| V10 | 0.967 (0.935–1.000) | 0.049 | 0.988 (0.952–1.026) | 0.529 |
| V20 | 0.960 (0.917–1.005) | 0.081 | ||
| V30 | 0.964 (0.904–1.029) | 0.269 | ||
| V40 | 0.887 (0.746–1.055) | 0.177 | ||
| REI of EAT (EAT-REI) | 1.002 (1.001–1.004) | 0.002 | 1.002 (1.000–1.004) | 0.028 |
| TAT volume (mL) | 0.999 (0.999–1.003) | 0.049 | 1.000 (0.999–1.000) | 0.322 |
| TAT mean dose (Gy) | 1.001 (0.999–1.000) | 0.294 | ||
| SAT volume (mL) | 0.999 (0.999–1.000) | 0.062 | ||
| SAT mean dose (Gy) | 1.001 (0.998–1.003) | 0.501 | ||
| VAT volume (mL) | 0.995 (0.991–1.000) | 0.054 | ||
| VAT mean dose (Gy) | 1.000 (1.000–1.001) | 0.472 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Characteristics | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Age | 1.014 (0.965–1.066) | 0.572 | ||
| BMI (kg/m2) | 0.974 (0.873–1.087) | 0.637 | ||
| Albumin (g/dL) | 0.694 (0.277–1.738) | 0.435 | ||
| Hb (g/dL) | 0.998 (0.719–1.385) | 0.989 | ||
| Lymphocyte (%) | 0.958 (0.904–1.015) | 0.149 | ||
| Cholesterol (mg/dL) | 0.992 (0.973–1.011) | 0.429 | ||
| Triglycerides (mg/dL) | 0.996 (0.986–1.007) | 0.510 | ||
| Metabolic diseases | 0.040 (0.000–20.334) | 0.311 | ||
| Cardiac diseases | 0.344 (0.078–1.522) | 0.160 | ||
| Clinical T (T1–2 vs. T3–4) | 0.904 (0.444–1.839) | 0.780 | ||
| Clinical N (N0–1 vs. N2–3) | 1.524 (0.689–3.375) | 0.299 | ||
| cTNM stage (II vs. III) | 0.828 (0.342–2.004) | 0.675 | ||
| Pathological response (non-pCR vs. pCR) | 0.694 (0.288–1.669) | 0.414 | ||
| Target Volume | 1.001 (1.000–1.002) | 0.164 | ||
| Heart mean dose | 1.000 (0.999–1.000) | 0.663 | ||
| Dose-volume of EAT * | ||||
| Volume (mL) | 0.982 (0.959–1.005) | 0.114 | ||
| Mean dose (Gy) | 1.000 (0.999–1.000) | 0.751 | ||
| V5 | 0.969 (0.943–0.996) | 0.026 | 1.131 (0.933–1.369) | 0.209 |
| V10 | 0.965 (0.935–0.996) | 0.049 | 0.796 (0.608–1.041) | 0.095 |
| V20 | 0.958 (0.918–1.000) | 0.049 | 1.116 (0.943–1.321) | 0.200 |
| V30 | 0.959 (0.902–1.029) | 0.182 | ||
| V40 | 0.871 (0.735–1.032) | 0.111 | ||
| REI of EAT (EAT-REI) | 1.003 (1.001–1.004) | 0.002 | 1.002 (1.000–1.004) | 0.030 |
| TAT volume (mL) | 0.999 (0.999–1.000) | 0.014 | 0.970 (0.998–1.000) | 0.060 |
| TAT mean dose (Gy) | 1.000 (0.998–1.002) | 0.896 | ||
| SAT volume (mL) | 0.999 (0.999–1.000) | 0.018 | ||
| SAT mean dose (Gy) | 1.000 (0.998–1.002) | 0.855 | ||
| VAT volume (mL) | 0.995 (0.991–1.000) | 0.035 | 1.003 (0.995–1.010) | 0.458 |
| VAT mean dose (Gy) | 1.000 (0.999–1.001) | 0.739 |
| Characteristics | EAT-REI ≥ 68.8 (n = 23) | EAT-REI < 68.8 (n = 13) | p Value |
