Lack of Adverse Effects of Cold Physical Plasma-Treated Blood from Leukemia Patients: A Proof-of-Concept Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Controls
2.2. Cold Plasma Jet Device and Sample Treatment
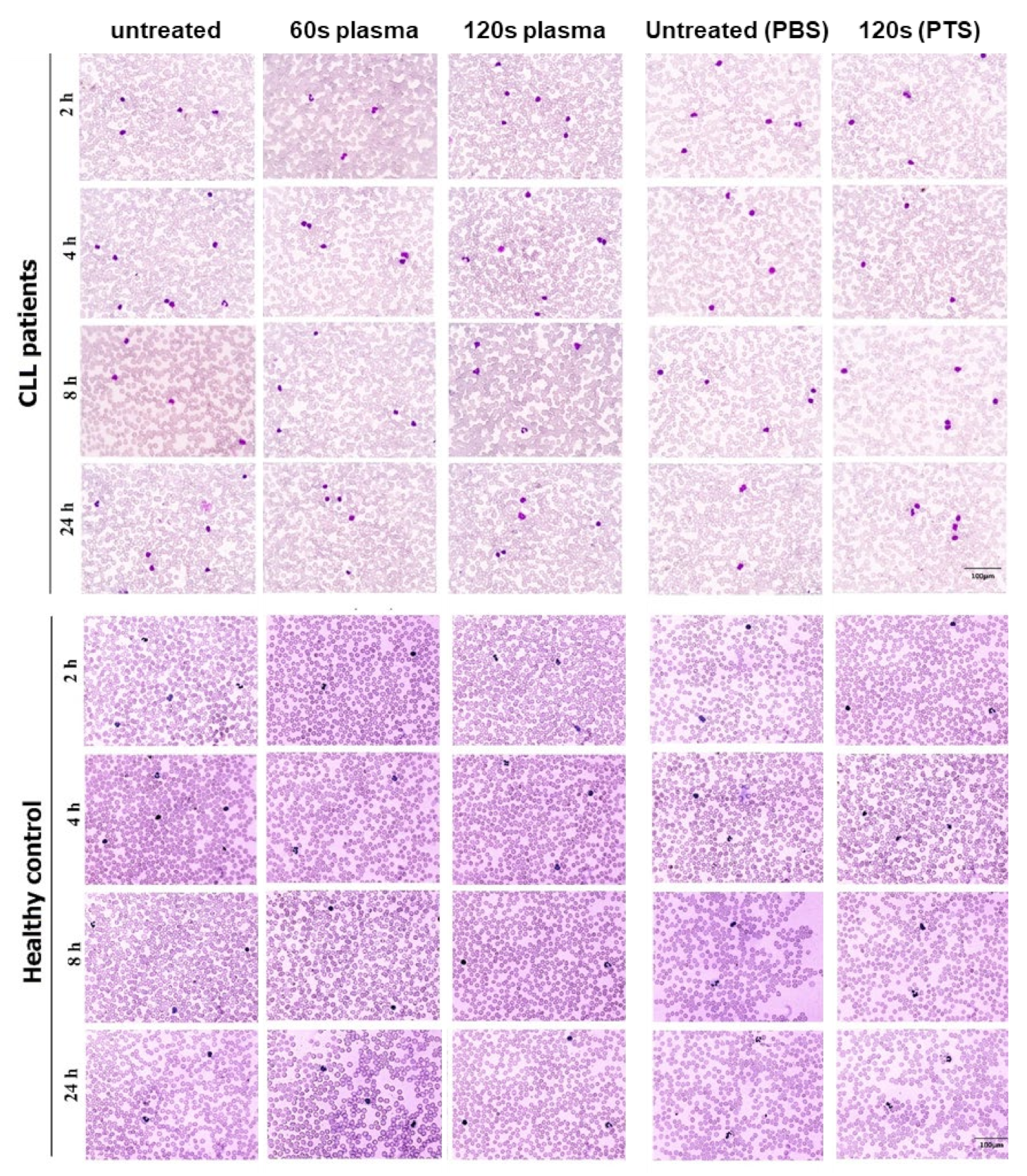
2.3. Complete Blood Cell Count (CBC) and Imaging of Peripheral Blood Smear
2.4. RNS and Lipid Peroxidation in Blood Plasma Treated with Cold Plasma or PTS
2.5. MTT Assay for Cytotoxicity of Cold Plasma and PTS Treatment
2.6. Prothrombin Time (PT) and Partial Thromboplastin Time (PTT) Measurements
2.7. Quantification of Hemolysis
2.8. Statistical Analysis
3. Results
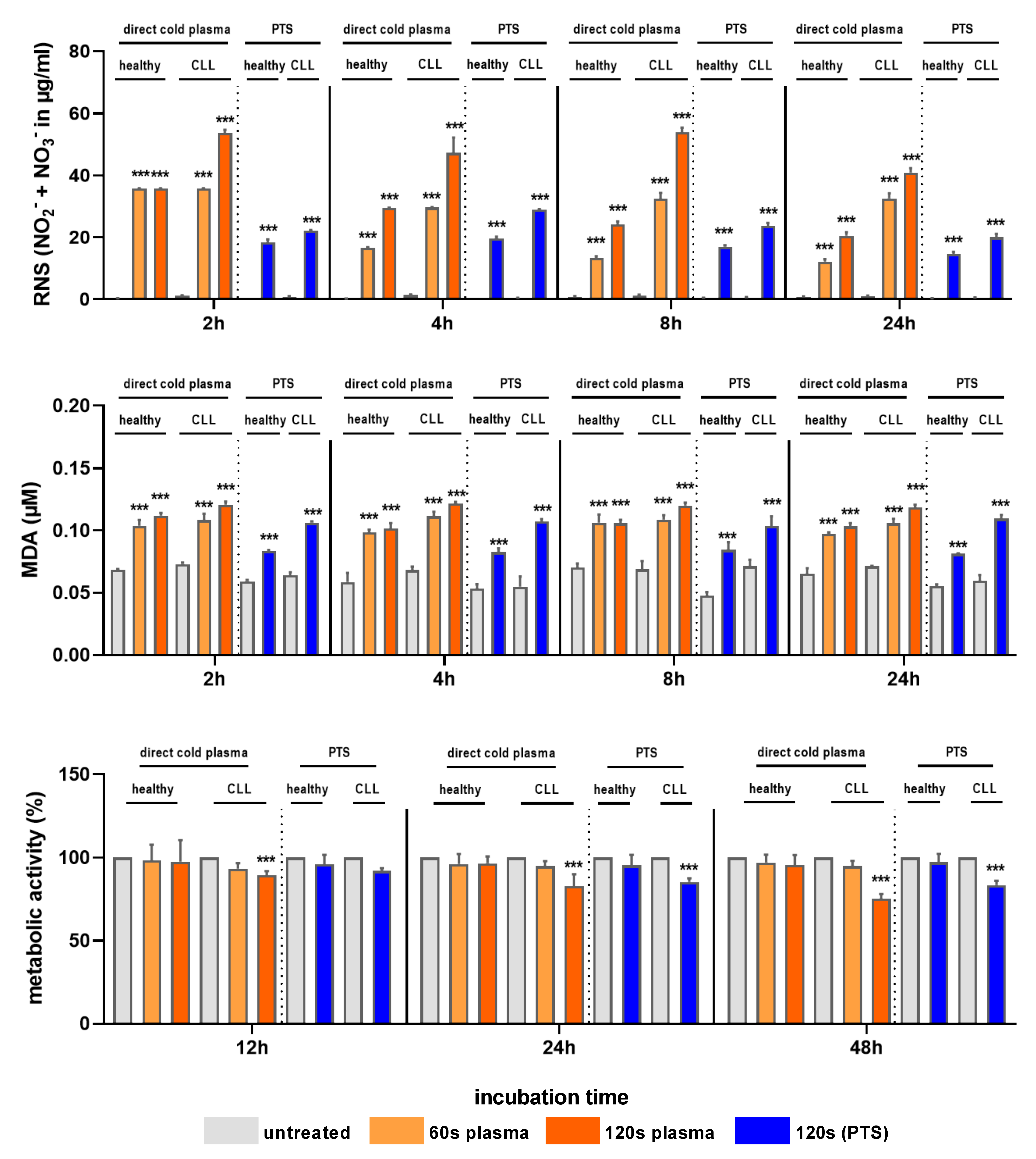
3.1. Cold Plasma and PTS Increased RNS Levels, Lipid Peroxidation, and Cytotoxicity
3.2. Cold Plasma and PTS Treatment Did Not Affect the PT, PTT, or Hemolysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amar, A. Chronic lymphocytic leukemia and second primary malignancies: A relationship revisited. Indian J. Med. Paediatr. Oncol. 2020, 41, 787–788. [Google Scholar] [CrossRef]
- Nabhan, C.; Rosen, S.T. Chronic lymphocytic leukemia: A clinical review. JAMA 2014, 312, 2265–2276. [Google Scholar] [CrossRef] [Green Version]
- Rezazadeh, H.; Astaneh, M.; Tehrani, M.; Hossein-Nataj, H.; Zaboli, E.; Shekarriz, R.; Asgarian-Omran, H. Blockade of PD-1 and TIM-3 immune checkpoints fails to restore the function of exhausted CD8+ T cells in early clinical stages of chronic lymphocytic leukemia. Immunol. Res. 2020, 68, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Hatton, M.P.; Rubin, P.A. Chronic lymphocytic leukemia of the orbit. Arch. Ophthalmol. 2002, 120, 990–991. [Google Scholar] [PubMed]
- Weniger, M.A.; Rizzatti, E.G.; Pérez-Galán, P.; Liu, D.; Wang, Q.; Munson, P.J.; Raghavachari, N.; White, T.; Tweito, M.M.; Dunleavy, K. Treatment-induced oxidative stress and cellular antioxidant capacity determine response to bortezomib in mantle cell lymphoma. Clin. Cancer Res. 2011, 17, 5101–5112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goel, A.; Spitz, D.R.; Weiner, G.J. Manipulation of cellular redox parameters for improving therapeutic responses in B-cell lymphoma and multiple myeloma. J. Cell. Biochem. 2012, 113, 419–425. [Google Scholar] [CrossRef] [Green Version]
