Open-Air Cold Plasma Device Leads to Selective Tumor Cell Cytotoxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture Conditions
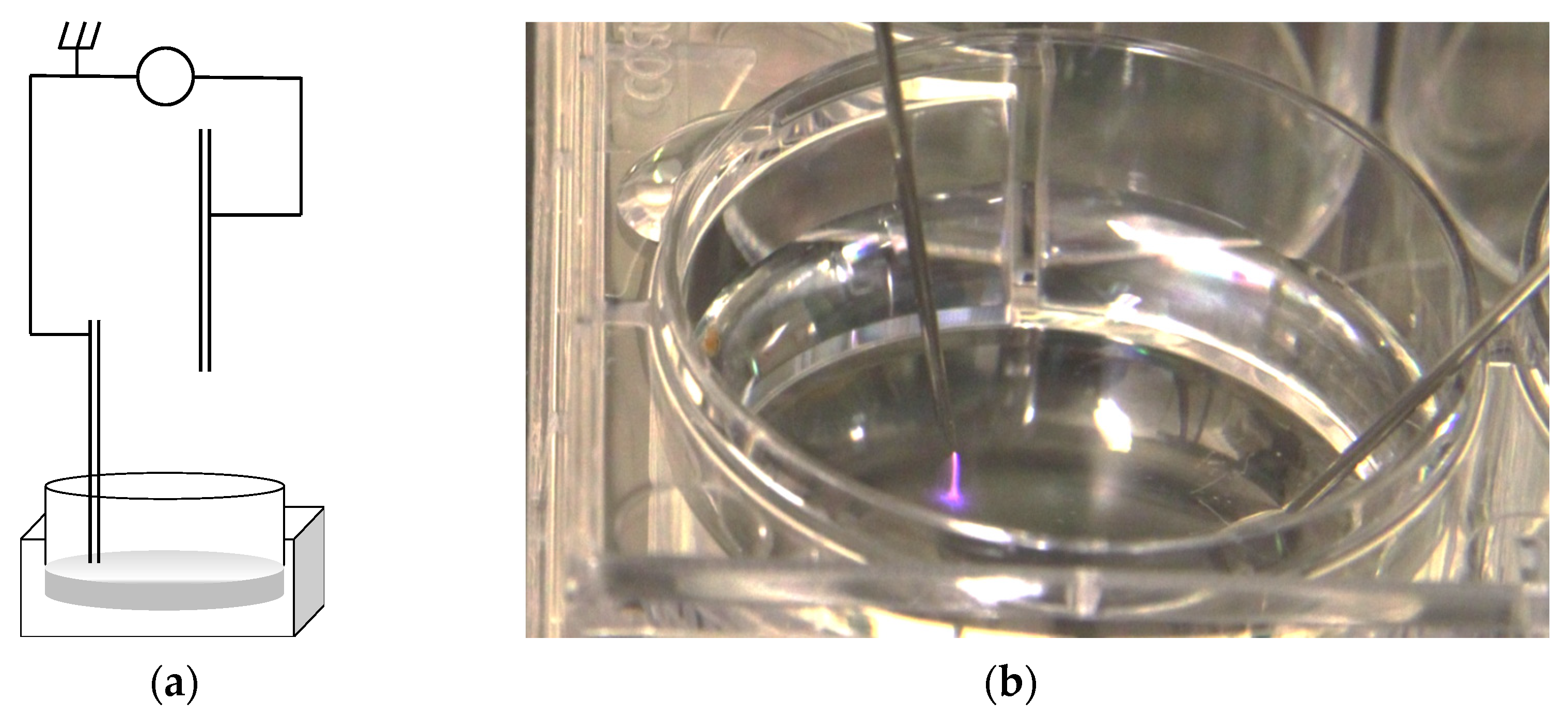
2.2. Cold Atmospheric Plasma Jet
2.3. Preparation of Plasma Activated Medium (PAM)
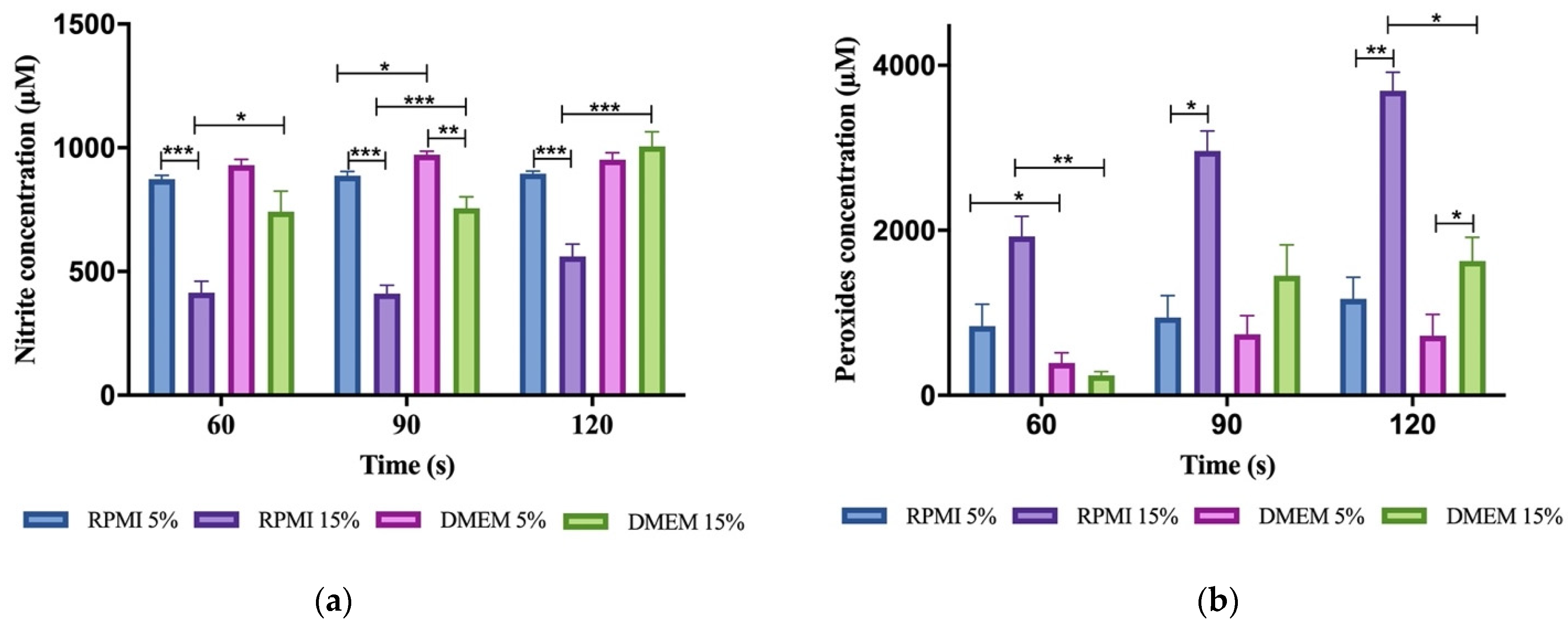
2.4. Quantification of Nitrite Concentration in PAM
2.5. Quantification of Peroxide Concentration in PAM
2.6. Plasma Treatment of Cell Cultures
2.7. SRB Assay
2.8. MTT Assay
2.9. Statistical Analysis
3. Results
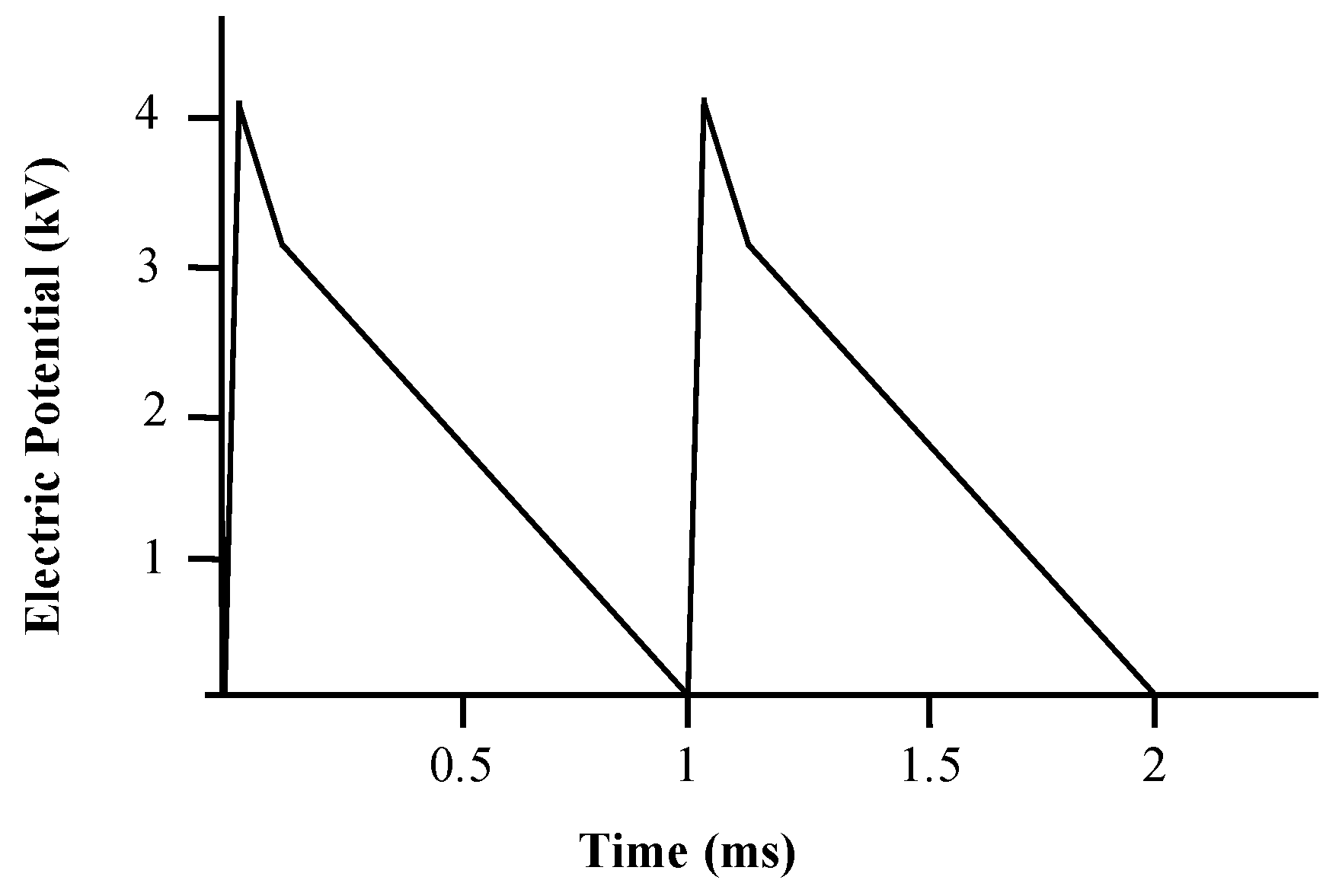
3.1. CAP Single Electrode Jet Generates High Voltage Electrical Pulses
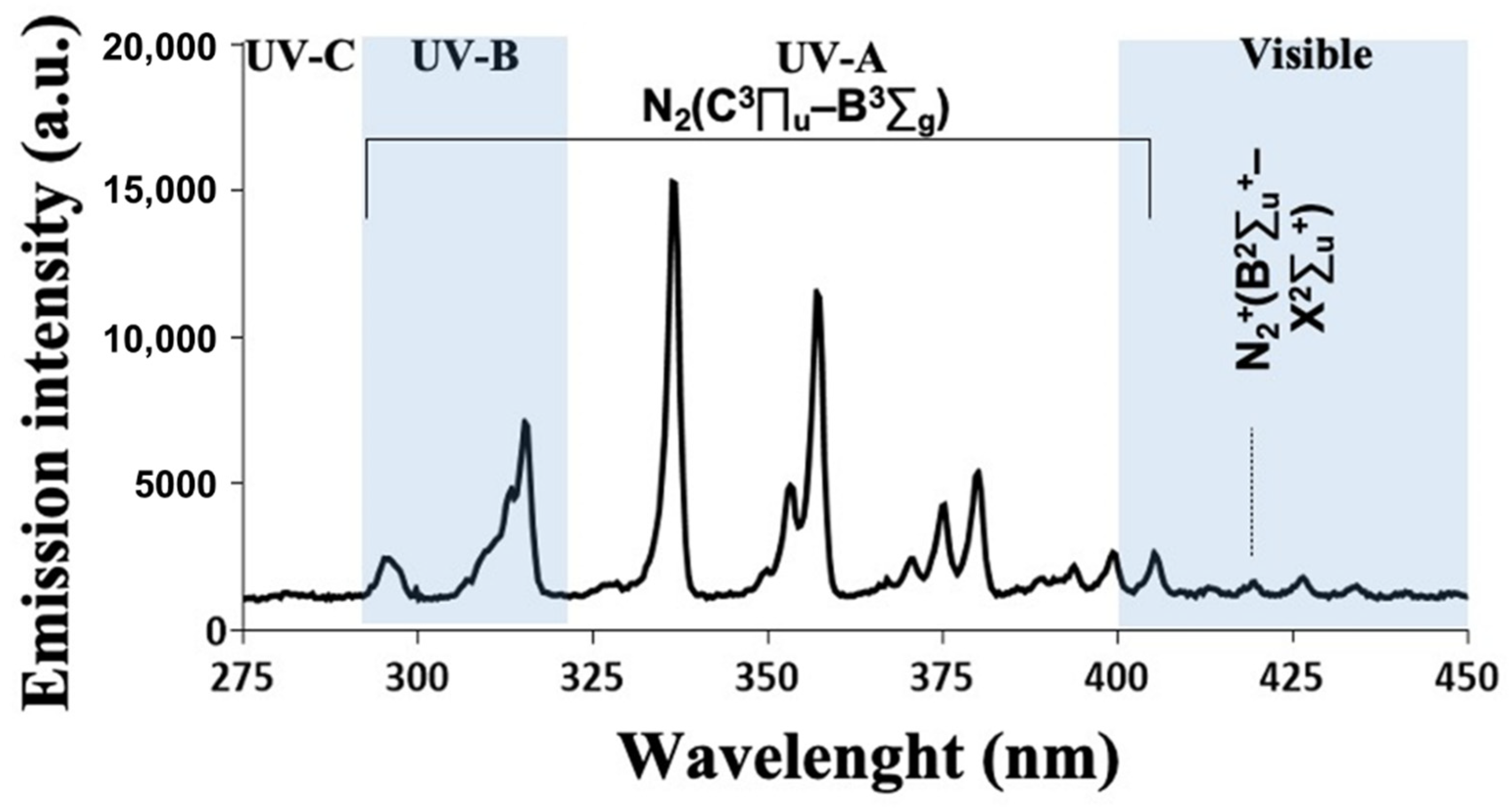
3.2. CAP Emission Spectrum Depicted Several Peaks in the UV Domain and Reactive Species
3.3. Reactive Oxygen and Nitrogen Species (RONS) Accumulate in Plasma-Activated Medium
3.4. Plasma-Activated Medium Did Not Alter Its pH Nor Its Temperature
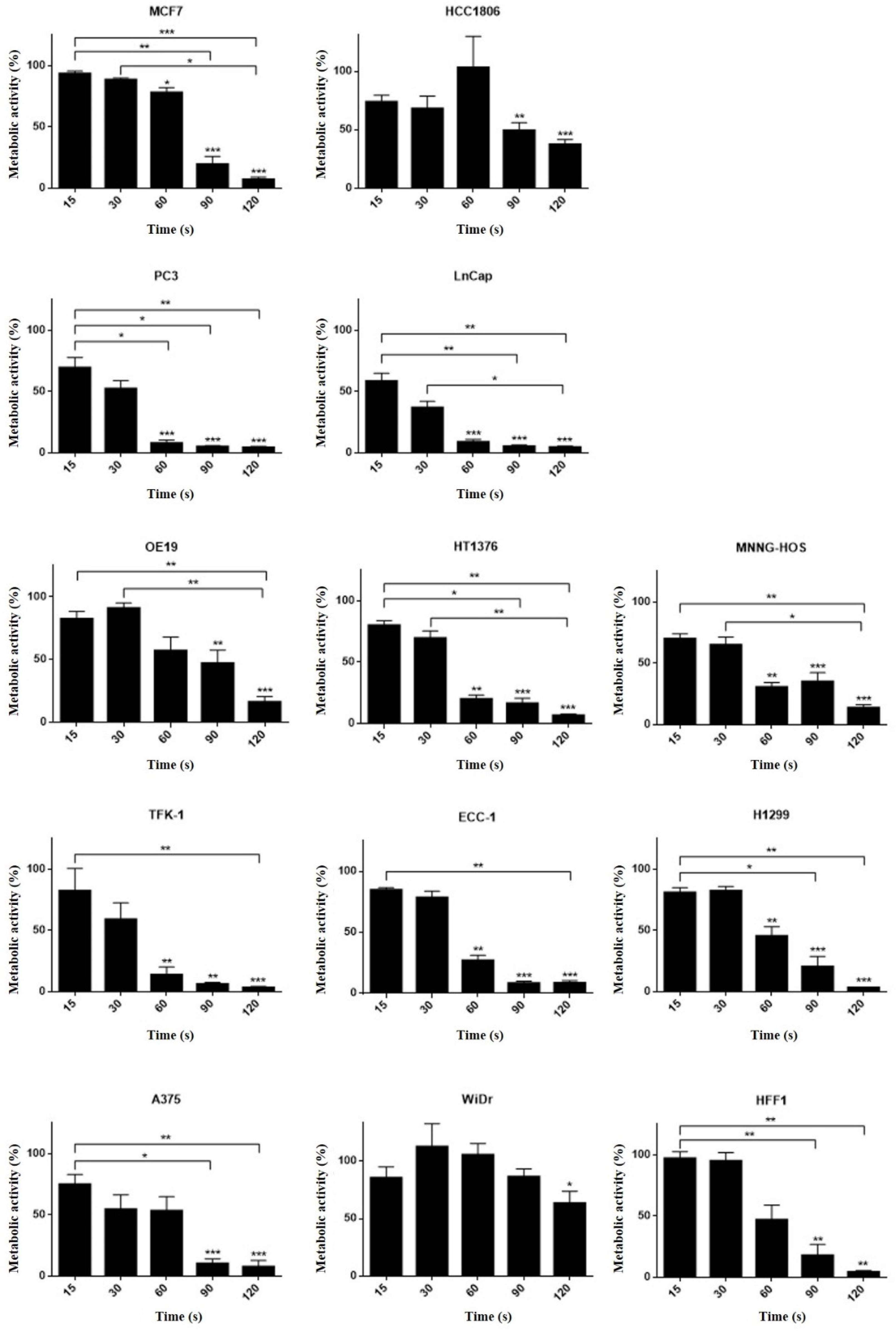
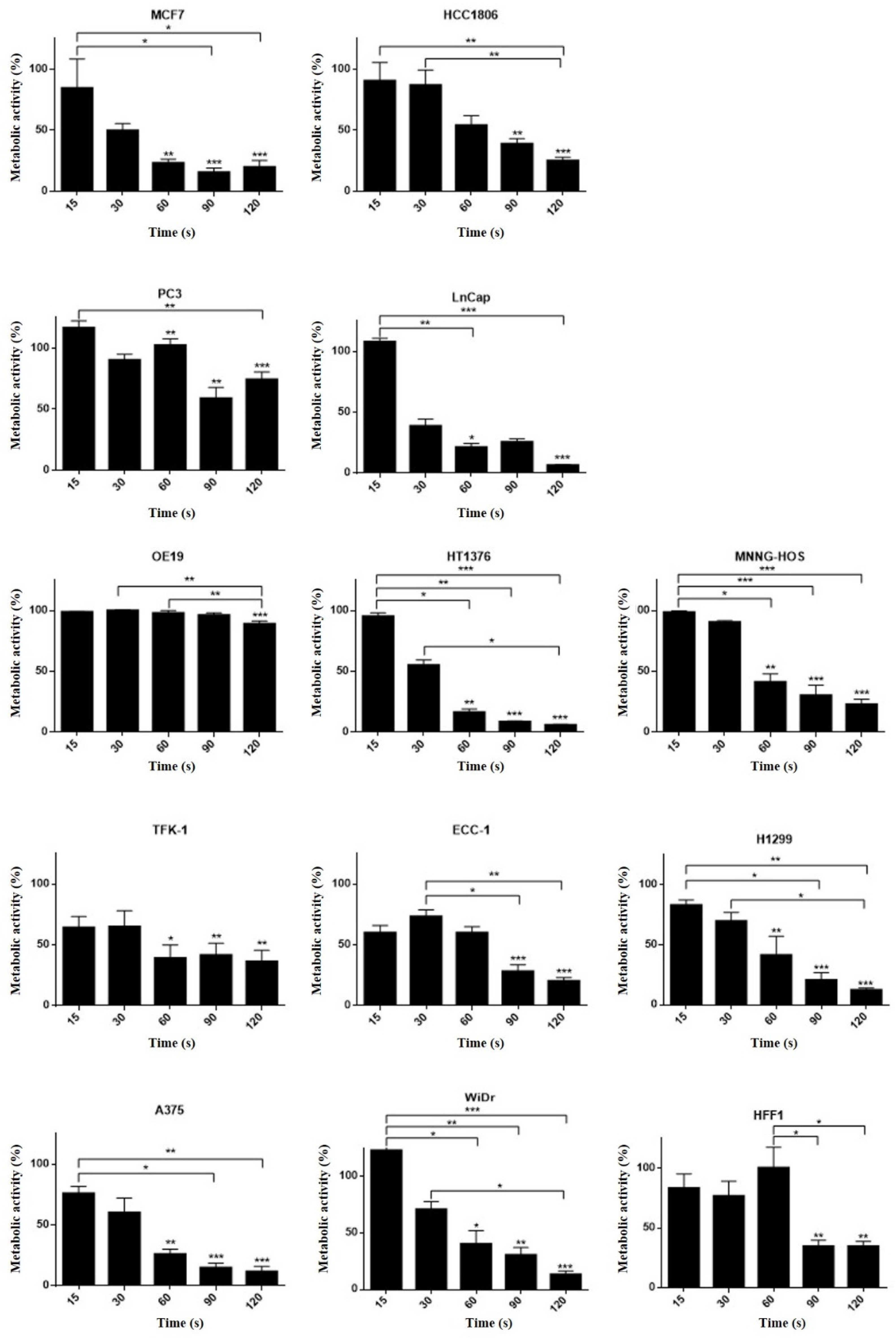
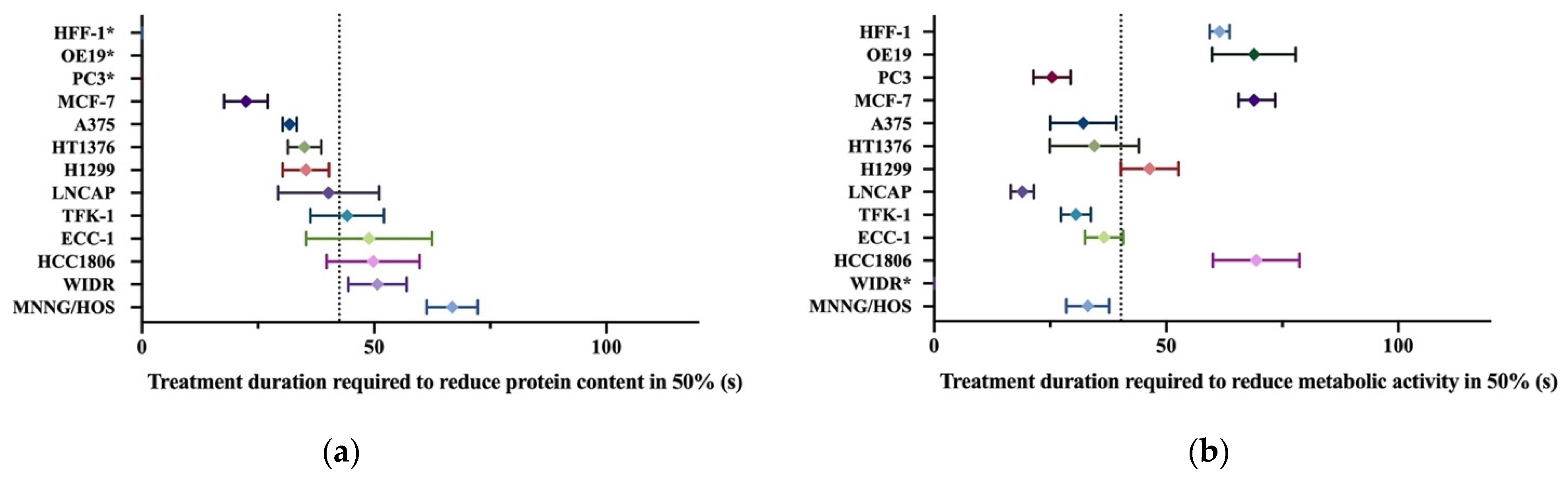
3.5. Different Cell Lines Displayed Different Responses to Cold Plasma
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2014, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Kong, P. Myrtle Atmospheric-Pressure Plasma Process and Applications. SOHN Int. Symp. Adv. Process. Metals Mater. Technol. Ind. Pract. 2006, 6, 493–506. [Google Scholar]
- Rahman, Z.; Rahman, H.; Rahman, M.A. Classification and Generation of Atmospheric Pressure Plasma and Its Principle Applications. Int. J. Math. Phys. Sci. Res. 2015, 2, 127–146. [Google Scholar]
- Kong, M.G.; Kroesen, G.; Morfill, G.; Nosenko, T.; Shimizu, T.; Van Dijk, J.; Zimmermann, J.L. Plasma medicine: An introductory review. New J. Phys. 2009, 11, 115012. [Google Scholar] [CrossRef]
- Köritzer, J.; Boxhammer, V.; Schäfer, A.; Shimizu, T.; Klämpfl, T.G.; Li, Y.-F.; Welz, C.; Schwenk-Zieger, S.; Morfill, G.E.; Zimmermann, J.L.; et al. Restoration of Sensitivity in Chemo — Resistant Glioma Cells by Cold Atmospheric Plasma. PLoS ONE 2013, 8, e64498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaushik, N.K.; Kaushik, N.; Park, D.; Choi, E.H. Altered Antioxidant System Stimulates Dielectric Barrier Discharge Plasma-Induced Cell Death for Solid Tumor Cell Treatment. PLoS ONE 2014, 9, e103349. [Google Scholar] [CrossRef]
- Tanaka, H.; Mizuno, M.; Katsumata, Y.; Ishikawa, K.; Kondo, H.; Hashizume, H.; Okazaki, Y.; Toyokuni, S.; Nakamura, K.; Yoshikawa, N.; et al. Oxidative stress-dependent and -independent death of glioblastoma cells induced by non-thermal plasma-exposed solutions. Sci. Rep. 2019, 9, 13657. [Google Scholar] [CrossRef] [Green Version]
- Conway, G.; He, Z.; Hutanu, A.L.; Cribaro, G.P.; Manaloto, E.; Casey, A.; Traynor, D.; Milosavljevic, V.; Howe, O.; Barcia, C.; et al. Cold Atmospheric Plasma induces accumulation of lysosomes and caspase-independent cell death in U373MG glioblastoma multiforme cells. Sci. Rep. 2019, 9, 12891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, L.; Xu, X.; Zhang, S.; Cai, D.; Dai, X. Cold atmospheric plasma conveys selectivity on triple negative breast cancer cells both in vitro and in vivo. Free. Radic. Biol. Med. 2018, 124, 205–213. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, S.; Zhang, H.; Kong, X.; Ding, L.; Shen, J.; Lan, Y.; Cheng, C.; Zhu, T.; Xia, W. Selective effects of non-thermal atmospheric plasma on triple-negative breast normal and carcinoma cells through different cell signaling pathways. Sci. Rep. 2017, 7, 7980. