Preparation of Freeze-Dried Porous Chitosan Microspheres for the Removal of Hexavalent Chromium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
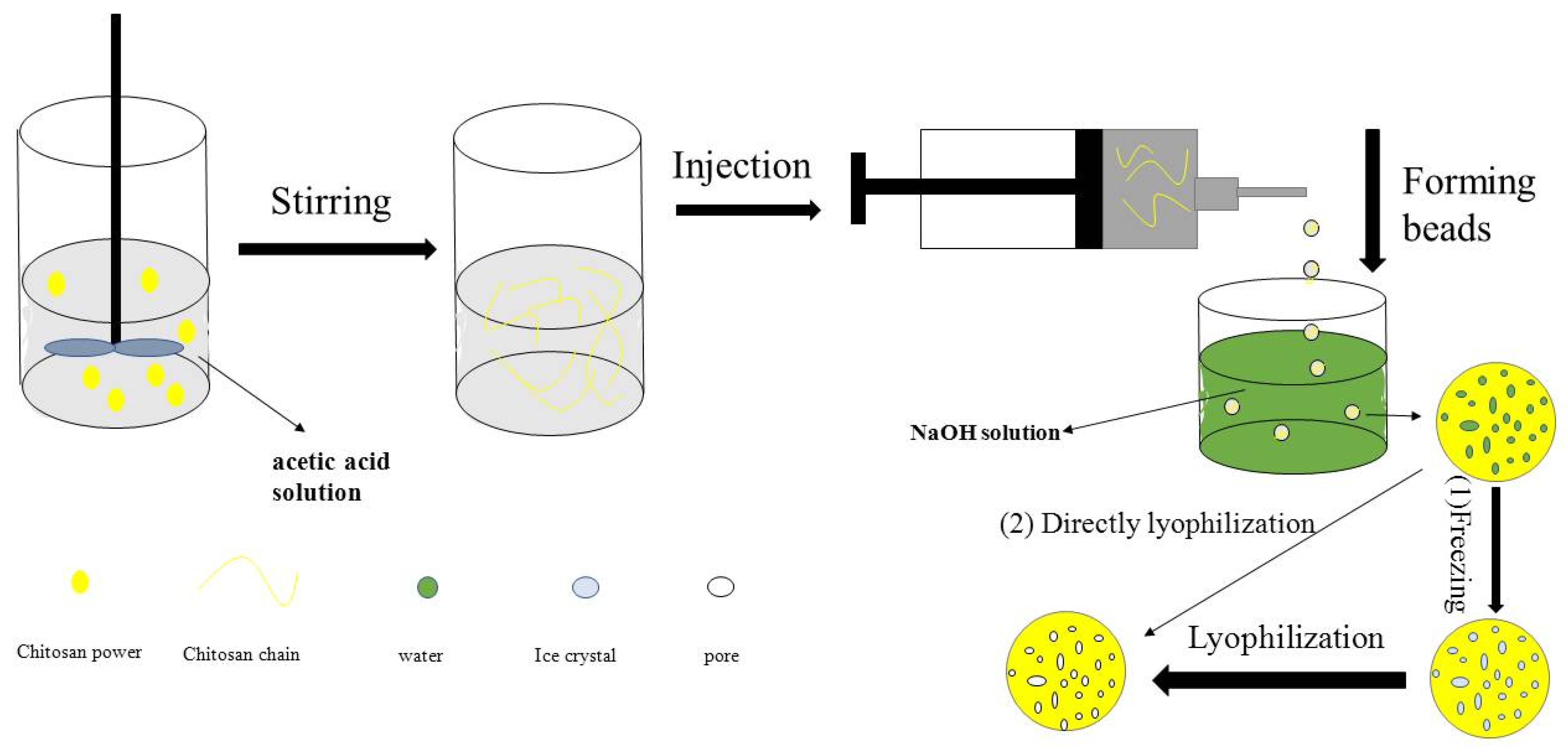
2.2. Preparation of Porous Chitosan Microspheres
2.3. Characterization of Porous Chitosan Microspheres
2.3.1. Scanning Electron Micrographs (SEM)
2.3.2. Porosity Analysis
2.3.3. Swelling and Acid–Base Resistance Experiment
2.3.4. Fourier Transform Infrared Spectra (FTIR)
2.3.5. Adsorption Kinetics Experiments
2.4. Statistical Analysis
3. Results
3.1. Porous Chitosan Microspheres Preparation
3.2. The Morphology of Porous Chitosan Microspheres
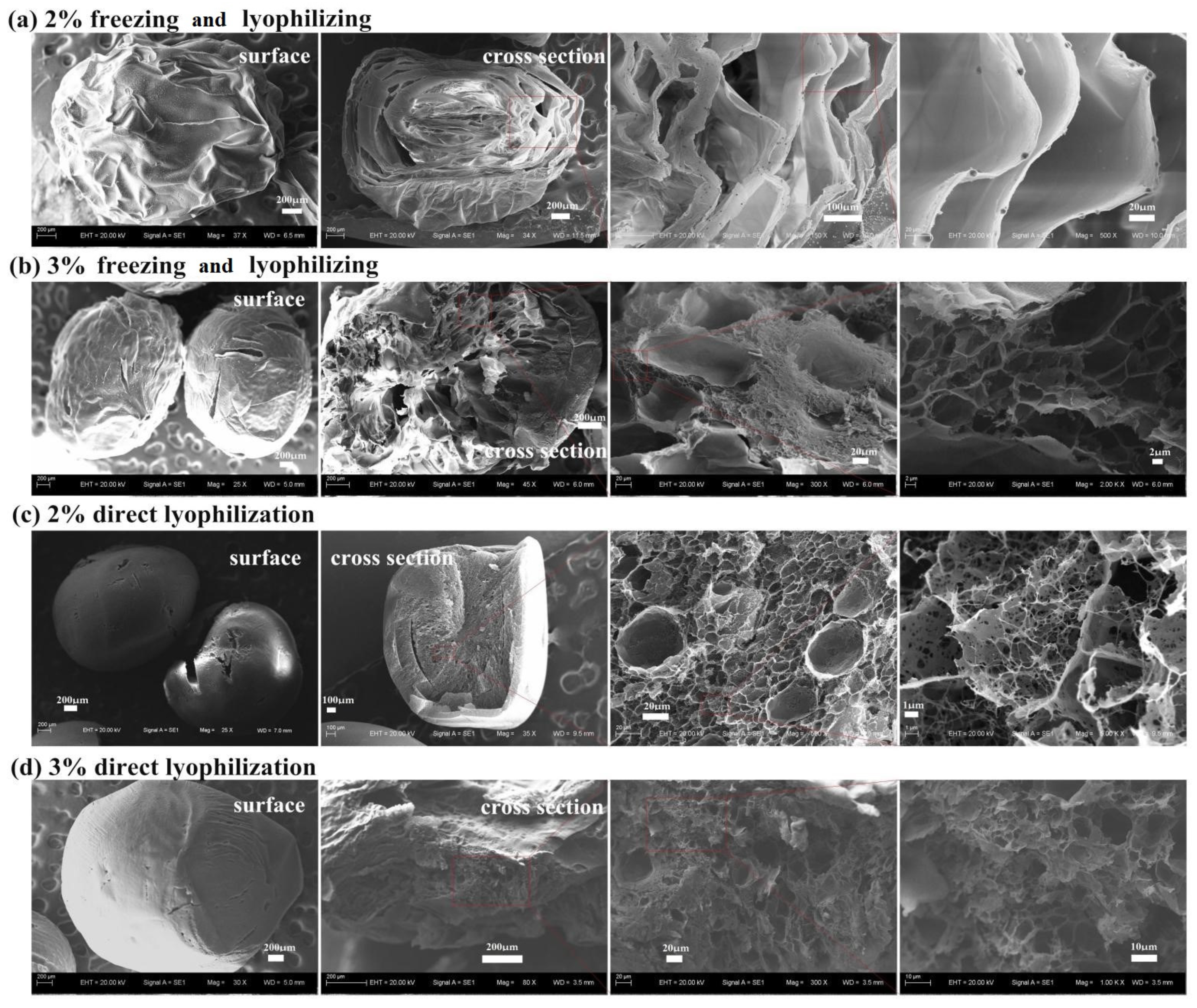
3.2.1. Effect of the Freezing Drying Method
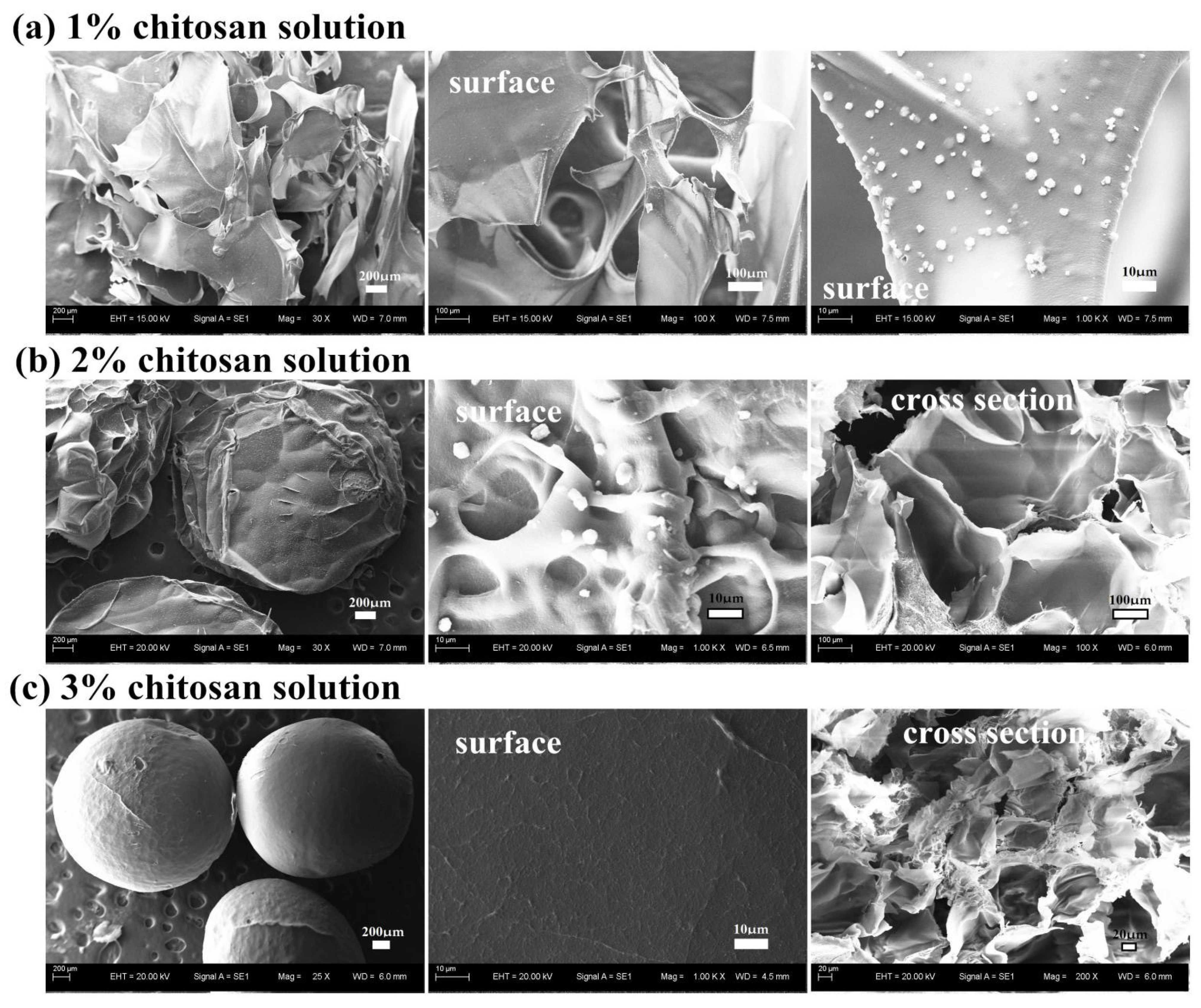
3.2.2. Effect of the Concentration of Chitosan Solution
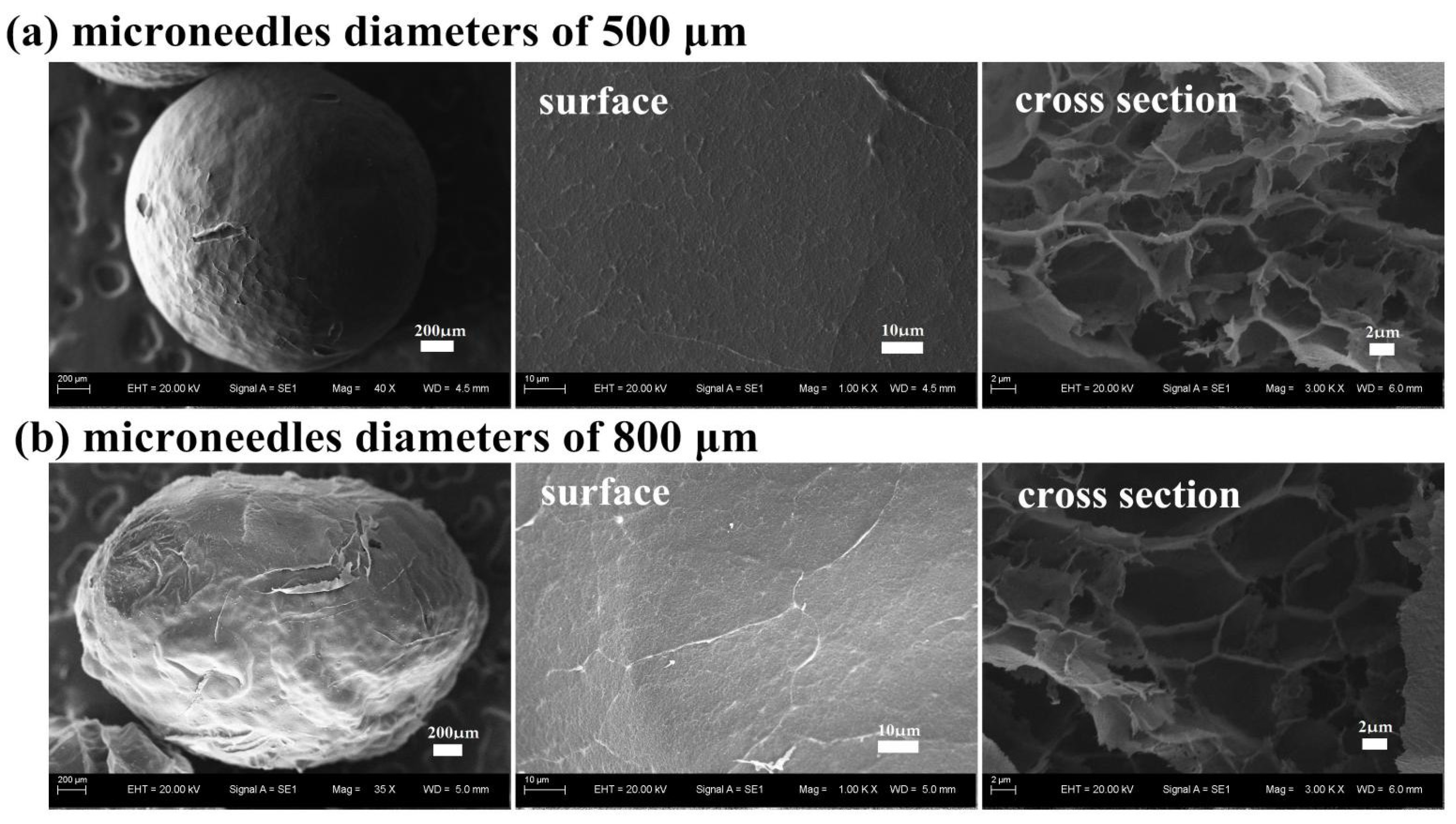
3.2.3. Effect of the Syringe Diameter
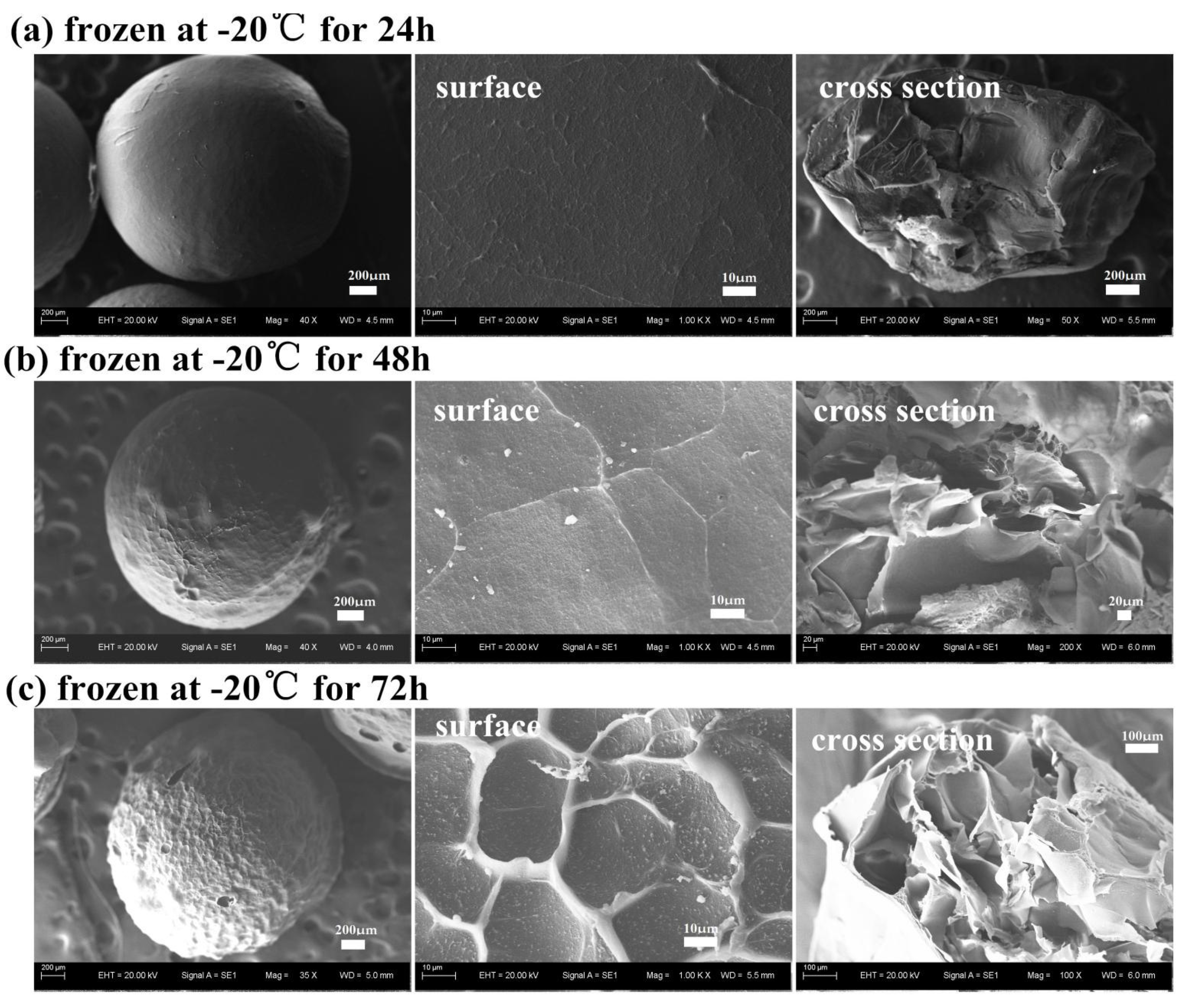
3.2.4. Effect of the Freezing Time
3.3. The Adsorption Properties of Porous Chitosan Microspheres
3.3.1. The Choice of pH of Initial Adsorption Solution
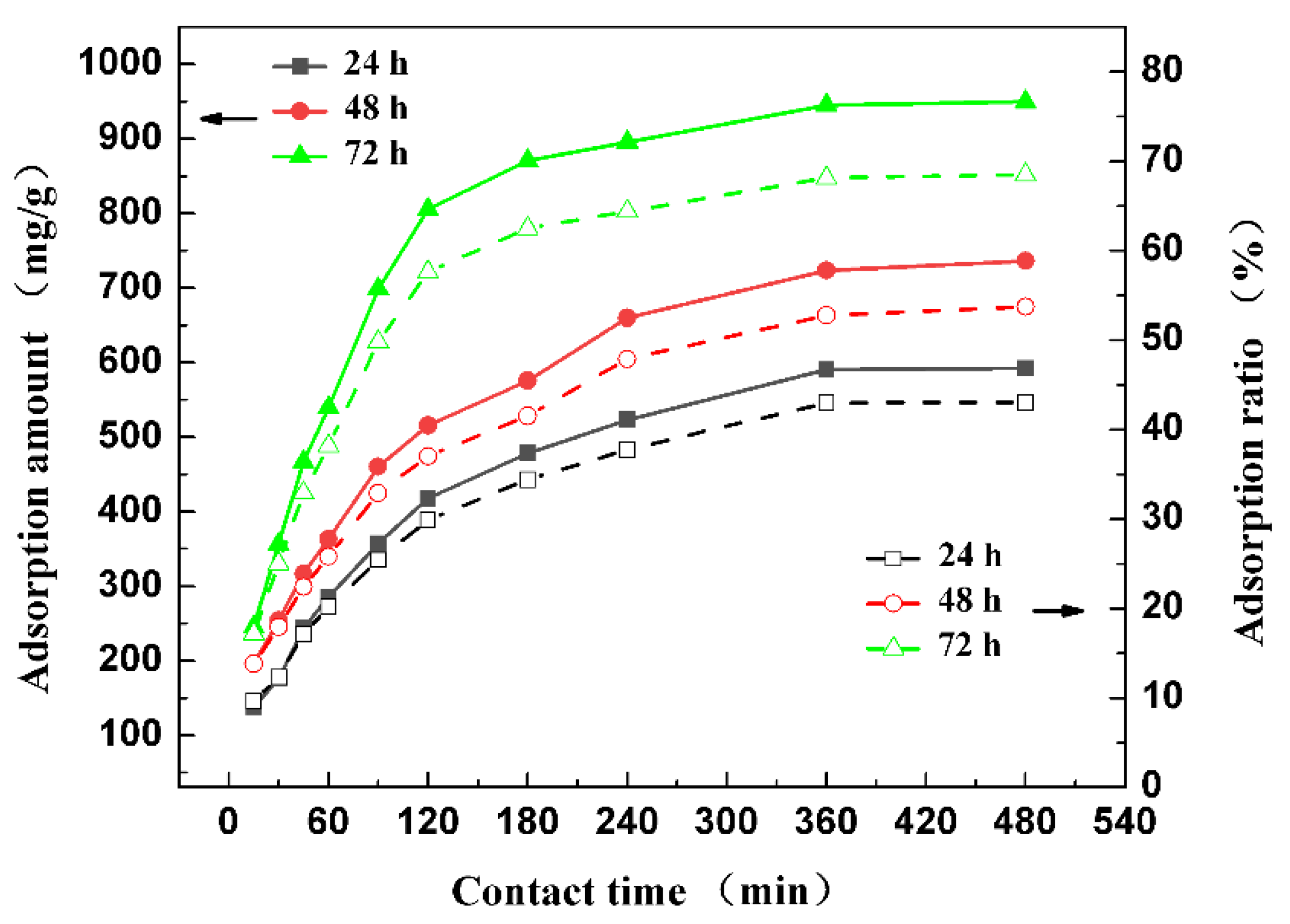
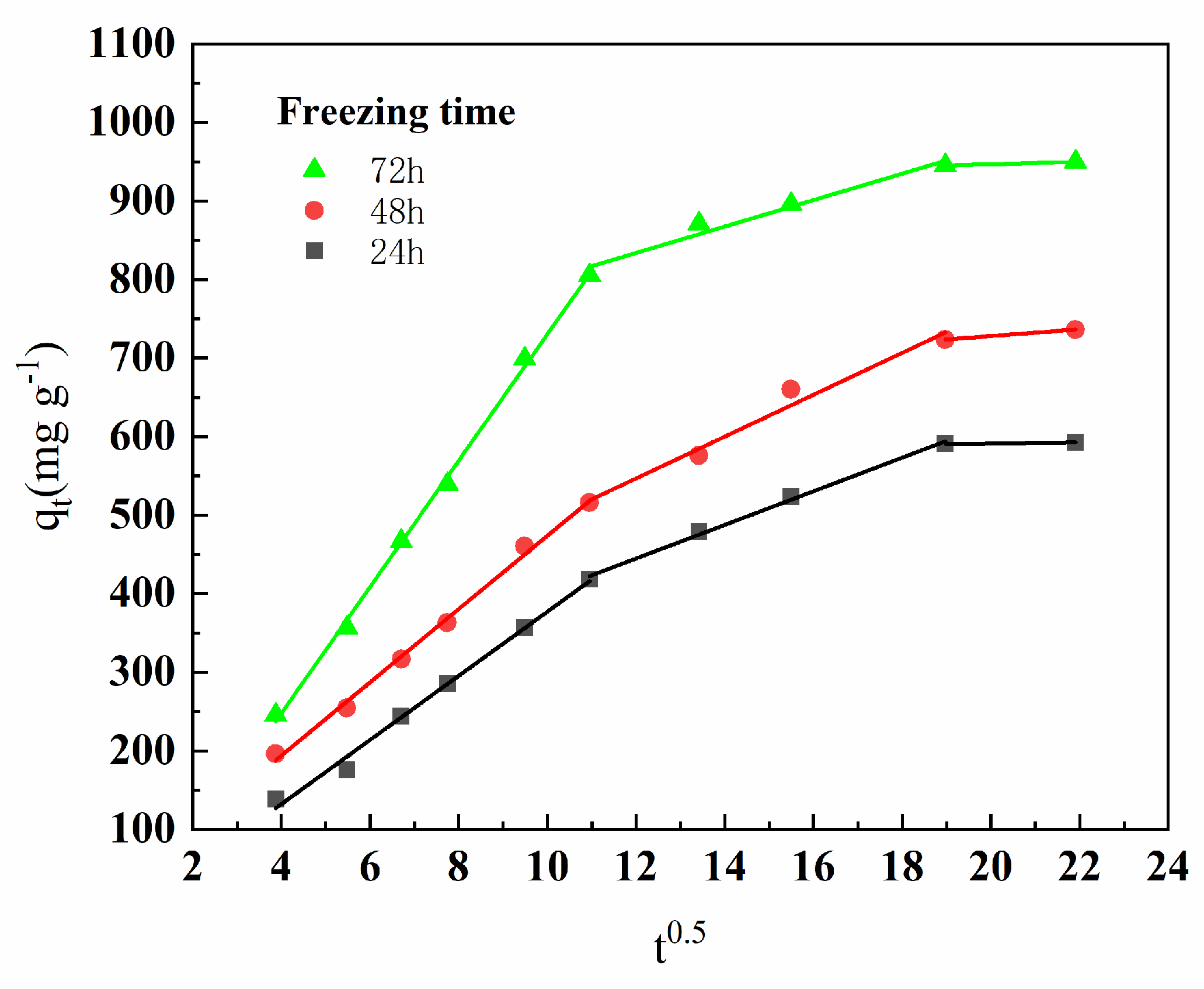
3.3.2. Effect of Freezing Time
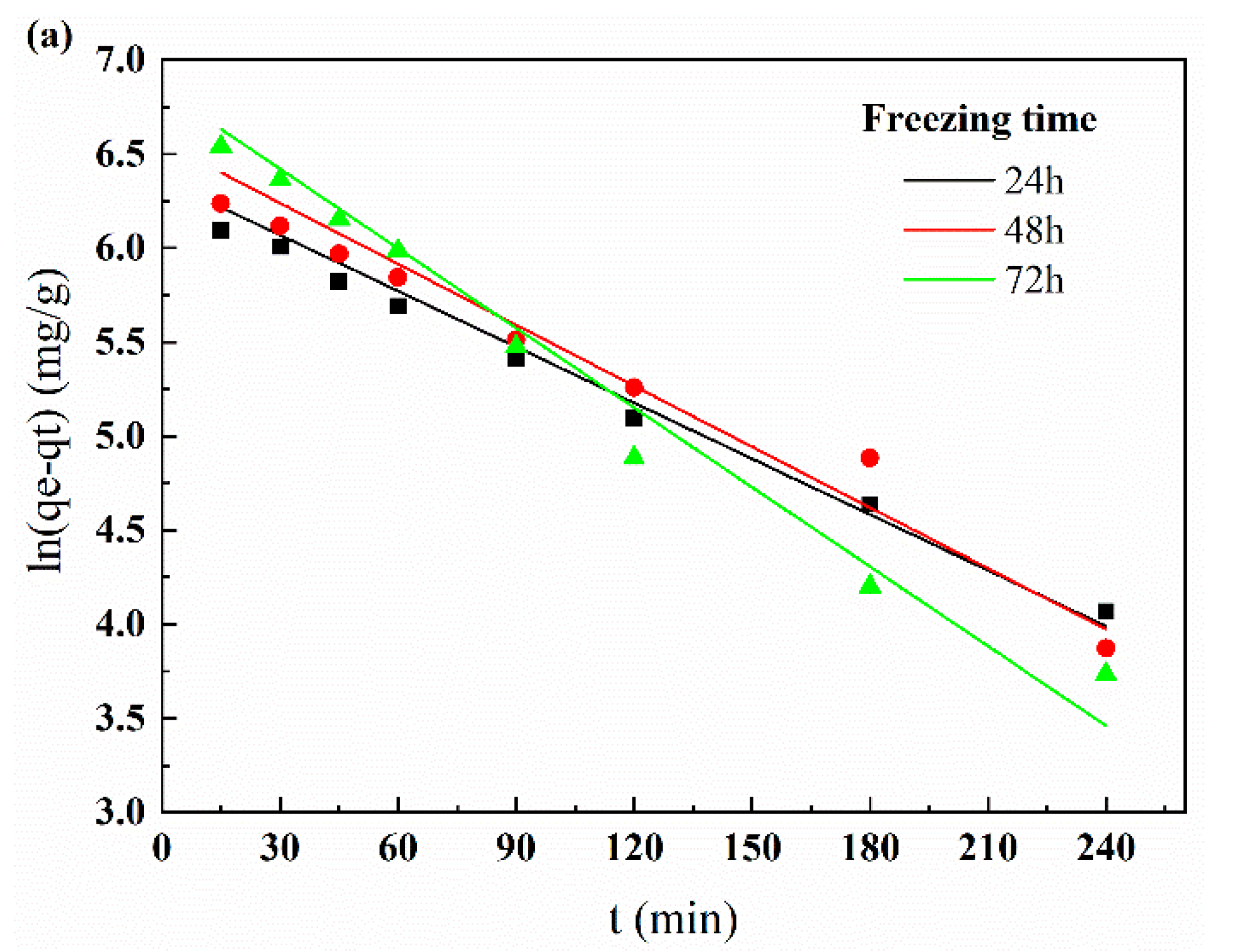
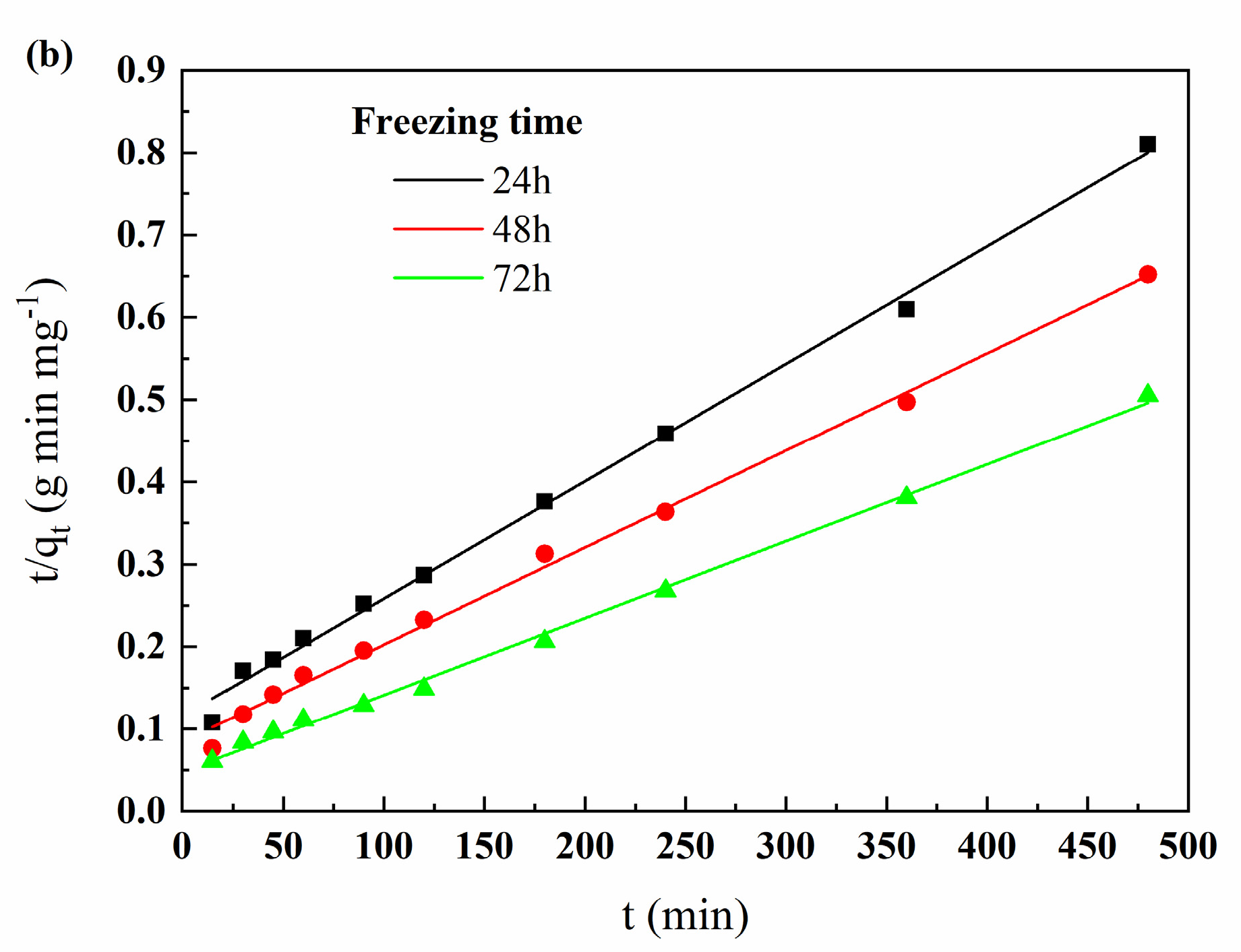
3.3.3. Adsorption Kinetic Analysis
3.4. Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Vareda, J.P.; Valente, J.M.; Durãesa, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef]
- Gómez, V.; Callao, M.P. Chromium determination and speciation since 2000, TRAC-Trend. Anal. Chem. 2006, 25, 1006–1015. [Google Scholar]