- Yosifov, D.Y.; Idler, I.; Bhattacharya, N.; Reichenzeller, M.; Close, V.; Ezerina, D.; Scheffold, A.; Jebaraj, B.M.C.; Kugler, S.; Bloehdorn, J. Oxidative stress as candidate therapeutic target to overcome microenvironmental protection of CLL. Leukemia 2020, 34, 115–127. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathi, A.K.; Tripathi, P.; Singh, R.; Singh, S.; Singh, R.K. Oxidative stress and antioxidant status in patients with chronic myeloid leukemia. Indian J. Clin. Biochem. 2008, 23, 328–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kay, N.E. ROS: Double-edged sword for leukemic cells. Blood 2006, 107, 2212–2213. [Google Scholar] [CrossRef]
- Samimi, A.; Kalantari, H.; Lorestani, M.Z.; Shirzad, R.; Saki, N. Oxidative stress in normal hematopoietic stem cells and leukemia. Apmis 2018, 126, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Privat-Maldonado, A.; Schmidt, A.; Lin, A.; Weltmann, K.D.; Wende, K.; Bogaerts, A.; Bekeschus, S. ROS from Physical Plasmas: Redox Chemistry for Biomedical Therapy. Oxid. Med. Cell. Longev. 2019, 2019, 9062098. [Google Scholar] [CrossRef] [Green Version]
- Bekeschus, S.; von Woedtke, T.; Emmert, S.; Schmidt, A. Medical gas plasma-stimulated wound healing: Evidence and mechanisms. Redox Biol. 2021, 46, 102116. [Google Scholar] [CrossRef]
- Dai, X.; Bazaka, K.; Richard, D.J.; Thompson, E.R.W.; Ostrikov, K.K. The Emerging Role of Gas Plasma in Oncotherapy. Trends Biotechnol. 2018, 36, 1183–1198. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.J.; Kim, K.I.; Hoan, N.N.; Kim, C.H.; Moon, E.; Choi, K.S.; Yang, S.S.; Lee, J.-S. Targeting cancer cells with reactive oxygen and nitrogen species generated by atmospheric-pressure air plasma. PLoS ONE 2014, 9, e86173. [Google Scholar]
- Ma, Y.; Ha, C.S.; Hwang, S.W.; Lee, H.J.; Kim, G.C.; Lee, K.-W.; Song, K. Non-thermal atmospheric pressure plasma preferentially induces apoptosis in p53-mutated cancer cells by activating ROS stress-response pathways. PLoS ONE 2014, 9, e91947. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.-H. Mechanisms of a novel anticancer therapeutic strategy involving atmospheric pressure plasma-mediated apoptosis and DNA strand break formation. Arch. Pharmacal Res. 2016, 39, 1–9. [Google Scholar] [CrossRef]
- Conway, G.E.; He, Z.; Hutanu, A.L.; Cribaro, G.P.; Manaloto, E.; Casey, A.; Traynor, D.; Milosavljevic, V.; Howe, O.; Barcia, C.; et al. Cold Atmospheric Plasma induces accumulation of lysosomes and caspase-independent cell death in U373MG glioblastoma multiforme cells. Sci. Rep. 2019, 9, 12891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, H.; Bekeschus, S.; Yan, D.; Hori, M.; Keidar, M.; Laroussi, M. Plasma-Treated Solutions (PTS) in Cancer Therapy. Cancers 2021, 13, 1737. [Google Scholar] [CrossRef]