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Kumar, N.; Hammerschmid, D.; Privat-Maldonado, A.; Dewilde, S.; Bogaerts, A. Synergistic Effects of Melittin and Plasma Treatment: A Promising Approach for Cancer Therapy. Cancers 2019, 11, 1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Holmes, B.; Cheng, X.; Zhu, W.; Keidar, M.; Zhang, L.G. Cold Atmospheric Plasma for Selectively Ablating Metastatic Breast Cancer Cells. PLoS ONE 2013, 8, e73741. [Google Scholar] [CrossRef]
- LeDuc, M.; Guay, D.; Leask, R.L.; Coulombe, S. Cell permeabilization using a non-thermal plasma. New J. Phys. 2009, 11, 115021. [Google Scholar] [CrossRef]
- Ryan, H.A.; Neuber, J.; Song, S.; Beebe, S.J.; Jiang, C.; Member, S. Effects of a non-thermal plasma needle device on HPV-16 positive cervical cancer cell viability in vitro. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Institute of Electrical and Electronics Engineers (IEEE), Lake Buena Vista (Orlando), FL, USA, 16–20 August 2016; Volume 2016, pp. 537–540. [Google Scholar]
- Kim, C.-H.; Kwon, S.; Bahn, J.H.; Lee, K.; Jun, S.I.; Rack, P.D.; Baek, S.J. Effects of atmospheric nonthermal plasma on invasion of colorectal cancer cells. Appl. Phys. Lett. 2010, 96, 243701. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.-H.; Bahn, J.H.; Lee, S.-H.; Kim, G.-Y.; Jun, S.-I.; Lee, K.; Baek, S.J. Induction of cell growth arrest by atmospheric non-thermal plasma in colorectal cancer cells. J. Biotechnol. 2010, 150, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, M.; Robert, E.; Lerondel, S.; Sarron, V.; Ries, D.; Dozias, S.; Sobilo, J.; Gosset, D.; Kieda, C.; Legrain, B.; et al. ROS implication in a new antitumor strategy based on non-thermal plasma. Int. J. Cancer 2012, 130, 2185–2194. [Google Scholar] [CrossRef]
- Ishaq, M.; Evans, M.D.; Ostrikov, K. (Ken) Atmospheric pressure gas plasma-induced colorectal cancer cell death is mediated by Nox2–ASK1 apoptosis pathways and oxidative stress is mitigated by Srx–Nrf2 anti-oxidant system. Biochim. Biophys. Acta (BBA) Bioenerg. 2014, 1843, 2827–2837. [Google Scholar] [CrossRef]
- Guerrero-Preston, R.; Ogawa, T.; Uemura, M.; Shumulinsky, G.; Valle, B.L.; Pirini, F.; Ravi, R.; Sidransky, D.; Keidar, M.; Trink, B. Cold atmospheric plasma treatment selectively targets head and neck squamous cell carcinoma cells. Int. J. Mol. Med. 2014, 34, 941–946. [Google Scholar] [CrossRef] [Green Version]
- Welz, C.; Emmert, S.; Canis, M.; Becker, S.; Baumeister, P.; Shimizu, T.; Morfill, G.E.; Harréus, U.; Zimmermann, J.L. Cold Atmospheric Plasma: A Promising Complementary Therapy for Squamous Head and Neck Cancer. PLoS ONE 2015, 10, e0141827. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.U.; Cho, J.-H.; Chang, J.W.; Shin, Y.S.; Kim, K.I.; Park, J.K.; Yang, S.S.; Lee, J.-S.; Moon, E.; Lee, K.; et al. Nonthermal plasma induces head and neck cancer cell death: The potential involvement of mitogen-activated protein kinase-dependent mitochondrial reactive oxygen species. Cell Death Dis. 2014, 5, e1056. [Google Scholar] [CrossRef]