- Baral, A.; Engelken, R.D. Chromium-based regulations and greening in metal finishing industries in the USA. Environ. Sci. Policy 2002, 5, 121–133. [Google Scholar] [CrossRef]
- Wang, Y.M.; Duan, L.; Sun, Y.; Hu, N. Adsorptive removal of Cr (VI) from aqueous solutions with an effective adsorbent: Cross-linked chitosan/montmorillonite nanocomposites in the presence of hydroxy-aluminum oligomeric cations. Desalin. Water Treat. 2015, 57, 1–9. [Google Scholar] [CrossRef]
- Habiba, U.; Siddique, T.A.; Talebian, S. Effect of deacetylation on property of electrospun chitosan/PVA nanofibrous membrane and removal of methyl orange, Fe (III) and Cr (VI) ions. Carbohyd. Polym. 2017, 177, 32–39. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; An, H.; Lin, J. Progress on the Photocatalytic Reduction Removal of Chromium Contamination. Chem. Rec. 2019, 5, 873–882. [Google Scholar] [CrossRef]
- Fu, H.R.; Wang, N.; Qin, J.H. Spatial confinement of a cationic MOF: A SC–SC approach for high capacity Cr (VI)-oxyanion capture in aqueous solution. Chem. Commun. 2018, 54, 11645. [Google Scholar] [CrossRef]
- Yan, E.Y.; Cao, M.L.; Jiang, J.Y. A novel adsorbent based on magnetic Fe3O4 contained polyvinyl alcohol/chitosan composite nanofibers for chromium (VI) removal. Solid State Sci. 2017, 72, 94–102. [Google Scholar] [CrossRef]
- Dui, J.N.; Zhu, G.Y.; Zhou, S.M. Facile and economical synthesis of large hollow ferrites and their applications in adsorption for As (V) and Cr (VI). ACS Appl. Mater. Interfaces 2013, 5, 10081–10089. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, W.; Sun, Z.; Pan, C.; Mi, X.; Zhao, G.; Gao, J. Fabrication of porous zeolite/chitosan monoliths and their applications for drug release and metal ions adsorption. Carbohyd. Polym. 