Trabecular Meshwork Motion Profile from Pulsatile Pressure Transients: A New Platform to Simulate Transitory Responses in Humans and Nonhuman Primates
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
- (1)
- Eyes and anterior segment perfusion setup
- (2)
- Imaging system and scanning procedures
- (3)
- Data analysis
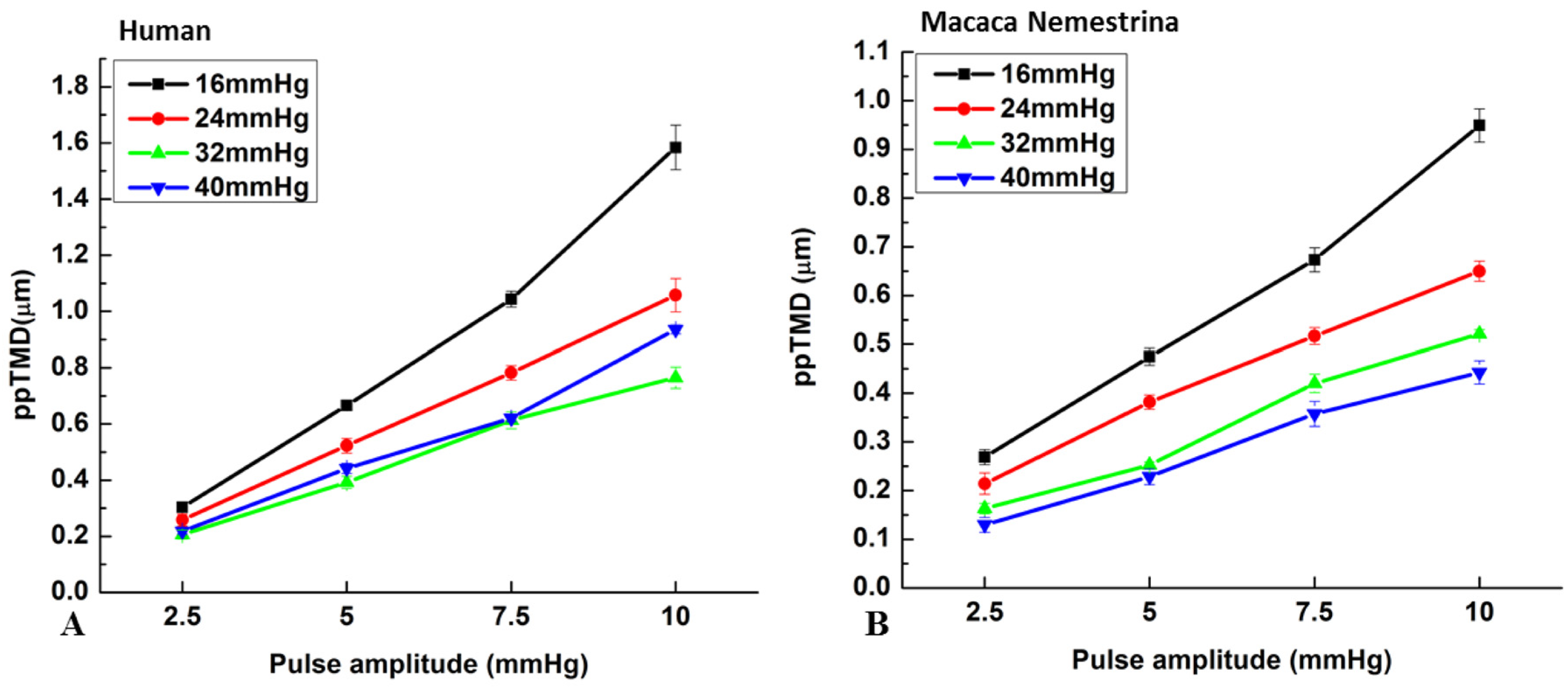
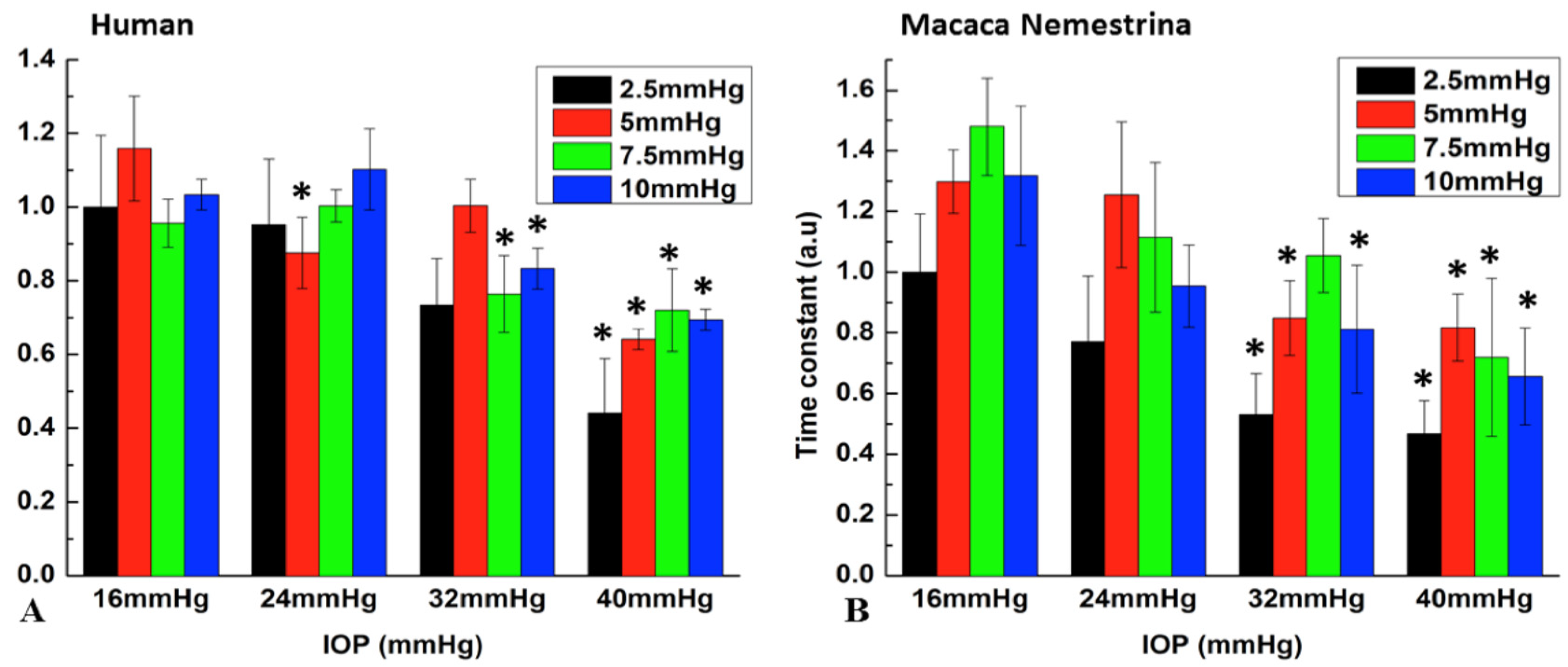
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [Green Version]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and metaanalysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Bahrami, H. Causal inference in primary open angle glaucoma: Specific discussion on intraocular pressure. Ophthalmic Epidemiol. 2006, 13, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Downs, J.C.; Burgoyne, C.F.; Seigfreid, W.P.; Reynaud, J.F.; Strouthidis, N.G.; Sallee, V. 24-Hour IOP Telemetry in the Nonhuman Primate: Implant System Performance and Initial Characterization of IOP at Multiple Timescales. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7365–7375. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, C. Ocular Pulse Amplitude in Healthy Subjects as Measured by Dynamic Contour Tonometry. Arch. Ophthalmol. 2006, 124, 1104. [Google Scholar] [CrossRef] [Green Version]
- Dastiridou, A.I.; Ginis, H.S.; De Brouwere, D.; Tsilimbaris, M.K.; Pallikaris, I.G. Ocular Rigidity, Ocular Pulse Amplitude, and Pulsatile Ocular Blood Flow: The Effect of Intraocular Pressure. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Rumpel, H.; Lim, W.E.H.; Baskaran, M.; Perera, S.A.; Nongpiur, M.E.; Aung, T.; Milea, D.; Girard, M.J.A. Finite Element Analysis Predicts Large Optic Nerve Head Strains During Horizontal Eye Movements. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2452–2462. [Google Scholar] [CrossRef]
- Turner, D.C.; Edmiston, A.M.; Zohner, Y.E.; Byrne, K.J.; Seigfreid, W.P.; Girkin, C.A.; Morris, J.S.; Downs, J.C. Transient Intraocular Pressure Fluctuations: Source, Magnitude, Frequency, and Associated Mechanical Energy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2572–2582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markert, J.E.; Jasien, J.V.; Turner, D.C.; Huisingh, C.; Girkin, C.A.; Downs, J.C. IOP, IOP transient impulse, ocular perfusion pressure, and mean arterial pressure relationships in nonhuman primates instrumented with telemetry. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4496–4505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnstone, M.; Xin, C.; Tan, J.; Martin, E.; Wen, J.; Wang, R.K. Aqueous outflow regulation-21st century concepts. Prog. Retin. Eye Res. 2021, 83, 100917. [Google Scholar] [CrossRef] [PubMed]