- Turrini, E.; Laurita, R.; Stancampiano, A.; Catanzaro, E.; Calcabrini, C.; Maffei, F.; Gherardi, M.; Colombo, V.; Fimognari, C. Cold Atmospheric Plasma Induces Apoptosis and Oxidative Stress Pathway Regulation in T-Lymphoblastoid Leukemia Cells. Oxidative Med. Cell. Longev. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Barekzi, N.; Laroussi, M. Dose-dependent killing of leukemia cells by low-temperature plasma. J. Phys. D Appl. Phys. 2012, 45, 422002. [Google Scholar] [CrossRef]
- Bekeschus, S.; Wende, K.; Hefny, M.M.; Rödder, K.; Jablonowski, H.; Schmidt, A.; Von Woedtke, T.; Weltmann, K.-D.; Benedikt, J. Oxygen atoms are critical in rendering THP-1 leukaemia cells susceptible to cold physical plasma-induced apoptosis. Sci. Rep. 2017, 7, 2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asif, M.; Liu, H.; Aziz, A.; Wang, H.; Wang, Z.; Ajmal, M.; Xiao, F.; Liu, H. Core-shell iron oxide-layered double hydroxide: High electrochemical sensing performance of H 2 O 2 biomarker in live cancer cells with plasma therapeutics. Biosens. Bioelectron. 2017, 97, 352–359. [Google Scholar] [CrossRef]
- Gweon, B.; Kim, M.; Kim, D.B.; Kim, D.; Kim, H.; Jung, H.; Shin, J.H.; Choe, W. Differential responses of human liver cancer and normal cells to atmospheric pressure plasma. Appl. Phys. Lett. 2011, 99, 063701. [Google Scholar] [CrossRef] [Green Version]
- Keidar, M.; Walk, R.M.; Shashurin, A.; Srinivasan, P.; Sandler, A.B.; Dasgupta, S.; Ravi, R.; Guerreropreston, R.; Trink, B. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br. J. Cancer 2011, 105, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Ballato, J.; Foy, P.; Hawkins, T.; Wei, Y.; Li, J.; Kim, S.-O. Apoptosis of lung carcinoma cells induced by a flexible optical fiber-based cold microplasma. Biosens. Bioelectron. 2011, 28, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Panngom, K.; Baik, K.Y.; Nam, M.K.; Han, J.H.; Rhim, H.; Choi, E.H. Preferential killing of human lung cancer cell lines with mitochondrial dysfunction by nonthermal dielectric barrier discharge plasma. Cell Death Dis. 2013, 4, e642. [Google Scholar] [CrossRef] [Green Version]
- Tabuchi, Y.; Uchiyama, H.; Zhao, Q.-L.; Yunoki, T.; Andocs, G.; Nojima, N.; Takeda, K.; Ishikawa, K.; Hori, M.; Kondo, T. Effects of nitrogen on the apoptosis of and changes in gene expression in human lymphoma U937 cells exposed to argon-based cold atmospheric pressure plasma. Int. J. Mol. Med. 2016, 37, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Klas, M.; Liu, Y.; Stack, M.S.; Ptasinska, S. DNA damage in oral cancer cells induced by nitrogen atmospheric pressure plasma jets. Appl. Phys. Lett. 2013, 102, 233703. [Google Scholar] [CrossRef]
- Chang, J.W.; Kang, S.U.; Shin, Y.S.; Kim, K.I.; Seo, S.J.; Yang, S.S.; Lee, J.-S.; Moon, E.; Baek, S.J.; Lee, K.; et al. Non-thermal atmospheric pressure plasma induces apoptosis in oral cavity squamous cell carcinoma: Involvement of DNA-damage-triggering sub-G1 arrest via the ATM/p53 pathway. Arch. Biochem. Biophys. 2014, 545, 133–140. [Google Scholar] [CrossRef]
- Gümbel, D.; Daeschlein, G.; Ekkernkamp, A.; Kramer, A.; Stope, M.B. Cold atmospheric plasma in orthopaedic and urologic tumor therapy. GMS Hyg Infect Control. 2017, 12, 12. [Google Scholar]
- Canal, C.; Fontelo, R.; Hamouda, I.; Guillem-Marti, J.; Cvelbar, U.; Ginebra, M.-P. Plasma-induced selectivity in bone cancer cells death. Free. Radic. Biol. Med. 2017, 110, 72–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koensgen, D.; Besic, I.; Gümbel, D.; Kaul, A.; Weiss, M.; Diesing, K.; Kramer, A.; Bekeschus, S.; Mustea, A.; Stope, M.B. Cold Atmospheric Plasma (CAP) and CAP-Stimulated Cell Culture Media Suppress Ovarian Cancer Cell Growth – A Putative Treatment Option in Ovarian Cancer Therapy. Anticancer Res. 2017, 37, 6739–6744. [Google Scholar] [CrossRef] [PubMed]