2015, 117, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Alsbaiee, A.; Smith, B.J.; Xiao, L.L.; Ling, Y.H.; Helbling, D.E.; Dichtel, W.R. Rapid removal of organic micropollutants from water by a porous β-cyclodextrin polymer. Nature 2016, 529, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xu, J.H.; Lan, W.J.; Wang, T.; Luo, G.S. Microflfluidic production of porous chitosan/silica hybrid microspheres and its Cu(II) adsorption performance. Chem. Eng. J. 2013, 229, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Hamedi, H.; Moradi, S.; Hudson, S.M. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohyd. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef]
- Song, W.; Xu, J.; Ren, L.L.; Guo, L.; Tong, J.; Wang, L.; Chang, Z. Traditional sensory evaluation and bionic electronic nose as innovative tools for the packaging performance evaluation of chitosan film. Polymers 2020, 12, 2310. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Heo, J.Y.; Han, J.H. Hexavalent chromium removal by various adsorbents: Powdered activated carbon, chitosan, and single/multi-walled carbon nanotubes. Sep. Purif. Technol. 2013, 106, 63–71. [Google Scholar] [CrossRef]
- Sankararamakrishnan, N.; Dixit, A. Removal of hexavalent chromium using a novel cross linked xanthated chitosan. Bioresour. Technol. 2006, 97, 2377–2382. [Google Scholar] [CrossRef]
- Bagheri, M.; Younesi, H.; Hajati, S. Application of chitosan-citric acid nanoparticles for removal of chromium (VI). Int. J. Biol. Macromol. 2015, 80, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Trikkaliotis, D.G.; Christoforidis, A.K.; Mitropoulos, A.C.; Kyzas, G.Z. Adsorption of copper ions onto chitosan/poly (vinyl alcohol) beads functionalized with poly (ethylene glycol). Carbohyd. Polym. 2020, 234, 115890. [Google Scholar] [CrossRef]