- Carreon, T.; van der Merwe, E.; Fellman, R.L.; Johnstone, M.; Bhattacharya, S.K. Aqueous outflow-A continuum from trabecular meshwork to episcleral veins. Prog. Retin. Eye Res. 2017, 57, 108–133. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.; Johnstone, M.; Wang, N.; Wang, R.K. Oct study of mechanical properties associated with trabecular meshwork and collector channel motion in human eyes. PLoS ONE 2016, 11, e0162048. [Google Scholar] [CrossRef] [Green Version]
- Xin, C.; Johnstone, M.A.; Song, S.; Padilla, S.; Wang, R.K. High-resolution oct platform to characterize tm stiffness relevant to glaucoma. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4704. [Google Scholar]
- Xin, C.; Wang, R.K.; Song, S.; Shen, T.; Wen, J.; Martin, E.; Jiang, Y.; Padilla, S.; Johnstone, M. Aqueous outflow regulation: Optical coherence tomography implicates pressure-dependent tissue motion. Exp. Eye Res. 2017, 158, 171–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, C.; Song, S.; Wang, N.; Wang, R.; Johnstone, M. Effects of Schlemm’s Canal Expansion: Biomechanics and MIGS Implications. Life 2021, 11, 176. [Google Scholar] [CrossRef]
- Hariri, S.; Johnstone, M.; Jiang, Y.; Padilla, S.; Zhou, Z.; Reif, R.; Wang, R.K. Platform to investigate aqueous outflow system structure and pressure-dependent motion using high-resolution spectral domain optical coherence tomography. J. Biomed. Opt. 2014, 19, 106013. [Google Scholar] [CrossRef]
- Li, G.; Lee, C.; Agrahari, V.; Wang, K.; Navarro, I.; Sherwood, J.M.; Crews, K.; Farsiu, S.; Gonzalez, P.; Lin, C.W.; et al. In vivo measurement of trabecular meshwork stiffness in a corticosteroid-induced ocular hypertensive mouse model. Proc. Natl. Acad. Sci. USA 2019, 116, 1714–1722. [Google Scholar] [CrossRef] [Green Version]
- Dragostinoff, N.; Werkmeister, R.M.; Klaizer, J.; Gröschl, M.; Schmetterer, L. Time Course and Topographic Distribution of Ocular Fundus Pulsation Measured by Low-Coherence Tissue Interferometry. J. Biomed. Opt. 2013, 18, 121502. [Google Scholar] [CrossRef]
- An, L.; Chao, J.; Johnstone, M.; Wang, R.K. Noninvasive imaging of pulsatile movements of the optic nerve head in normal human subjects using phase-sensitive spectral domain optical coherence tomography. Opt. Lett. 2013, 38, 1512–1514. [Google Scholar] [CrossRef]
- Li, P.; Reif, R.; Zhi, Z.; Martin, E.; Shen, T.T.; Johnstone, M.; Wang, R.K. Phase-sensitive optical coherence tomography characterization of pulse-induced trabecular meshwork displacement in ex vivo non-human primate eyes. Med. Opt. 2012, 17, 076026. [Google Scholar]