- Iseki, S.; Nakamura, K.; Hayashi, M.; Tanaka, H.; Kondo, H.; Kajiyama, H.; Kano, H.; Kikkawa, F.; Hori, M. Selective killing of ovarian cancer cells through induction of apoptosis by nonequilibrium atmospheric pressure plasma. Appl. Phys. Lett. 2012, 100, 113702. [Google Scholar] [CrossRef]
- Utsumi, F.; Kajiyama, H.; Nakamura, K.; Tanaka, H.; Hori, M.; Kikkawa, F. Selective cytotoxicity of indirect nonequilibrium atmospheric pressure plasma against ovarian clear-cell carcinoma. SpringerPlus 2014, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Brullé, L.; Vandamme, M.; Riès, D.; Martel, E.; Robert, E.; Lerondel, S.; Trichet, V.; Richard, S.; Pouvesle, J.-M.; Le Pape, A. Effects of a Non Thermal Plasma Treatment Alone or in Combination with Gemcitabine in a MIA PaCa2-luc Orthotopic Pancreatic Carcinoma Model. PLoS ONE 2012, 7, e52653. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Schreiber, C.A.; Knutson, G.J.; El Khattouti, A.; Sakiyama, M.J.; Hassan, M.; Charlesworth, M.C.; Madden, B.J.; Zhou, X.; Vuk-Pavlovic, S.; et al. Effects of oxygen on the antigenic landscape of prostate cancer cells. BMC Res. Notes 2015, 8, 687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.J.; Shon, C.H.; Kim, Y.S.; Kim, S.; Kim, G.C.; Kong, M.G. Degradation of adhesion molecules of G361 melanoma cells by a non-thermal atmospheric pressure microplasma. New J. Phys. 2009, 11, 115026. [Google Scholar] [CrossRef]
- Schneider, C.; Gebhardt, L.; Arndt, S.; Karrer, S.; Zimmermann, J.L.; Fischer, M.J.M.; Bosserhoff, A.-K. Acidification is an Essential Process of Cold Atmospheric Plasma and Promotes the Anti-Cancer Effect on Malignant Melanoma Cells. Cancers 2019, 11, 671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurake, N.; Tanaka, H.; Ishikawa, K.; Kondo, T.; Sekine, M.; Nakamura, K.; Kajiyama, H.; Kikkawa, F.; Mizuno, M.; Hori, M. Cell survival of glioblastoma grown in medium containing hydrogen peroxide and/or nitrite, or in plasma-activated medium. Arch. Biochem. Biophys. 2016, 605, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Tanaka, H.; Nonomura, S.; Hara, H.; Kondo, S.-I.; Hori, M. Plasma-activated medium induces A549 cell injury via a spiral apoptotic cascade involving the mitochondrial–nuclear network. Free. Radic. Biol. Med. 2015, 79, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Tresp, H.; Hammer, M.U.; Iseni, S.; Kupsch, S.; Schmidt-Bleker, A.; Wende, K.; Dünnbier, M.; Masur, K.; Weltmann, K.-D.; et al. Tracking plasma generated H2O2 from gas into liquid phase and revealing its dominant impact on human skin cells. J. Phys. D Appl. Phys. 2014, 47, 285401. [Google Scholar] [CrossRef]
- Bekeschus, S.; Kolata, J.; Winterbourn, C.; Kramer, A.; Turner, R.; Weltmann, K.D.; Bröker, B.; Masur, K. Hydrogen peroxide: A central player in physical plasma-induced oxidative stress in human blood cells. Free. Radic. Res. 2014, 48, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Yokoyama, M.; Johkura, K. A key inactivation factor of HeLa cell viability by a plasma flow. J. Phys. D Appl. Phys. 2011, 44, 44. [Google Scholar] [CrossRef]
