Fermented Antler Recovers Stamina, Muscle Strength and Muscle Mass in Middle-Aged Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Antler
2.2. Lactic Acid Bacteria and Growth Conditions
2.3. Preparation of Fermented Antler
2.4. Animals, Diet and Experimental Design
2.5. Treadmill Exercise Performance Test and Grip Strength Measurement
2.6. Swimming Endurance
2.7. Tissue Collection and Serum Biochemistry
2.8. Histological Analysis
2.9. Real-Time RT-PCR
2.10. Statistical Analysis
3. Results
3.1. FA Supplementation Improves Muscle Strength
3.2. FA Supplementation Ameliorated Exercise Performance and Related Blood Indices
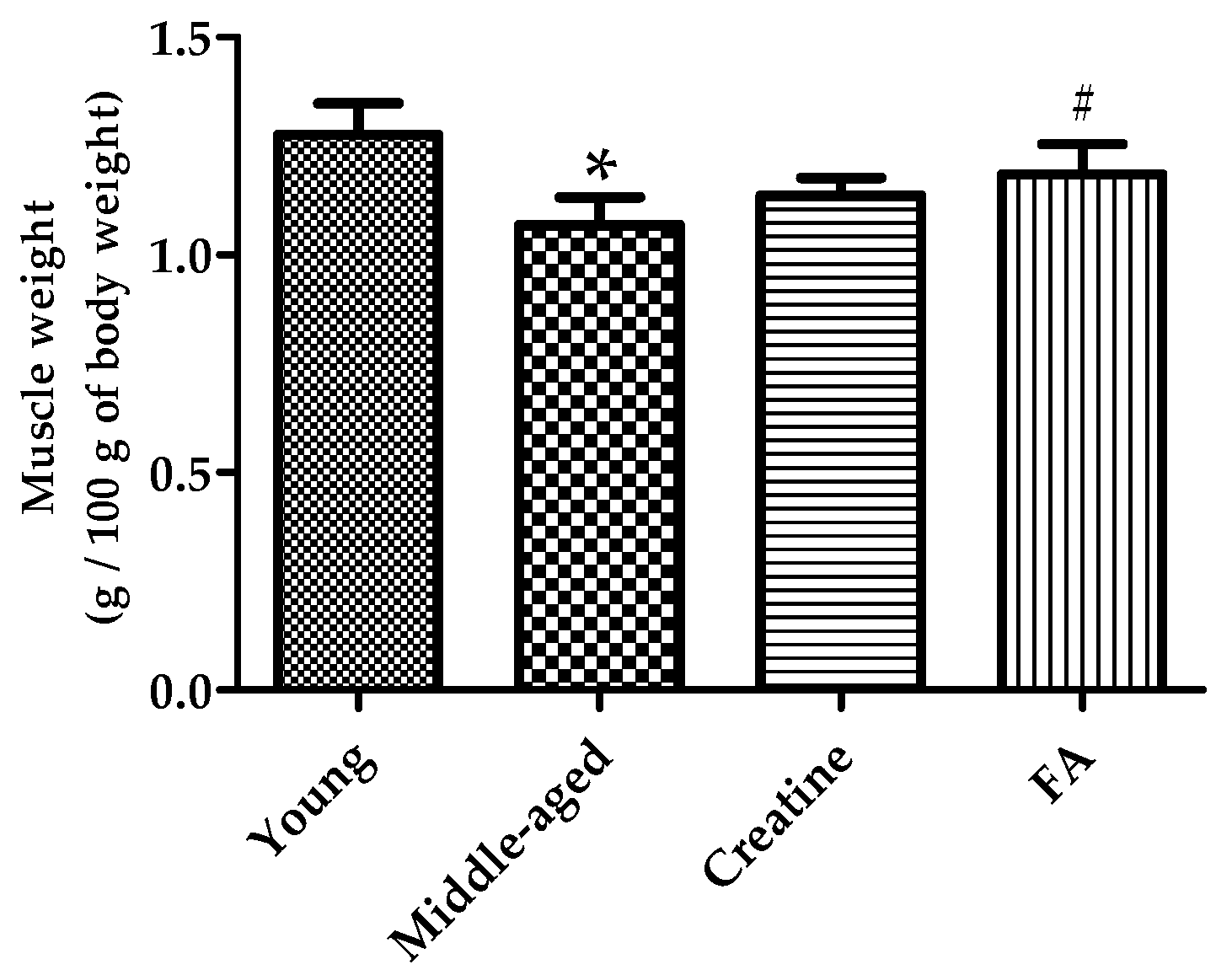
3.3. FA Supplementation Increased Muscle Mass and Histological Section Area of Muscle Fiber
3.4. FA Supplementation Altered mRNA Expression Related in Muscle Hypertrophy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ivankina, N.F.; Isay, S.V.; Busarova, N.G.; Mischenko, T. Prostaglandin-like activity, fatty acid and phospholipid composition of sika deer (Cervus nippon) antlers at different growth stages. Comp. Biochem. Physiol. B Comp. Biochem. 1993, 106, 159–162. [Google Scholar] [CrossRef]
- Jung, S.; Kim, S.-H.; Jeung, W.; Ra, J.; Heo, K.; Shim, J.-J.; Lee, J.-L. Fermented Antler Improves Endurance during Exercise Performance by Increasing Mitochondrial Biogenesis and Muscle Strength in Mice. Appl. Sci. 2021, 11, 5386. [Google Scholar] [CrossRef]
- Jang, D.W.; Ameer, K.; Oh, J.H.; Park, M.K. Optimization and Pretreatment for Hot Water Extraction of Korean Deer (Cervus canadensis Erxleben) Velvet Antlers. J. Microbiol. Biotechnol. 2020, 30, 1116–1123. [Google Scholar] [CrossRef]
- Earnest, C.P.; Quindry, J.; Panton, L.; Broeder, C. Effect of Deer Antler Velvet on Aerobic, Anaerobic and Strength Performance. Cent. Eur. J. Sport Sci. Med. 2015, 9, 17–26. [Google Scholar]
- Jo, K.; Jang, W.Y.; Yun, B.S.; Kim, J.S.; Lee, H.S.; Chang, Y.B.; Suh, H.J. Effect of Deer Antler Extract on Muscle Differentiation and 5-Aminoimidazole-4-Carboxamide Ribonucleoside (AICAR)-Induced Muscle Atrophy in C2C12 Cells. Food Sci. Anim. Resour. 2021, 41, 623–635. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, M.K.; Kim, Y.K.; Jung, E.Y.; Park, C.S.; Woo, M.J.; Lee, S.H.; Kim, J.S.; Suh, H.J. Stimulation of osteoblastic differentiation and mineralization in MC3T3-E1 cells by antler and fermented antler using Cordyceps militaris. J. Ethnopharmacol. 2011, 133, 710–717. [Google Scholar] [CrossRef]