- Bauer, G. The synergistic effect between hydrogen peroxide and nitrite, two long-lived molecular species from cold atmospheric plasma, triggers tumor cells to induce their own cell death. Redox Biol. 2019, 26, 101291. [Google Scholar] [CrossRef] [PubMed]
- Alimohammadi, M.; Golpour, M.; Sohbatzadeh, F.; Hadavi, S.; Bekeschus, S.; Niaki, H.A.; Valadan, R.; Rafiei, A. Cold atmospheric plasma is a potent tool to improve chemotherapy in melanoma in vitro and in vivo. Biomolecules 2020, 10, 1011. [Google Scholar] [CrossRef]
- Rafiei, A.; Sohbatzadeh, F.; Hadavi, S.; Bekeschus, S.; Alimohammadi, M.; Valadan, R. Inhibition of murine melanoma tumor growth in vitro and in vivo using an argon-based plasma jet. Clin. Plasma Med. 2020, 19, 100102. [Google Scholar] [CrossRef]
- Yazdani, Z.; Mehrabanjoubani, P.; Rafiei, A.; Biparva, P.; Kardan, M. Combined Effect of Cold Atmospheric Plasma and Curcumin in Melanoma Cancer. BioMed Res. Int. 2021, 2021, 1969863. [Google Scholar] [CrossRef]
- Gjika, E.; Pal-Ghosh, S.; Kirschner, M.E.; Lin, L.; Sherman, J.H.; Stepp, M.A.; Keidar, M. Combination therapy of cold atmospheric plasma (CAP) with temozolomide in the treatment of U87MG glioblastoma cells. Sci. Rep. 2020, 10, 1969863. [Google Scholar] [CrossRef] [PubMed]
- Mahdikia, H.; Saadati, F.; Freund, E.; Gaipl, U.S.; Majidzadeh-a, K.; Shokri, B.; Bekeschus, S. Gas plasma irradiation of breast cancers promotes immunogenicity, tumor reduction, and an abscopal effect in vivo. Oncoimmunology 2021, 10, 1859731. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, Z.; Mehrabanjoubani, P.; Biparva, P.; Rafiei, A. Cytotoxicity Effect of Cold Atmospheric Plasma on Melanoma (B16-F10), Breast (MCF-7) and Lung (A549) Cancer Cell Lines Compared with Normal Cells. J. Maz. Univ. Med. Sci. 2020, 30, 38–48. [Google Scholar]
- Takeda, S.; Yamada, S.; Hattori, N.; Nakamura, K.; Tanaka, H.; Kajiyama, H.; Kanda, M.; Kobayashi, D.; Tanaka, C.; Fujii, T. Intraperitoneal administration of plasma-activated medium: Proposal of a novel treatment option for peritoneal metastasis from gastric cancer. Ann. Surg. Oncol. 2017, 24, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Gümbel, D.; Daeschlein, G.; Ekkernkamp, A.; Kramer, A.; Stope, M.B. Cold atmospheric plasma in orthopaedic and urologic tumor therapy. GMS Hyg. Infect. Control. 2017, 12, 1–10. [Google Scholar] [CrossRef]