- Gibson, A.R.; McCarthy, H.O.; Ali, A.A.; O’Connell, D.; Graham, W.G. Interactions of a Non-Thermal Atmospheric Pressure Plasma Effluent with PC-3 Prostate Cancer Cells. Plasma Process. Polym. 2014, 11, 1142–1149. [Google Scholar] [CrossRef]
- Yan, D.; Cui, H.; Zhu, W.; Talbot, A.; Zhang, L.G.; Sherman, J.H.; Keidar, M. The Strong Cell-based Hydrogen Peroxide Generation Triggered by Cold Atmospheric Plasma. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, H.; Cheng, C.; Shen, J.; Bao, L.; Han, W. Contribution of hydrogen peroxide to non-thermal atmospheric pressure plasma induced A549 lung cancer cell damage. Plasma Process. Polym. 2017, 14, 1600162. [Google Scholar] [CrossRef]
- Bekeschus, S.; Schmidt, A.; Niessner, F.; Gerling, T.; Weltmann, K.-D.; Wende, K. Basic Research in Plasma Medicine—A Throughput Approach from Liquids to Cells. J. Vis. Exp. 2017, e56331. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Liu, D.; Wang, B.; Chen, C.; Chen, Z.; Li, D.; Yang, Y.; Chen, H.; Kong, M.G. In Situ OH Generation from O2− and H2O2 Plays a Critical Role in Plasma-Induced Cell Death. PLoS ONE 2015, 10, e0128205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohades, S.; Laroussi, M.; Sears, J.; Barekzi, N.; Razavi, H. Evaluation of the effects of a plasma activated medium on cancer cells. Phys. Plasmas 2015, 22, 122001. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, K.I.; Hoan, N.N.; Kim, C.H.; Moon, E.; Choi, K.S.; Yang, S.S.; Lee, J.-S. Targeting Cancer Cells with Reactive Oxygen and Nitrogen Species Generated by Atmospheric-Pressure Air Plasma. PLoS ONE 2014, 9, e86173. [Google Scholar] [CrossRef]
- Tanaka, H.; Mizuno, M.; Ishikawa, K.; Toyokuni, S.; Kajiyama, H.; Kikkawa, F.; Hori, M. Molecular mechanisms of non-thermal plasma-induced effects in cancer cells. Biol. Chem. 2018, 400, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Boehm, D.; Bourke, P. Safety implications of plasma-induced effects in living cells – a review of in vitro and in vivo findings. Biol. Chem. 2018, 400, 3–17. [Google Scholar] [CrossRef]
- International Commission on Non-Ionizing Radiation Protection Guidelines for Limiting Exposure to Time-Varying Electric and Magnetic Fields (1 Hz TO 100 kHz). Health Phys. 2010, 99, 818–836. [CrossRef] [PubMed]
- Kielbassa, C.; Roza, L.; Epe, B. Wavelength dependence of oxidative DNA damage induced by UV and visible light. Carcinogenesis 1997, 18, 811–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nijdam, S.; Van Veldhuizen, E.; Bruggeman, P.; Ebert, U. An Introduction to Nonequilibrium Plasmas at Atmospheric Pressure. In Plasma Chemistry and Catalysis in Gases and Liquids; Wiley: Hoboken, NJ, USA, 2012; pp. 1–44. [Google Scholar]
- Chang, J.-S.; Lawless, P.A.; Yamamoto, T. Corona discharge processes. IEEE Trans. Plasma Sci. 1991, 19, 1152–1166. [Google Scholar] [CrossRef] [Green Version]
- Yousfi, M.; Merbahi, N.; Pathak, A.; Eichwald, O. Low-temperature plasmas at atmospheric pressure: Toward new pharmaceutical treatments in medicine. Fundam. Clin. Pharmacol. 2013, 28, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Kolpaková, A.; Kudrna, P.; Tichý, M. Study of Plasma System by OES (Optical Emission Spectroscopy). In Proceedings of the 20th Annual Conference of Doctoral Students, Prague, Czech Republic, 31 May–3 June 2011; pp. 180–185. [Google Scholar]
