Investigation of Volatile Compounds in Combination with Multivariate Analysis for the Characterization of Monofloral Honeys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Honey Extraction and Isolation of the Components
2.3. Gas Chromatography–Mass Spectrometry (GC–MS) Conditions
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Radovic, B.; Careri, M.; Mangia, A.; Musci, M.; Gerboles, M.; Anklam, E. Contribution of dynamic headspace GC-MS analysis of aroma compounds to authenticity testing of honey. Food Chem. 2001, 72, 511–520. [Google Scholar] [CrossRef]
- Serra-Bonvehi, J.; Coll, F.V. Flavour index and aroma profiles of fresh and processed honeys. J. Sci. Food Agric. 2003, 83, 275–282. [Google Scholar] [CrossRef]
- Castro-Vazquez, L.; Leon-Ruiz, V.; Alañon, M.E.; Pérez-Coello, M.S.; González-Porto, A.V. Floral origin markers for authenticating Lavandin honey (Lavandula angustifolia x latifolia). Discrimination from Lavender honey (Lavandula latifolia). Food Control 2014, 37, 362–370. [Google Scholar] [CrossRef]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Glory, L.F.; Pino, J.A.; Santiago, L.S.; Sauri-Duch, E. A review of volatile analytical methods for determining the botanical origin of honey. Food Chem. 2007, 103, 1032–1043. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Díaz-Maroto, M.; González-Viñas, M.; Pérez-Coello, M. Differentiation of monofloral citrus, rosemary, eucalyptus, lavender, thyme and heather honeys based on volatile composition and sensory descriptive analysis. Food Chem. 2009, 112, 1022–1030. [Google Scholar] [CrossRef]
- Jerković, I.; Tuberoso, C.; Marijanović, Z.; Jelić, M.; Kasum, A. Headspace, volatile and semi-volatile patterns of Paliurus spina-christi unifloral honey as markers of botanical origin. Food Chem. 2009, 112, 239–245. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Venskutonis, P.R. Floral Markers in Honey of Various Botanical and Geographic Origins: A Review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 620–634. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Ndip, R.N.; Clarke, A.M. Volatile Compounds in Honey: A Review on Their Involvement in Aroma, Botanical Origin Determination and Potential Biomedical Activities. Int. J. Mol. Sci. 2011, 12, 9514–9532. [Google Scholar] [CrossRef] [Green Version]
- Soria, A.C.; Martínez-Castro, I.; Sanz, J. Study of the precision in the purge-and-trap–gas chromatography–mass spectrometry analysis of volatile compounds in honey. J. Chromatogr. A 2009, 1216, 3300–3304. [Google Scholar] [CrossRef] [Green Version]
- Dimou, M.; Katsaros, J.; Klonari, K.T.; Thrasyvoulou, A. Discriminating pine and fir honeydew honeys by microscopic characteristics. J. Apic. Res. 2006, 45, 16–21. [Google Scholar] [CrossRef]
- El-Sofany, A.; Al Naggar, Y.; Naiem, E.; Giesy, J.P.; Seif, A. Authentication of the botanical and geographic origin of Egyptian honey using pollen analysis methods. J. Apic. Res. 2020, 59, 946–955. [Google Scholar] [CrossRef]
- Bogdanov, S.; Ruoff, K.; Oddo, L.P. Physico-chemical methods for the characterisation of unifloral honeys: A review. Apidologie 2004, 35, S4–S17. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Miret, M.L.; Terrab, A.; Hernanz, D.; Fernandez-Recamales, M.A.; Heredia, F.J. Multivariate correlation between colour and mineral composition of honeys and by their botanical origin. J. Agric. Food Chem. 2005, 53, 2574–2580. [Google Scholar] [CrossRef]