- Canady, J.; Gordon, S.; Zhuang, T.; Wigh, S.; Rowe, W.; Shashurin, A.; Chiu, D.; Jones, S.; Wiley, K.; Cohen, E. Cold Atmospheric Plasma (CAP) Combined with Chemo-Radiation and Cytoreductive Surgery: The First Clinical Experience for Stage IV Metastatic Colon Cancer. In Comprehensive Clinical Plasma Medicine; Springer: Berlin/Heidelberg, Germany, 2018; pp. 163–183. [Google Scholar]
- Metelmann, H.-R.; Seebauer, C.; Miller, V.; Fridman, A.; Bauer, G.; Graves, D.B.; Pouvesle, J.-M.; Rutkowski, R.; Schuster, M.; Bekeschus, S.; et al. Clinical experience with cold plasma in the treatment of locally advanced head and neck cancer. Clin. Plas. Med. 2018, 9, 6–13. [Google Scholar] [CrossRef]
- Witzke, K.; Seebauer, C.; Jesse, K.; Kwiatek, E.; Berner, J.; Semmler, M.L.; Boeckmann, L.; Emmert, S.; Weltmann, K.D.; Metelmann, H.R.; et al. Plasma medical oncology: Immunological interpretation of head and neck squamous cell carcinoma. Plasma Process. Polym. 2020, 17, e1900258. [Google Scholar] [CrossRef]
- Jaffe, E.S. The 2008 WHO classification of lymphomas: Implications for clinical practice and translational research. ASH Educ. Program Book 2009, 2009, 523–531. [Google Scholar]
- Rostami, S.; Fathollahpour, A.; Abdi, M.; Naderi, K. Alteration in Prooxidant-antioxidant Balance Associated with Selenium Concentration in Patients with Congenital Hypothyroidism. J. Med. Biochem. 2018, 37, 355–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Zhang, X.; Broderick, M.; Fein, H. Measurement of nitric oxide production in biological systems by using Griess reaction assay. Sensors 2003, 3, 276–284. [Google Scholar] [CrossRef] [Green Version]
- Ratovitski, E.A.; Cheng, X.; Yan, D.; Sherman, J.H.; Canady, J.; Trink, B.; Keidar, M. Anti-cancer therapies of 21st century: Novel approach to treat human cancers using cold atmospheric plasma. Plasma Process. Polym. 2014, 11, 1128–1137. [Google Scholar] [CrossRef]