- Humphrey, J.D. Cardiovascular Solid Mechanics: Cells, Tissues, and Organs, 1st ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Samples, J.R.; Knepper, P.A. Glaucoma Research and Clinical Advances: 2016 to 2018; Kugler Publications: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Chi, H.H.; Katzin, H.M.; Teng, C.C. Primary degeneration in the vicinity of the chamber angle; as an etiologic factor in wide-angle glaucoma. Am. J. Ophthalmol. 1957, 43, 193–203. [Google Scholar] [PubMed]
- Hamanaka, T.; Matsuda, A.; Sakurai, T.; Kumasaka, T. Morphological Abnormalities of Schlemm’s Canal in Primary Open-Angle Glaucoma From the Aspect of Aging. Invest. Ophthalmol. Vis. Sci. 2016, 57, 692–706. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, P.L.; Bárány, E.H. Residual pilocarpine effects on outflow facility after ciliary muscle disinsertion in the synomolgus monkey. Investig. Ophthalmol. Vis. Sci. 1976, 15, 558–561. [Google Scholar]
- Kaufman, P.L.; Bárány, E.H. Loss of acute pilocarpine effect on outflow facility following surgical disinsertion and retrodisplacement of the ciliary muscle from the scleral spur in the cynomolgus monkey. Invest Ophthalmol. 1976, 15, 793–807. [Google Scholar] [PubMed]
- Béla, G.L. Instrument Engineers’ Handbook: Process Control and Optimization, 4th ed.; CRC Press: Boca Raton, FL, USA, 2003; p. 100. [Google Scholar]
- Park, J.; Shin, A.; Jafari, S.; Demer, J.L. Material properties and effect of preconditioning of human sclera, optic nerve, and optic nerve sheath. Biomech. Model. Mechanobiol. 2021, 20, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Razaghi, R.; Sera, T.; Kudo, S. A combination of the finite element analysis and experimental indentation via the cornea. J. Mech. Behav. Biomed. Mater. 2019, 90, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Man, X.; Arroyo, E.; Dunbar, M.; Reed, D.M.; Shah, N.; Kagemann, L.; Kim, W.; Moroi, S.E.; Argento, A. Perilimbal sclera mechanical properties: Impact on intraocular pressure in porcine eyes. PLoS ONE 2018, 13, e0195882. [Google Scholar] [CrossRef] [Green Version]
- Nibourg, L.M.; Sharma, P.K.; van Kooten, T.G.; Koopmans, S.A. Changes in lens stiffness due to capsular opacification in accommodative lens refilling. Exp. Eye Res. 2015, 134, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Boazak, E.M.; d’Humières, J.; Read, A.T.; Ethier, C.R. Compressive mechanical properties of rat and pig optic nerve head. J. Biomech. 2019, 93, 204–208. [Google Scholar] [CrossRef]