|---|---|---|---|
| Age (years), | 57.3 ± 7.9 | 61.6 ± 9.4 | 0.15 |
| Sex | 0.60 | ||
| Man | 22 (95.7%) | 12 (92.3%) | |
| Woman | 1 (4.3%) | 1 (7.7%) | |
| BMI (kg/m2) | 21.2 ± 4.4 | 23.6 ± 3.7 | 0.11 |
| CRP (mg/dL) | 7.0 ± 6.2 | 6.2 ± 5.5 | 0.66 |
| Albumin (g/dL) | 3.9 ± 0.5 | 4.0 ± 0.6 | 0.71 |
| Hb (g/dL) | 11.9 ± 1.4 | 12.3 ± 1.5 | 0.38 |
| Lymphocyte (number/uL) | 1414.4 ± 770.5 | 1744.2 ± 631.4 | 0.20 |
| Cholesterol (mg/dL) | 178.6 ± 48.6 | 186.4 ± 18.8 | 0.60 |
| Triglycerides (mg/dL) | 115.8 ± 52.9 | 116.8 ± 66.1 | 0.77 |
| Metabolic diseases | 4 (17.4%) | 2 (15.4%) | 1.00 |
| Cardiac diseases | 4 (17.4%) | 5 (38.5%) | 0.24 |
| Clinical T stage | 0.69 | ||
| cT1–2 | 5 (21.7%) | 4 (30.8%) | |
| cT3–4 | 18 (78.3%) | 9 (69.2%) | |
| Clinical N stage | 0.92 | ||
| cN0–1 | 12 (52.2%) | 7 (53.8%) | |
| cN2–3 | 11 (47.8%) | 6 (46.2%) | |
| cTNM stage | 0.72 | ||
| II | 7 (30.4%) | 3 (23.1%) | |
| III | 16 (69.6%) | 10 (76.9%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tai, H.-C.; Lee, J.; Huang, W.-C.; Liu, H.-C.; Chen, C.-H.; Huang, Y.-C.; Lee, C.-J.; Yun, C.-H.; Hsu, S.-M.; Chen, Y.-J. The Impact of Radiation to Epicardial Adipose Tissue on Prognosis of Esophageal Squamous Cell Carcinoma Receiving Neoadjuvant Chemoradiotherapy and Esophagectomy. Appl. Sci. 2021, 11, 4023. https://doi.org/10.3390/app11094023
Tai H-C, Lee J, Huang W-C, Liu H-C, Chen C-H, Huang Y-C, Lee C-J, Yun C-H, Hsu S-M, Chen Y-J. The Impact of Radiation to Epicardial Adipose Tissue on Prognosis of Esophageal Squamous Cell Carcinoma Receiving Neoadjuvant Chemoradiotherapy and Esophagectomy. Applied Sciences. 2021; 11(9):4023. https://doi.org/10.3390/app11094023
Chicago/Turabian StyleTai, Hung-Chi, Jie Lee, Wen-Chien Huang, Hung-Chang Liu, Chao-Hung Chen, Yu-Chuen Huang, Chi-Jung Lee, Chun-Ho Yun, Shih-Ming Hsu, and Yu-Jen Chen. 2021. "The Impact of Radiation to Epicardial Adipose Tissue on Prognosis of Esophageal Squamous Cell Carcinoma Receiving Neoadjuvant Chemoradiotherapy and Esophagectomy" Applied Sciences 11, no. 9: 4023. https://doi.org/10.3390/app11094023
APA StyleTai, H. -C., Lee, J., Huang, W. -C., Liu, H. -C., Chen, C. -H., Huang, Y. -C., Lee, C. -J., Yun, C. -H., Hsu, S. -M., & Chen, Y. -J. (2021). The Impact of Radiation to Epicardial Adipose Tissue on Prognosis of Esophageal Squamous Cell Carcinoma Receiving Neoadjuvant Chemoradiotherapy and Esophagectomy. Applied Sciences, 11(9), 4023. https://doi.org/10.3390/app11094023