- Yan, D.; Talbot, A.; Nourmohammadi, N.; Cheng, X.; Canady, J.; Sherman, J.; Keidar, M. Principles of using cold atmospheric plasma stimulated media for cancer treatment. Sci. Rep. 2015, 5, 18339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salunkhe, S.; Mishra, S.V.; Ghorai, A.; Hole, A.; Chandrani, P.; Dutt, A.; Chilakapati, M.; Dutt, S. Metabolic rewiring in drug resistant cells exhibit higher OXPHOS and fatty acids as preferred major source to cellular energetics. Biochim. Biophys. Acta (BBA)-Bioenerg. 2020, 1861, 148300. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Turrini, E.; Laurita, R.; Simoncelli, E.; Stancampiano, A.; Catanzaro, E.; Calcabrini, C.; Carulli, G.; Rousseau, M.; Gherardi, M.; Maffei, F. Plasma-activated medium as an innovative anticancer strategy: Insight into its cellular and molecular impact on in vitro leukemia cells. Plasma Process. Polym. 2020, 17, 2000007. [Google Scholar] [CrossRef]
- Jezeh, M.A.; Tayebi, T.; Khani, M.R.; Niknejad, H.; Shokri, B. Direct cold atmospheric plasma and plasma-activated medium effects on breast and cervix cancer cells. Plasma Process. Polym. 2020, 17, 1900241. [Google Scholar] [CrossRef]
- Rozman, C.; Montserrat, E. Chronic lymphocytic leukemia. N. Engl. J. Med. 1995, 333, 1052–1057. [Google Scholar] [CrossRef] [Green Version]
- Jawaid, P.; Rehman, M.U.; Zhao, Q.L.; Takeda, K.; Ishikawa, K.; Hori, M.; Shimizu, T.; Kondo, T. Helium-based cold atmospheric plasma-induced reactive oxygen species-mediated apoptotic pathway attenuated by platinum nanoparticles. J. Cell. Mol. Med. 2016, 20, 1737–1748. [Google Scholar] [CrossRef]
- Wolff, C.M.; Kolb, J.F.; Weltmann, K.-D.; von Woedtke, T.; Bekeschus, S. Combination treatment with cold physical plasma and pulsed electric fields augments ROS production and cytotoxicity in lymphoma. Cancers 2020, 12, 845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turrini, E.; Laurita, R.; Stancampiano, A.; Catanzaro, E.; Calcabrini, C.; Maffei, F.; Gherardi, M.; Colombo, V.; Fimognari, C. Cold Atmospheric Plasma Induces Apoptosis and Oxidative Stress Pathway Regulation in T-Lymphoblastoid Leukemia Cells. Oxidative Med. Cell. Longev. 2017, 2017, 4271065. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Schmidt, A.; Kramer, A.; Metelmann, H.R.; Adler, F.; von Woedtke, T.; Niessner, F.; Weltmann, K.D.; Wende, K. High throughput image cytometry micronucleus assay to investigate the presence or absence of mutagenic effects of cold physical plasma. Environ. Mol. Mutagen. 2018, 59, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Ning, N.; Xu, Y.; Wang, B.; Cui, Q.; Liu, Z.; Wang, X.; Liu, D.; Chen, H.; Kong, M.G. Effect of cold atmospheric plasma treatment on the metabolites of human leukemia cells. Cancer Cell Int. 2019, 19, 135. [Google Scholar] [CrossRef]