- Kowalski, S.; Lukasiewicz, M.; Berski, W. Applicability of physico-chemical parameters of honey for identification of the botanical origin. Acta Sci. Pol. Technol. Aliment. 2014, 12, 51–59. [Google Scholar]
- Adgaba, N.; Al-Ghamdi, A.A.; Getachew, A.; Tadesse, Y.; Belay, A.; Ansari, M.J.; Radloff, S.E.; Sharma, D. Characterization of honeys by their botanical and geographical origins based on physico-chemical properties and chemo-metrics analysis. J. Food Meas. Charact. 2017, 11, 1106–1117. [Google Scholar] [CrossRef]
- Marcazzan, G.L.; Mucignat-Caretta, C.; Marchese, C.M.; Piana, M.L. A review of methods for honey sensory analysis. J. Apic. Res. 2017, 57, 75–87. [Google Scholar] [CrossRef]
- Perna, A.M.; Intaglietta, I.; Simonetti, A.; Gambacorta, E. A comparative study on phenolic profile, vitamin C content and antioxidant activity of Italian honeys of different botanical origin. Int. J. Food Sci. Technol. 2013, 48, 1899–1908. [Google Scholar] [CrossRef]
- Oroian, M.; Sorina, R. Honey authentication based on physicochemical parameters and phenolic compounds. Comput. Electron. Agric. 2017, 138, 148–156. [Google Scholar] [CrossRef]
- Chua, L.S.; Lee, S.C.; Chan, G.F. Characterization of the Proteins in Honey. Anal. Lett. 2015, 48, 697–709. [Google Scholar] [CrossRef]
- Yao, L.; Singanusong, R.; Data, N.; Raymont, K. Phenolics acids and abscidic acid in Australian Eucalyptus honeys and their potential for the floral authentication. Food Chem. 2004, 86, 169–177. [Google Scholar] [CrossRef]
- Del Nozal, M.; Bernal, J.; Diego, J.; Gómez, L.; Ruiz, J.; Higes, M. Determination of oxalate, sulfate and nitrate in honey and honeydew by ion-chromatography. J. Chromatogr. A 2000, 881, 629–638. [Google Scholar] [CrossRef]
- Anklam, E. A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chem. 1998, 63, 549–562. [Google Scholar] [CrossRef]
- Amtmann, M. The chemical relationship between the scent features of goldenrod (Solidago canadensis L.) flower and its unifloral honey. J. Food Compos. Anal. 2010, 23, 122–129. [Google Scholar] [CrossRef]
- Overton, S.; Manura, J.J. Flavor and aroma in commercial bee honey. A purge-and-trap thermal desorption technique for the identification and quantification of volatiles and semivolatiles in honey. Am. Lab. 1994, 56, 45–53. [Google Scholar]
- Ferreres, F.; Giner, J.M.; Tómas-Barberán, F.A. A comparative study of hesperetin and methyl anthranilate as markers of the floral origin of citrus honey. J. Sci. Food Agric. 1994, 65, 371–372. [Google Scholar] [CrossRef]
- Alissandrakis, E.; Tarantilis, P.A.; Harizanis, P.C.; Polissiou, M. Evaluation of four isolation techniques for honey aroma compounds. J. Food Sci. Agric. 2005, 85, 91. [Google Scholar] [CrossRef]
- Piasenzotto, L.; Gracco, L.; Conte, L. Solid phase microextraction (SPME) applied to honey quality control. J. Sci. Food Agric. 2003, 83, 1037–1044. [Google Scholar] [CrossRef]
- Soria, A.C.; Martínez-Castro, I.; Sanz, J. Analysis of volatile composition of honey by solid phase microextraction and gas chromatography-mass spectrometry. J. Sep. Sci. 2003, 26, 793–801. [Google Scholar] [CrossRef]