- Jeon, C.; Holl, W.H. Chemical modification of chitosan and equilibrium study for mercury ion removal. Water Res. 2003, 37, 4770–4780. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. A review and experimental verification of using chitosan and its derivatives as adsorbents for selected heavy metals. J. Environ. Manag. 2010, 91, 798–806. [Google Scholar] [CrossRef]
- Ye, H.L.; Cheng, J.W.; Yu, K. In situ reduction of silver nanoparticles by gelatin to obtain porous silver nanoparticle/chitosan composites with enhanced antimicrobial and wound-healing activity. Int. J. Biol. Macromol. 2019, 121, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Kanimozhi, K.; Basha, S.K.; Kumari, V.S. Processing and characterization of chitosan/PVA and methylcellulose porous scaffolds for tissue engineering. Mater. Sci. Eng. C 2016, 61, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Chen, G.H.; Murray, P. Porous chitosan by crosslinking with tricarboxylic acid and tuneable release. SN Appl. Sci. 2020, 2, 435. [Google Scholar] [CrossRef] [Green Version]
- Madihally, S.V.; Matthew, H.W.T. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999, 20, 1133–1142. [Google Scholar] [CrossRef]
- Ren, L.L.; Xu, J.; Zhang, Y.C. Preparation and characterization of porous chitosan microspheres and adsorption performance for hexavalent chromium. Int. J. Biol. Macromol. 2019, 135, 898–906. [Google Scholar] [CrossRef]