- Park, Y.; Choi, H.-S.; Lee, H.-S.; Suh, H.J. Hematopoietic effect of deer antler extract fermented by Bacillus subtilis on murine marrow cells. Nutr. Res. Pract. 2015, 9, 451–458. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Kim, Y.; Joo, H.-G. Fermented antler extract enhances the viability and interleukin-12 production of spleen cells. Korean J. Vet. Res. 2016, 56, 183–187. [Google Scholar] [CrossRef][Green Version]
- Critchley, M. Neurological disabilities in extreme old age. Pa Med. J. 1963, 66, 35–37. [Google Scholar]
- De Beas-Jiménez, J.D.; López-Lluch, G.; Sánchez-Martínez, I.; Muro-Jiménez, A.; Rodríguez-Bies, E.; Navas, P. Sarcopenia, implications of physical exercise in its pathophysiology. prevention and treatment. Rev. Andal. De Med. Del Deporte 2011, 4, 158–166. [Google Scholar]
- Nair, K.S. Age-Related Changes in Muscle. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2000; Volume 75, pp. S14–S18. [Google Scholar]
- Cruz-Jentoft, A.J.; Hughes, B.D.; Scott, D.; Sanders, K.M.; Rizzoli, R. Nutritional strategies for maintaining muscle mass and strength from middle age to later life: A narrative review. Maturitas 2020, 132, 57–64. [Google Scholar] [CrossRef]
- Tanimoto, Y.; Watanabe, M.; Kono, R.; Hirota, C.; Takasaki, K.; Kono, K. Aging changes in muscle mass of Japanese. Nihon Ronen Igakkai Zasshi. Jpn. J. Geriatr. 2010, 47, 52–57. [Google Scholar] [CrossRef]
- Mijnarends, D.M.; Luiking, Y.C.; Halfens, R.J.G.; Evers, S.; Lenaerts, E.L.A.; Verlaan, S.; Wallace, M.; Schols, J.M.G.A.; Meijers, J.M.M. Muscle, health and costs: A glance at their relationship. J. Nutr. Health Aging 2018, 22, 766–773. [Google Scholar] [CrossRef]
- Jones, T.E.; Stephenson, K.W.; King, J.G.; Knight, K.R.; Marshall, T.L.; Scott, W.B. Sarcopenia—Mechanisms and treatments. J. Geriatr. Phys. 2009, 32, 83–89. [Google Scholar] [CrossRef]
- Rizzoli, R.; Reginster, J.-Y.; Arnal, J.-F.; Bautmans, I.; Beaudart, C.; Bischoff-Ferrari, H.; Biver, E.; Boonen, S.; Brandi, M.-L.; Chines, A.; et al. Quality of life in sarcopenia and frailty. Calcif. Tissue Int. 2013, 93, 101–120. [Google Scholar] [CrossRef]
- Beaudart, C.; Locquet, M.; Reginster, J.-Y.; Delandsheere, L.; Petermans, J.; Bruyère, O. Quality of life in sarcopenia measured with the SarQoL®: Impact of the use of different diagnosis definitions. Aging Clin. Exp. Res. 2018, 30, 307–313. [Google Scholar] [CrossRef]
- Woo, T.; Yu, S.; Adams, R.; Visvanathan, R. The Association Between Sarcopenia and Quality of Life is Different in Community Dwelling Older Australian Men and Women. Geriatr. Med. Care 2018, 2, 31. [Google Scholar] [CrossRef]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Min. Bone Metab. 2014, 11, 177–180. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Bloom, I.; Shand, C.; Cooper, C.; Robinson, S.; Baird, J. Diet Quality and Sarcopenia in Older Adults: A Systematic Review. Nutrients 2018, 10, 308. [Google Scholar] [CrossRef]