- Volotskova, O.; Hawley, T.S.; Stepp, M.A.; Keidar, M. Targeting the cancer cell cycle by cold atmospheric plasma. Sci. Rep. 2012, 2, 636. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.; Talbot, A.; Nourmohammadi, N.; Cheng, X.; Canady, J.; Sherman, J.H.; Keidar, M. Principles of using Cold Atmospheric Plasma Stimulated Media for Cancer Treatment. Sci. Rep. 2015, 5, 18339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, M.; Gümbel, D.; Hanschmann, E.-M.; Mandelkow, R.; Gelbrich, N.; Zimmermann, U.; Walther, R.; Ekkernkamp, A.; Sckell, A.; Kramer, A.; et al. Cold Atmospheric Plasma Treatment Induces Anti-Proliferative Effects in Prostate Cancer Cells by Redox and Apoptotic Signaling Pathways. PLoS ONE 2015, 10, e0130350. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kang, S.U.; Kim, K.I.; Kang, S.; Shin, Y.S.; Chang, J.W.; Yang, S.S.; Lee, K.; Lee, J.-S.; Moon, E.; et al. Nonthermal Plasma Induces Apoptosis in ATC Cells: Involvement of JNK and p38 MAPK-Dependent ROS. Yonsei Med J. 2014, 55, 1640–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishaq, M.; Kumar, S.; Varinli, H.; Han, Z.J.; Rider, A.E.; Evans, M.D.M.; Murphy, A.B.; Ostrikov, K. Atmospheric gas plasma–induced ROS production activates TNF-ASK1 pathway for the induction of melanoma cancer cell apoptosis. Mol. Biol. Cell 2014, 25, 1523–1531. [Google Scholar] [CrossRef]
- Michels, A.J.; Frei, B. Myths, Artifacts, and Fatal Flaws: Identifying Limitations and Opportunities in Vitamin C Research. Nutrients 2013, 5, 5161–5192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gstraunthaler, G.; Lindl, T.; Van Der Valk, J. A plea to reduce or replace fetal bovine serum in cell culture media. Cytotechnology 2013, 65, 791–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Valk, J.; Brunner, D.; De Smet, K.; Svenningsen, Å.F.; Honegger, P.; Knudsen, L.; Lindl, T.; Noraberg, J.; Price, A.; Scarino, M. Optimization of chemically defined cell culture media – Replacing fetal bovine serum in mammalian in vitro methods. Toxicol. Vitr. 2010, 24, 1053–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boehm, D.; Heslin, C.; Cullen, P.J.; Bourke, P. Cytotoxic and mutagenic potential of solutions exposed to cold atmospheric plasma. Sci. Rep. 2016, 6, 21464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adan, A.; Kiraz, Y.; Baran, Y. The international international journal journal for timely for timely in-depth reviews in in-depth reviews in Pharmaceutical Pharmaceutical Biotechnology Impact Factor: 1.802 BENTHAM. Curr. Biotechnol. Pharm. 2016, 17, 1873–4316. [Google Scholar]
- Orellana, E.A.; Kasinski, A.L. Sulforhodamine B (SRB) Assay in Cell Culture to Investigate Cell Proliferation. Bio Protoc. 2016, 6, e1984. [Google Scholar] [CrossRef] [Green Version]
- Keepers, Y.P.; Pizao, P.E.; Peters, G.J.; van Ark-Otte, J.; Winograd, B.; Pinedo, H.M. Comparison of the sulforhodamine B protein and tetrazolium (MTT) assays for in vitro chemosensitivity testing. Eur. J. Cancer Clin. Oncol. 1991, 27, 897–900. [Google Scholar] [CrossRef] [Green Version]