- Lan, W.J.; Li, S.W.; Xu, J.H.; Luo, G.S. One-step synthesis of chitosan-silica hybrid microspheres in a microfluidic device. Biomed. Microdevices 2010, 12, 1087–1095. [Google Scholar] [CrossRef]
- Wu, J.J.; Zhao, Q. Preparation of poly (ethylene glycol) aligned porous cryogels using a unidirectional freezing technique. Soft Matter 2012, 8, 3620. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; García-Carvajal, Z. Y Poly (vinyl alcohol) Scaffolds with Tailored Morphologies for Drug Delivery and Controlled Release. Adv. Funct. Mater. 2017, 17, 3505–3513. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.J.; Zhang, Y.; Yan, S.F.; Liu, Z.W.; He, S.M.; Cui, S.M.; Yin, J.B. Poly (L-glutamic acid)/chitosan polyelectrolyte complex porous microspheres as cell microcarriers for cartilage regeneration. Acta Biomater. 2014, 10, 276–288. [Google Scholar] [CrossRef]
- Menger, F.M.; Zhang, H. Gemini-Induced Columnar Jointing in Vitreous Ice. Cryo-HRSEM as a Tool for Discovering New Colloidal Morphologies. J. Am. Chem. Soc. 2002, 124, 1140–1141. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Kostoglou, M.; Lazaridis, N.K. Copper and chromium (VI) removal by chitosan derivatives-equilibrium and kinetic studies. Chem. Eng. J. 2009, 152, 440–448. [Google Scholar] [CrossRef]
- Zia, Q.; Tabassum, M. Porous poly (L–lactic acid)/chitosan nanofibres for copper ion adsorption. Carbohyd. Polym. 2020, 227, 115343. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, A.C.; Mecabô, A. Adsorption of Cr(VI) using Fe-crosslinked chitosan complex (Ch-Fe). J. Hazard. Mater. 2010, 179, 192–196. [Google Scholar] [CrossRef]
- Wu, Z.J.; Li, S.Y. Cr (VI) adsorption on an improved synthesised cross-linked chitosan resin. J. Mol. Liq. 2012, 170, 25–29. [Google Scholar] [CrossRef]