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of muscle atrophy and hypertrophy: Implications in health and disease. Nat. Commun. 2021, 12, 330. [Google Scholar] [CrossRef]
- Shen, L.; Meng, X.; Zhang, Z.; Wang, T. Physical Exercise for Muscle Atrophy. Adv. Exp. Med. Biol. 2018, 1088, 529–545. [Google Scholar] [CrossRef]
- Stene, G.B.; Helbostad, J.L.; Balstad, T.R.; Riphagen, I.I.; Kaasa, S.; Oldervoll, L.M. Effect of physical exercise on muscle mass and strength in cancer patients during treatment—a systematic review. Crit. Rev. Oncol. Hematol. 2013, 88, 573–593. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Corriere, M.; Ferrucci, L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet. Diabetes Endocrinol. 2014, 2, 819–829. [Google Scholar] [CrossRef]
- Moore, D.R.; Kelly, R.P.; Devries, M.C.; Churchward-Venne, T.A.; Phillips, S.M.; Parise, G.; Johnston, A.P. Low-load resistance exercise during inactivity is associated with greater fibre area and satellite cell expression in older skeletal muscle. J. Cachexia Sarcopenia Muscle 2018, 9, 747–754. [Google Scholar] [CrossRef]
- Morley, J.E.; Argiles, J.M.; Evans, W.J.; Bhasin, S.; Cella, D.; Deutz, N.E.P.; Doehner, W.; Fearon, K.C.H.; Ferrucci, L.; Hellerstein, M.K. Nutritional recommendations for the management of sarcopenia. J. Am. Med. Dir. Assoc. 2010, 11, 391–396. [Google Scholar] [CrossRef]
- Malafarina, V.; Uriz-Otano, F.; Iniesta, R.; Gil-Guerrero, L. Effectiveness of nutritional supplementation on muscle mass in treatment of sarcopenia in old age: A systematic review. J. Am. Med. Dir. Assoc. 2013, 14, 10–17. [Google Scholar] [CrossRef]
- Saraswati, S.; Sitaraman, R. Aging and the human gut microbiota—From correlation to causality. Front. Microbiol. 2015, 5, 764. [Google Scholar] [CrossRef]
- Kim, S.; Jazwinski, S.M. The gut microbiota and healthy aging: A mini-review. Gerontology 2018, 64, 513–520. [Google Scholar] [CrossRef]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented foods: Definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef]
- Taylor, B.C.; Lejzerowicz, F.; Poirel, M.; Shaffer, J.P.; Jiang, L.; Aksenov, A.; Litwin, N.; Humphrey, G.; Martino, C.; Miller-Montgomery, S. Consumption of fermented foods is associated with systematic differences in the gut microbiome and metabolome. Msystems 2020, 5, e00901–e00919. [Google Scholar]