- Qu, J.; Chen, H.; Zhu, L.; Ambalavanan, N.; Girkin, C.A.; Murphy-Ullrich, J.E.; Downs, J.C.; Zhou, Y. High-Magnitude and/or High-Frequency Mechanical Strain Promotes Peripapillary Scleral Myofibroblast Differentiation. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7821–7830. [Google Scholar] [CrossRef] [Green Version]
- Lutjen-Drecoll, E.; Tamm, E.; Kaufman, P.L. Age-related loss of morphologic responses to pilocarpine in rhesus monkey ciliary muscle. Arch. Ophthalmol. 1988, 106, 1591–1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnstone, M.; Martin, E.; Jamil, A. Pulsatile flow into the aqueous veins: Manifestations in normal and glaucomatous eyes. Exp. Eye Res. 2011, 92, 318–327. [Google Scholar] [CrossRef] [Green Version]
- Bradley, J.M.; Vranka, J.; Colvis, C.M.; Conger, D.M.; Alexander, J.P.; Fisk, A.S.; Samples, J.R.; Acott, T.S. Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2649–2658. [Google Scholar]
- Acott, T.S.; Kelley, M.J.; Keller, K.E.; Vranka, J.A.; Abu-Hassan, D.W.; Li, X.; Aga, M.; Bradley, J.M. Intraocular pressure homeostasis: Maintaining balance in a high-pressure environment. J. Ocul. Pharm. Ther. 2014, 30, 94–101. [Google Scholar] [CrossRef] [PubMed]



| Human Eye | Monkey Eye | |||||||
|---|---|---|---|---|---|---|---|---|
| MP (mmHg) | PA (2.5) | PA (5) | PA (7.5) | PA (10) | PA (2.5) | PA (5) | PA (7.5) | PA (10) |
| 16–24 | 0.975 | 0.005 ** | 0.868 | 0.492 | 0.258 | 0.978 | 0.109 | 0.074 |
| 16–32 | 0.153 | 0.145 | 0.036 * | 0.005 ** | 0.008 ** | 0.007 ** | 0.054 | 0.011 ** |
| 16–40 | 0.002 ** | <0.001 ** | 0.01 * | <0.001 ** | 0.003 ** | 0.004 ** | 0.001 ** | 0.002 ** |
| 24–32 | 0.284 | 0.272 | 0.009 ** | <0.001 ** | 0.222 | 0.014 * | 0.975 | 0.712 |
| 24–40 | 0.004 ** | 0.02 * | 0.003 ** | <0.001 ** | 0.078 | 0.008 ** | 0.076 | 0.167 |
| 32–40 | 0.104 | <0.001 ** | 0.891 | 0.051 | 0.913 | 0.991 | 0.151 | 0.655 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, C.; Wang, X.; Wang, N.; Wang, R.; Johnstone, M. Trabecular Meshwork Motion Profile from Pulsatile Pressure Transients: A New Platform to Simulate Transitory Responses in Humans and Nonhuman Primates. Appl. Sci. 2022, 12, 11. https://doi.org/10.3390/app12010011
Xin C, Wang X, Wang N, Wang R, Johnstone M. Trabecular Meshwork Motion Profile from Pulsatile Pressure Transients: A New Platform to Simulate Transitory Responses in Humans and Nonhuman Primates. Applied Sciences. 2022; 12(1):11. https://doi.org/10.3390/app12010011
Chicago/Turabian StyleXin, Chen, Xiaofei Wang, Ningli Wang, Ruikang Wang, and Murray Johnstone. 2022. "Trabecular Meshwork Motion Profile from Pulsatile Pressure Transients: A New Platform to Simulate Transitory Responses in Humans and Nonhuman Primates" Applied Sciences 12, no. 1: 11. https://doi.org/10.3390/app12010011