- Abdel-Mohsen, A.M.; Jancar, J.; Kalina, L.; Hassan, F. Comparative study of chitosan and silk fibroin staple microfibers on removal of chromium (VI): Fabrication, kinetics and thermodynamic studies. Carbohyd. Polym. 2020, 234, 115861. [Google Scholar] [CrossRef] [PubMed]
- Kousalya, G.N.; Gandhi, M.R.; Meenakshi, S. Sorption of chromium (VI) using modified forms of chitosan beads. Int. J. Biol. Macromol. 2010, 47, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Sankararamakrishnan, N.; Sanghi, R. Preparation and characterization of a novel xanthated chitosan. Carbohyd. Polym. 2006, 66, 160–167. [Google Scholar] [CrossRef]
- Chauhan, D.; Jaiswal, M. Removal of cadmium and hexavalent chromium from electroplating waste water using thiocarbamoyl chitosan. Carbohyd. Polym. 2012, 88, 670–675. [Google Scholar] [CrossRef]
- Yavuz, A.G.; Dincturk-Atalay, E. A comparison study of adsorption of Cr (VI) from aqueous solutions onto alkyl-substituted polyaniline/chitosan composites. Desalination 2011, 279, 325–331. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, D.; Wang, A. Chitosan-g-poly (acrylic acid) hydrogel with crosslinked polymeric networks for Ni2+ recovery. Anal. Chim. Acta 2011, 687, 193–200. [Google Scholar] [CrossRef]
- Sahebjamee, N.; Soltanieh, M. Removal of Cu2+, Cd2+ and Ni2+ ions from aqueous solution using a novel chitosan/polyvinyl alcohol adsorptive membrane. Carbohyd. Polym. 2019, 210, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, H.C.; Patel, V.M.; Patel, R.D. Adsorption of cellulose triacetate on calcium silicate. Eur. Polym. J. 1973, 9, 525–531. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Y.X. Removal of heavy metal ions using chitosan and modified chitosan: A review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Cheng, Z.H.; Liu, X.H.; Han, M.; Ma, W. Adsorption kinetic character of copper ion onto a modified chitosan transparent thin membrane from aqueous solution. J. Hazard. Mat. 2010, 182, 408–415. [Google Scholar] [CrossRef]
- Debnath, S.; Maity, A. Magnetic chitosan-GO nanocomposite: Synthesis, characterization and batch adsorber design for Cr (VI) removal. J. Environ. Chem. Eng. 2014, 2, 963–973. [Google Scholar] [CrossRef]
- Habiba, U.; Afifi, A.M.; Salleh, A.; Ang, B.C. Chitosan/(polyvinyl alcohol)/zeolite electrospun composite nanofibrous membrane for adsorption of Cr6+, Fe3+ and Ni2+. J. Hazard. Mater. 2017, 322, 182–194. [Google Scholar] [CrossRef]
- Costa-Júnior, E.D.S.; Pereira, M.M.; Mansur, H.S. Properties and biocompatibility of chitosan films modified by blending with PVA and chemically crosslinked. J. Mater. Sci. Mat. Med. 2009, 20, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Sivakami, M.S.; Gomathi, T.; Venkatesan, J.; Jeong, H.S.; Kim, S.K.; Sudha, P.N. Preparation and characterization of nano chitosan for treatment wastewaters. Int. J. Biol. Macromol. 2013, 57, 204–212. [Google Scholar] [CrossRef]

| Kinetic Model | Parameters | Freezing Time of the Chitosan Microspheres | ||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||
| qe, exp (mg g−1) | 590.6 | 723.6 | 945.2 | |
| Pseudo first order | qe, cal (mg g−1) | 581.7 | 708.12 | 937.8 |
| k1 (min−1) | 0.0111 | 0.0122 | 0.0155 | |
| r2 | 0.982 | 0.961 | 0.992 | |