- Bianchi, F.; Careri, M.; Musci, M. Volatile norisoprenoids as markers of botanical origin of Sardinian strawberry-tree (Arbutus unedo L.) honey: Characterisation of aroma compounds by dynamic headspace extraction and gas chromatography–mass spectrometry. Food Chem. 2005, 89, 527–532. [Google Scholar] [CrossRef]
- Guyot, C.; Scheirman, V.; Collin, S. Floral origin markers of heather honeys: Calluna vulgaris and Erica arborea. Food Chem. 1999, 64, 3–11. [Google Scholar] [CrossRef]
- Tananaki, C.; Thrasyvoulou, A.; Giraudel, J.; Montury, M. Determination of volatile characteristics of Greek and Turkish pine honey samples and their classification by using Kohonen self organising maps. Food Chem. 2007, 101, 1687–1693. [Google Scholar] [CrossRef]
- Ampuero, S.; Bogdanov, S.; Bosset, J.-O. Classification of unifloral honeys with an MS-based electronic nose using different sampling modes: SHS, SPME and INDEX. Eur. Food Res. Technol. 2004, 218, 198–207. [Google Scholar] [CrossRef]
- Lammertyn, J.; Veraverbeke, E.A.; Irudayaraj, J. zNose™ technology for the classification of honey based on rapid aroma profiling. Sens. Actuators B Chem. 2004, 98, 54–62. [Google Scholar] [CrossRef]
- Wang, X.; Yang, S.; He, J.; Chen, L.; Zhang, J.; Jin, Y.; Zhou, J.; Zhang, Y. A green triple-locked strategy based on volatile-compound imaging, chemometrics, and markers to discriminate winter honey and sapium honey using headspace gas chromatography-ion mobility spectrometry. Food Res. Int. 2019, 119, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Bouseta, A.; Collin, S. Optimized Likens-Nickerson Methodology for Quantifying Honey Flavors. J. Agric. Food Chem. 1995, 43, 1890–1897. [Google Scholar] [CrossRef]
- Bentivenga, M.; Coltorti, M.; Prosser, G.; Tavarnelli, E. A new interpretation of terraces in the Taranto Gulf: The role of extensional faulting. Geomorphology 2004, 60, 383–402. [Google Scholar] [CrossRef]
- Panseri, S.; Manzo, A.; Chiesa, L.M.; Giorgi, A. Melissopalynological and Volatile Compounds Analysis of Buckwheat Honey from Different Geographical Origins and Their Role in Botanical Determination. J. Chem. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Da Silva, P.M.; Gonzaga, L.V.; de Azevedo, M.S.; Biluca, F.C.; Schulz, M.; Costa, A.C.O.; Fett, R. Stability of volatile compounds of honey during prolonged storage. J. Food Sci. Technol. 2020, 57, 1167–1182. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Badeka, A.; Kontominas, M.G. A decisive strategy for monofloral honey authentication using analysis of volatile compounds and pattern recognition techniques. Microchem. J. 2020, 152, 104263. [Google Scholar] [CrossRef]
- Tananaki, C. The Study of Factors Affective the Volatile Compounds from Honeydew Honeys. Ph.D. Thesis, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2006. [Google Scholar]
- Cavalli, J.-F.; Fernandez, X.; Lizzani-Cuvelier, L.; Loiseau, A.-M. Comparison of Static Headspace, Headspace Solid Phase Microextraction, Headspace Sorptive Extraction, and Direct Thermal Desorption Techniques on Chemical Composition of French Olive Oils. J. Agric. Food Chem. 2003, 51, 7709–7716. [Google Scholar] [CrossRef]
- LECO Corporation. Rapid Qualitative GC/TOFMS Analysis of Unleaded Gasoline. 2003. Available online: https://gcms.cz/labrulez-bucket-strapi-h3hsga3/b6020741dd614849b6fdb53086c76608/PEG_UNLEADED-GASOLINE_203-821-064.pdf (accessed on 20 December 2021).