- Xu, D.; Ning, N.; Xu, Y.; Xia, W.; Liu, D.; Chen, H.; Kong, M.G. Effect of He Plasma Jet Versus Surface Plasma on the Metabolites of Acute Myeloid Leukemia Cells. Front. Oncol. 2021, 11, 552480. [Google Scholar] [CrossRef]
- Maikho, T.; Patwardhan, R.S.; Das, T.N.; Sharma, D.; Sandur, S.K. Cold atmospheric plasma-modulated phorbol 12-myristate 13-acetate-induced differentiation of U937 cells to macrophage-like cells. Free Radic. Res. 2018, 52, 212–222. [Google Scholar] [CrossRef]
- Tabuchi, Y.; Uchiyama, H.; Zhao, Q.-L.; Yunoki, T.; Andocs, G.; Nojima, N.; Takeda, K.; Ishikawa, K.; Hori, M.; Kondo, T. Effects of nitrogen on the apoptosis of and changes in gene expression in human lymphoma U937 cells exposed to argon-based cold atmospheric pressure plasma. Int. J. Mol. Med. 2016, 37, 1706–1714. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.W.; Kim, H.; Kim, H.W.; Yun, S.H.; Park, J.E.; Choi, E.H.; Kim, S.J. Genome-wide comparison of the target genes of the reactive oxygen species and non-reactive oxygen species constituents of cold atmospheric plasma in cancer cells. Cancers 2020, 12, 2640. [Google Scholar] [CrossRef]
- Moniruzzaman, R.; Rehman, M.U.; Zhao, Q.-L.; Jawaid, P.; Takeda, K.; Ishikawa, K.; Hori, M.; Tomihara, K.; Noguchi, K.; Kondo, T. Cold atmospheric helium plasma causes synergistic enhancement in cell death with hyperthermia and an additive enhancement with radiation. Sci. Rep. 2017, 7, 11659. [Google Scholar] [CrossRef] [PubMed]
- Bundscherer, L.; Bekeschus, S.; Tresp, H.; Hasse, S.; Reuter, S.; Weltmann, K.-D.; Lindequist, U.; Masur, K. Viability of Human Blood Leukocytes Compared with Their Respective Cell Lines after Plasma Treatment. Plasma Med. 2013, 3, 71–80. [Google Scholar] [CrossRef]
- Clemen, R.; Freund, E.; Mrochen, D.; Miebach, L.; Schmidt, A.; Rauch, B.H.; Lackmann, J.W.; Martens, U.; Wende, K.; Lalk, M.; et al. Gas Plasma Technology Augments Ovalbumin Immunogenicity and OT-II T Cell Activation Conferring Tumor Protection in Mice. Adv. Sci. 2021, 8, 2003395. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Kolata, J.; Winterbourn, C.; Kramer, A.; Turner, R.; Weltmann, K.D.; Broker, B.; Masur, K. Hydrogen peroxide: A central player in physical plasma-induced oxidative stress in human blood cells. Free Radic. Res. 2014, 48, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Freund, E.; Bekeschus, S. Gas Plasma-Oxidized Liquids for Cancer Treatment: Preclinical Relevance, Immuno-Oncology, and Clinical Obstacles. IEEE Trans. Radiat. Plasma Med. Sci. 2021, 5, 761–774. [Google Scholar] [CrossRef]