- Rai, Y.; Pathak, R.; Kumari, N.; Sah, D.K.; Pandey, S.; Kalra, N.; Soni, R.; Dwarakanath, B.S.; Bhatt, A.N. Mitochondrial biogenesis and metabolic hyperactivation limits the application of MTT assay in the estimation of radiation induced growth inhibition. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.J.; Chung, T.H. Cold atmospheric plasma jet-generated RONS and their selective effects on normal and carcinoma cells. Sci. Rep. 2016, 6, 20332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Quan, Y.; Wang, W.; Fu, Q.; Wu, J.; Mei, T.; Li, J.; Tang, Y.; Luo, C.; Ouyang, Q.; et al. Dynamic equilibrium between cancer stem cells and non-stem cancer cells in human SW620 and MCF-7 cancer cell populations. Br. J. Cancer 2012, 106, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Dalerba, P.; Cho, R.W.; Clarke, M.F. Cancer Stem Cells: Models and Concepts. Annu. Rev. Med. 2007, 58, 267–284. [Google Scholar] [CrossRef] [Green Version]
- Mathew, R.; Karantza-Wadsworth, V.; White, E. Role of autophagy in cancer. Nat. Rev. Cancer 2007, 7, 961–967. [Google Scholar] [CrossRef]
- Mizushima, N. Autophagy: Process and function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgescu, N.; Lupu, A.R. Tumoral and Normal Cells Treatment with High-Voltage Pulsed Cold Atmospheric Plasma Jets. IEEE Trans. Plasma Sci. 2010, 38, 1949–1955. [Google Scholar] [CrossRef]
- Szili, E.J.; Hong, S.-H.; Oh, J.-S.; Gaur, N.; Short, R.D. Tracking the Penetration of Plasma Reactive Species in Tissue Models. Trends Biotechnol. 2018, 36, 594–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Cancer Types | Cell Lines | References |
|---|---|---|
| Brain cancer | LN18, LN229, U87MG, T98G, U251SP, U373MG-CD14 | [6,7,8,9] |
| Breast cancer | MDA-MB-231, MCF7, MDAMB468, MDA-MB-453 | [10,11,12,13] |
| Cervical cancer | HeLa, CaSki | [14,15] |
| Colorectal cancer | HCT-116, SW480, LoVo, Caco2, HT29 | [16,17,18,19] |
| Head and neck cancer | JHU-022, JHU-028, JHU-029, SCC25, FaDu, SNU1041, SNU899, HN9, OSC 19 | [20,21,22] |
| Leukemia | CCRFCEM, Jurkat, THP-1 | [23,24,25] |
| Liver cancer | SK-HEP-1, H-22 | [26,27] |
| Lung cancer | SW900, TC-1, H460, HCC1588, A549 | [28,29,30] |
| Lymphoma | U937 | [31] |
| Oral cancer | KB, SCC25, MSK QLL1, SCC1483, SCC15 | [7,32,33] |
| Osteosarcoma | U2-OS, MNNG-HOS, SaOS-2 | [34,35] |
| Ovarian cancer | OHFC, HPMC, SKOV3 and HRA, OVCAR-3, TOV-21G, TOV-112D | [36,37,38] |
| Pancreatic cancer | MIA PaCa2 | [39] |
| Prostate cancer | LNCaP, PC-3, VCaP | [34,40] |
| Skin cancer | G361, A375 | [41,42] |
| Thyroid cancer | SNU80, BHP10-3, TPC1 | [7] |
| Device | Gap Length (mm) | Volume (μL) | H2O2 (μM) | NO2 (μM) | Medium | Ref. |
|---|---|---|---|---|---|---|
| Ar 60 Hz 7 kV NEAPP | 13 | 3000 | 17 ± 3 | 642 ± 10 | DMEM #5796 | [43] |
| Ar 60 Hz 2–6 kVNEAPP | 3 | 6000 | 227 ± 42 | 265 | DMEM #5796 | [44] |
| Ar 1 MHz 2–6 kV plasma jet (kinpen GmbH) | 9 | 5000 | 33 | n.d. | RPMI + FBS(8%) + P/S(1%) | [45] |
| Ar 1.1 MHz plasma jet (kinpen 09 GmbH) | 5 from the top of the well | 1000 | 60 | 26 | RPMI + FBS(10%) + P/S(2%) +glutamine(1%) | [46] |
| Air 5 kHz 7.5 kV Liquid surface | 1,5 | 1000 | 87 | n.d. | MEM + FBS (10%) + P/S | [47] |
| He + 0.3%O2 13.56 MHz | 20 | 1500 | n.a. | 7.4 ± 1 | MEM + FBS 10% | [48] |
| Ar 60 Hz AC 7 kV | 13 | 3000 | 1.8 × 104 | 6.3 × 105 | DMEM #5796 + P/S + 10% FBS | [43] |
| Ar 60 Hz AC 7 kV | 13 | 3000 | 2.1 × 104 | 6.3 × 105 | DMEM #5796 + 10% FBS | [43] |
| He 30 KHz 3.85 kV | 30 | 30 | 50 | n.a. | DMEM #11965-118 | [49] |
| He 24 kKz 12 kV | 5 | 2000 | 2.3 × 103 ± 240 | 55 ± 9 | DMEM + 15% FBS | [50] |
| Ar 2 kV 1.1 MHz | n.d. | 100 | 60 | 22 | RPMI #1640 + 10% FBS | [51] |
| Ar 2 kV 1.1 MHz | n.d. | 100 | 60 | 31 | RPMI #1640 | [51] |