| pseudo second order | qe, cal (mg g−1) | 701.3 | 847.9 | 1070.5 |
| k2 (g mg−1 min−1) | 0.0000176 | 0.0000165 | 0.0000183 | |
| r2 | 0.996 | 0.995 | 0.997 | |
| Intra-Particle Diffusion | Parameters | Freezing Time of the Chitosan Microspheres | ||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||
| first step | kwm (mg g−1 min−0.5) | 40.85 | 46.67 | 80.46 |
| C (mg g−1) | 31.39 | 6.98 | 74.45 | |
| r2 | 0.993 | 0.996 | 0.998 | |
| second step | kwm (mg/g min−0.5) | 21.4 | 26.65 | 16.83 |
| C (mg g−1) | 188.01 | 229.87 | 631.87 | |
| r2 | 0.996 | 0.977 | 0.967 | |
| third step | kwm (mg g−1 min−0.5) | 0.62 | 4.31 | 1.55 |
| C (mg g−1) | 578.9 | 641.9 | 915.8 | |
| r2 | 1 | 1 | 1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, W.; Xu, J.; Gao, L.; Zhang, Q.; Tong, J.; Ren, L. Preparation of Freeze-Dried Porous Chitosan Microspheres for the Removal of Hexavalent Chromium. Appl. Sci. 2021, 11, 4217. https://doi.org/10.3390/app11094217
Song W, Xu J, Gao L, Zhang Q, Tong J, Ren L. Preparation of Freeze-Dried Porous Chitosan Microspheres for the Removal of Hexavalent Chromium. Applied Sciences. 2021; 11(9):4217. https://doi.org/10.3390/app11094217
Chicago/Turabian StyleSong, Wei, Jian Xu, Lepeng Gao, Qingzhu Zhang, Jin Tong, and Lili Ren. 2021. "Preparation of Freeze-Dried Porous Chitosan Microspheres for the Removal of Hexavalent Chromium" Applied Sciences 11, no. 9: 4217. https://doi.org/10.3390/app11094217
APA StyleSong, W., Xu, J., Gao, L., Zhang, Q., Tong, J., & Ren, L. (2021). Preparation of Freeze-Dried Porous Chitosan Microspheres for the Removal of Hexavalent Chromium. Applied Sciences, 11(9), 4217. https://doi.org/10.3390/app11094217