- Del Campo, A.; Contreras-Hernández, I.; Castro-Sepúlveda, M.; Campos, C.A.; Figueroa, R.; Tevy, M.F.; Eisner, V.; Casas, M.; Jaimovich, E. Muscle function decline and mitochondria changes in middle age precede sarcopenia in mice. Aging 2018, 10, 34. [Google Scholar] [CrossRef]
- Flurkey, K.; Currer, J.M.; Harrison, D.E. Chapter 20—Mouse Models in Aging Research. In The Mouse in Biomedical Research, 2nd ed.; Fox, J.G., Davisson, M.T., Quimby, F.W., Barthold, S.W., Newcomer, C.E., Smith, A.L., Eds.; Academic Press: Burlington, MA, USA, 2007; pp. 637–672. [Google Scholar] [CrossRef]
- Kim, H.; Rafiuddin-Shah, M.; Tu, H.C.; Jeffers, J.R.; Zambetti, G.P.; Hsieh, J.J.; Cheng, E.H. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat. Cell Biol. 2006, 8, 1348–1358. [Google Scholar] [CrossRef]
- Daniel, P.T.; Schulze-Osthoff, K.; Belka, C.; Güner, D. Guardians of cell death: The Bcl-2 family proteins. Essays Biochem. 2003, 39, 73–88. [Google Scholar] [CrossRef]
- Kwak, H.B.; Song, W.; Lawler, J.M. Exercise training attenuates age-induced elevation in Bax/Bcl-2 ratio, apoptosis, and remodeling in the rat heart. Faseb J. 2006, 20, 791–793. [Google Scholar] [CrossRef]
- Starkey, D.B.; Pollock, M.L.; Ishida, Y.; Welsch, M.A.; Brechue, W.F.; Graves, J.E.; Feigenbaum, M.S. Effect of resistance training volume on strength and muscle thickness. Med. Sci. Sports Exerc. 1996, 28, 10. [Google Scholar] [CrossRef]
- Hughes, S.M.; Schiaffino, S. Control of muscle fibre size: A crucial factor in ageing. Acta Physiol. Scand. 1999, 167, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Salahshoor, T.; Farzanegi, P.; Habibian, M. Synergistic effects of omega 3 supplementation and exercise on markers of liver (ALP, AST, and ALT) and muscle (LDH and CK) damage in male karate athletes. J. Appl. Sci. Agric. 2014, 9, 245–249. [Google Scholar]
- Wu, R.-E.; Huang, W.-C.; Liao, C.-C.; Chang, Y.-K.; Kan, N.-W.; Huang, C.-C. Resveratrol protects against physical fatigue and improves exercise performance in mice. Molecules 2013, 18, 4689. [Google Scholar] [CrossRef]
- Kan, N.-W.; Ho, C.-S.; Chiu, Y.-S.; Huang, W.-C.; Chen, P.-Y.; Tung, Y.-T.; Huang, C.-C. Effects of resveratrol supplementation and exercise training on exercise performance in middle-aged mice. Molecules 2016, 21, 661. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wen, Y.-T.; Lee, M.-C.; Ho, H.-M.; Huang, C.-C.; Hsu, Y.-J. Effects of isolated soy protein and strength exercise training on exercise performance and biochemical profile in postpartum mice. Metabolism 2019, 94, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-C.; Liao, Y.-C.; Lee, M.-C.; Lin, K.-J.; Hsu, H.-Y.; Chiou, S.