| He + H2O 10 KHz 8 kV | 1500 | 300 | 50–60 | n.d. | RPMI #1640 | [52] |
| Ar 10 kHZ 10 kV | 1500 | 300 | <20 | n.d. | RPMI #1640 | [52] |
| He 5 kHz 5–9 kV | 2500 from the bottom of the well | 500 | 28 | n.d. | MEM | [53] |
| Air 1 kHz 4 kV | 2 | 200 | 8.4 × 103 ± 2.7 × 103 | 873 ± 15 | RPMI #1640 + 5% FBS | Author’s |
| Air 1 kHz 4 kV | 2 | 200 | 1.9 × 104 ± 0.3 × 104 | 414 ± 47 | RPMI #1640 + 15% FBS | Author’s |
| Air 1 kHz 4 kV | 2 | 200 | 3.9 × 103 ± 1.3 × 103 | 929 ± 24 | DMEM #5648 + 5% FBS | Author’s |
| Air 1 kHz 4 kV | 2 | 200 | 2.4 × 103 ± 1.3 × 103 | 742 ± 82 | DMEM #5648 + 15% FBS | Author’s |
| Cell Line | Cells Plated (Cells/mL) | Organ | Histology | Culture Medium | MTT (IT50 (s)) | SRB (IT50 (s)) |
|---|---|---|---|---|---|---|
| A375 | 100,000 | Skin | Melanoma | DMEM 5% | 32.1 ± 7.1 | 31.8 ± 1.5 |
| OE19 | 100,000 | Esophagus | Adenocarcinoma | DMEM 5% | 68.9 ± 9.0 | nc. |
| HT1376 | 80,0000 | Urinary bladder | Transitional carcinoma grade 3 | DMEM 10% | 34.6 ±3.7 | 35.0 ± 3.6 |
| MNNG/HOS | 50,000 | Bone | Osteosarcoma | DMEM 5% | 33.1 ± 4.6 | 66.8 ± 5.5 |
| TFK-1 | 80,000 | Extra-hepatic bile duct | Carcinoma | RPMI 15% | 30.5 ± 3.2 | 44.2 ± 7.9 |
| PC3 | 100,000 | Prostate (derived from bone) | Adenocarcinoma grade IV | RPMI 5% | 25.4 ± 4.0 | nc. |
| WIDR | 50,000 | Colon | Adenocarcinoma | DMEM 10% | nc. | 50.7 ± 6.3 |
| LNCAP | 100,000 | Prostate (derived from left supraclavicular lymph node) | Adenocarcinoma | RPMI 10% | 19.0 ± 2.5 | 40.2 ± 10.9 |
| ECC-1 | 100,000 | Endometrium | Adenocarcinoma | RPMI 10% | 36.6 ± 4.1 | 48.9 ± 13.6 |
| H1299 | 100,000 | Lung (derived from lymph node) | Large cell carcinoma | DMEM 10% | 46.4 ± 6.2 | 35.3 ± 5.0 |
| MCF-7 | 100,000 | Breast (derived from Pleural effusion) | Invasive ductal carcinoma | DMEM 10% | 65.6 ± 7.9 | 22.4 ± 4.7 |
| HCC 1806 | 100,000 | Breast | Squamous cell breast carcinoma, acantholytic variant | RPMI 10% | 69.4 ± 9.3 | 49.8 ± 10.0 |
| HFF-1 | 250,000 | Connective tissue | Fibroblasts | DMEM 15% | 61.5 ± 2.1 | nc. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida-Ferreira, C.; Silva-Teixeira, R.; Laranjo, M.; Almeida, N.; Brites, G.; Dias-Ferreira, J.; Marques, I.; Neves, R.; Serambeque, B.; Teixo, R.; et al. Open-Air Cold Plasma Device Leads to Selective Tumor Cell Cytotoxicity. Appl. Sci. 2021, 11, 4171. https://doi.org/10.3390/app11094171
Almeida-Ferreira C, Silva-Teixeira R, Laranjo M, Almeida N, Brites G, Dias-Ferreira J, Marques I, Neves R, Serambeque B, Teixo R, et al. Open-Air Cold Plasma Device Leads to Selective Tumor Cell Cytotoxicity. Applied Sciences. 2021; 11(9):4171. https://doi.org/10.3390/app11094171
Chicago/Turabian StyleAlmeida-Ferreira, Catarina, Rafael Silva-Teixeira, Mafalda Laranjo, Nuno Almeida, Gonçalo Brites, João Dias-Ferreira, Inês Marques, Rita Neves, Beatriz Serambeque, Ricardo Teixo, and et al. 2021. "Open-Air Cold Plasma Device Leads to Selective Tumor Cell Cytotoxicity" Applied Sciences 11, no. 9: 4171. https://doi.org/10.3390/app11094171
APA StyleAlmeida-Ferreira, C., Silva-Teixeira, R., Laranjo, M., Almeida, N., Brites, G., Dias-Ferreira, J., Marques, I., Neves, R., Serambeque, B., Teixo, R., Abrantes, A. M., Caramelo, F., & Botelho, M. F. (2021). Open-Air Cold Plasma Device Leads to Selective Tumor Cell Cytotoxicity. Applied Sciences, 11(9), 4171. https://doi.org/10.3390/app11094171