| Sex | Age | WBC (103/mm3) | RBC (106/mm3) | Hb (g/DL) | HCT (%) | MCHC (g/DL) | RDW (FL) |
PLT (103/mm3) | CD19 (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 63 | 6.6 | 5.1 | 14.2 | 42.2 | 33.6 | 12.3 | 126 | NA |
| 2 | M | 55 | 10.1 | 4.0 | 8.8 | 39.3 | 34.8 | 13.7 | 139 | 65 |
| 3 | M | 71 | 17.8 | 5.1 | 12.1 | 40.3 | 33.2 | 13.2 | 111 | 71 |
| 4 | M | 90 | 41.8 | 3.0 | 13.1 | 32.3 | 33.4 | 12.6 | 157 | 91 |
| 5 | M | 48 | 16.0 | 4.3 | 13.2 | 37.7 | 35.0 | 12.8 | 254 | 80 |
| 6 | F | 72 | 51.9 | 5.3 | 10.6 | 35.6 | 29.8 | 17.5 | 287 | 68 |
| 7 | F | 56 | 19.4 | 4.7 | 13.4 | 39.9 | 33.6 | 13.3 | 227 | 65 |
| 8 | F | 52 | 107.2 | 3.1 | 12.3 | 37.8 | 34.9 | 13.0 | 93 | NA |
| 9 | M | 64 | 70.7 | 3.6 | 11.0 | 31.1 | 35.4 | 13.8 | 127 | 78 |
| Ref | - | - | 8.3 | 4.6 | 12.3 | 37.4 | 32.5 | 13.3 | 334 | (14) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golpour, M.; Alimohammadi, M.; Mohseni, A.; Zaboli, E.; Sohbatzadeh, F.; Bekeschus, S.; Rafiei, A. Lack of Adverse Effects of Cold Physical Plasma-Treated Blood from Leukemia Patients: A Proof-of-Concept Study. Appl. Sci. 2022, 12, 128. https://doi.org/10.3390/app12010128
Golpour M, Alimohammadi M, Mohseni A, Zaboli E, Sohbatzadeh F, Bekeschus S, Rafiei A. Lack of Adverse Effects of Cold Physical Plasma-Treated Blood from Leukemia Patients: A Proof-of-Concept Study. Applied Sciences. 2022; 12(1):128. https://doi.org/10.3390/app12010128
Chicago/Turabian StyleGolpour, Monireh, Mina Alimohammadi, Alireza Mohseni, Ehsan Zaboli, Farshad Sohbatzadeh, Sander Bekeschus, and Alireza Rafiei. 2022. "Lack of Adverse Effects of Cold Physical Plasma-Treated Blood from Leukemia Patients: A Proof-of-Concept Study" Applied Sciences 12, no. 1: 128. https://doi.org/10.3390/app12010128
APA StyleGolpour, M., Alimohammadi, M., Mohseni, A., Zaboli, E., Sohbatzadeh, F., Bekeschus, S., & Rafiei, A. (2022). Lack of Adverse Effects of Cold Physical Plasma-Treated Blood from Leukemia Patients: A Proof-of-Concept Study. Applied Sciences, 12(1), 128. https://doi.org/10.3390/app12010128