-Y.; Young, S.-L.; Lin, J.-S.; Huang, C.-C.; Watanabe, K. Lactobacillus plantarum TWK10 attenuates aging-associated muscle weakness, bone loss, and cognitive impariment by modulating the gut microbiome in mice. Front. Nutr. 2021, 8, 708096. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Yang, X.; Zheng, L.; Wang, Z.; Wu, L.; Jiang, J.; Yang, T.; Ma, L.; Fu, Z. Lactobacillus and Bifidobacterium improves physiological function and cognitive ability in aged mice by the regulation of gut microbiota. Mol. Nutr. Food Res. 2019, 63, e1900603. [Google Scholar] [CrossRef]





| Young (One Group) | Middle-Aged (Three Groups) | |||
|---|---|---|---|---|
| Treatments | Diet Only | Diet Only | +Creatine | +FA |
| Body weight (g) | 25.20 ± 1.14 (range, 23.43–27.18) | 31.69 ± 2.15 (range, 28.37–36.75) | ||
| Grip strength (N) | 1.04 ± 0.22 (range, 0.65–1.25) | 0.94 ± 0.20 (range, 0.55–1.35) | ||
| Lactate (mmol/L) | 3.56 ± 0.78 (range, 2.9–4.9) | 3.26 ± 0.62 (range, 2.5–4.9) | ||
| Young | Middle-Aged | Creatine | FA | |
|---|---|---|---|---|
| ALT (U/L) | 16.3 ± 1.4 | 21.2 ± 2.3 * | 19.1 ± 2.8 | 16.8 ± 3.0 # |
| ALP (U/L) | 169 ± 26 | 222 ± 19 * | 177 ± 24 # | 159 ± 14 # |
| LDH (U/L) | 135 ± 9 | 226 ± 54 * | 194 ± 11 | 141 ± 24 # |
| CK (U/L) | 29 ± 3 | 60 ± 14 * | 46 ± 10 | 32 ± 6 # |
| Lactate (mg/dL) | 106.1 ± 13.4 | 163.4 ± 30.1 * | 118.2 ± 8.6 # | 10.6 ± 17.5 # |
| Creatinine (mg/dL) | 0.45 ± 0.04 | 0.49 ± 0.02 * | 0.48 ± 0.02 | 0.45 ± 0.02 # |
| Glucose (mg/dL) | 257 ± 28 | 357 ± 60 * | 329 ± 61 | 284 ± 63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-T.; Jeon, H.; Kim, S.-H.; Heo, K.; Shim, J.-J.; Lee, J.-L.; Yang, D.-C.; Kang, S.C. Fermented Antler Recovers Stamina, Muscle Strength and Muscle Mass in Middle-Aged Mice. Appl. Sci. 2022, 12, 106. https://doi.org/10.3390/app12010106
Kim Y-T, Jeon H, Kim S-H, Heo K, Shim J-J, Lee J-L, Yang D-C, Kang SC. Fermented Antler Recovers Stamina, Muscle Strength and Muscle Mass in Middle-Aged Mice. Applied Sciences. 2022; 12(1):106. https://doi.org/10.3390/app12010106
Chicago/Turabian StyleKim, Yong-Tae, Hyejin Jeon, Sung-Hwan Kim, Keon Heo, Jae-Jung Shim, Jung-Lyoul Lee, Deok-Chun Yang, and Se Chan Kang. 2022. "Fermented Antler Recovers Stamina, Muscle Strength and Muscle Mass in Middle-Aged Mice" Applied Sciences 12, no. 1: 106. https://doi.org/10.3390/app12010106
APA StyleKim, Y.-T., Jeon, H., Kim, S.-H., Heo, K., Shim, J.-J., Lee, J.-L., Yang, D.-C., & Kang, S. C. (2022). Fermented Antler Recovers Stamina, Muscle Strength and Muscle Mass in Middle-Aged Mice. Applied Sciences, 12(1), 106. https://doi.org/10.3390/app12010106