- Von Kovαts, E. Gas-chromatographische Charakterisierung organischer Verbindungen. Teil 1: Retentionsindices aliphatischer Halogenide, Alkohole, Aldehyde und Ketone. Helv. Chim. Acta 1958, 41, 1915–1932. [Google Scholar] [CrossRef]
- Kim, J.S. Einfluss der Temperatur Beim Rφsten von Sesam Auf Aroma und Antioxidative Eigenschaften des Fls. Ph.D. Thesis, Technischen Universitδt Berlin zur Erlangung des Akademischen Grades, Berlin, Germany, 2001. [Google Scholar]
- Censullo, A.C.; Jones, D.R.; Wills, M.T. Speciation of the volatile organic compounds (VOCs) in solventborne aerosol coatings by solid phase microextraction-gas chromatography. J. Coat. Technol. 2003, 75, 47–53. [Google Scholar] [CrossRef]
- Place, R.B.; Imhof, M.; Teuber, M.; Olivier Bosset, J. Distribution of the volatile (flavour) compounds in Raclette cheese produced with different staphylococci in the smear. Mitt. Lebensm. Hyg. 2003, 94, 192–211. [Google Scholar]
- Pino, J.A.; Marbot, R.; Bello, A. Volatile Compounds of Psidium salutare (H.B.K.) Berg. Fruit. J. Agric. Food Chem. 2002, 50, 5146–5148. [Google Scholar] [CrossRef] [PubMed]
- Osorio, C.; Alarcon, M.; Moreno, C.; Bonilla, A.; Barrios, J.; Garzon, C.; Duque, C. Characterization of Odor-Active Volatiles in Champa (Campomanesia lineatifolia R. & P.). J. Agric. Food Chem. 2006, 54, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Rochat, S.; de Saint Laumer, J.Y.; Chaintreau, A. Analysis of sulfur compounds from the in-oven roast beef aroma by comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2007, 1147, 85–94. [Google Scholar] [CrossRef]
- Bartley, J.P.; Schwede, A.M. Production of volatile componds in ripening kiwi fruit (Actinidia chinensis). J. Agric. Food Chem. 1989, 37, 1023–1025. [Google Scholar] [CrossRef]
- Bianchini, A.; Tomi, P.; Bernardini, A.F.; Morelli, I.; Flamini, G.; Cioni, P.L.; Usai, M.; Marchetti, M. A comparative study of volatile constituents of two Helichrysum italicum (Roth) Guss. Don Fil subspecies growing in Corsica (France), Tuscany and Sardinia (Italy). Flavour Fragr. J. 2003, 18, 487–491. [Google Scholar] [CrossRef]
- Schreyen, L.; Dirinck, P.; Sandra, P.; Schamp, N. Flavor analysis of quince. J. Agric. Food Chem. 1979, 27, 872–876. [Google Scholar] [CrossRef]
- Pang, T.; Zhu, S.; Lu, X.; Xu, G. Identification of unknown compounds on the basis of retention index data in comprehensive two-dimensional gas chromatography. J. Sep. Sci. 2007, 30, 868–874. [Google Scholar] [CrossRef] [PubMed]
- LIB2Nistv1.0.2.2 (Beta).—Nist Standard Reference Database Version 2.0. Mass Spectral Data Conversioning Program. Beta-Release 14/7/2008. Available online: https://www.nist.gov/system/files/documents/srd/NIST1a11Ver2-0Man.pdf (accessed on 20 December 2021).
- Boneva, S. Gas chromatographic retention indices for C6 alkanols on OV-101 and Carbowax 20M capillary columns. Chromatographia 1987, 23, 50–52. [Google Scholar] [CrossRef]
- Politeo, O.; Jukic, M.; Milos, M. Chemical composition and antioxidant capacity of free volatile aglycones from basil (Ocimum basilicum L.) compared with its essential oil. Food Chem. 2007, 101, 379–385. [Google Scholar] [CrossRef]
- Duquesnoy, E.; Castola, V.; Casanova, J. Composition and chemical variability of the twig oil ofAbies alba Miller from Corsica. Flavour Fragr. J. 2007, 22, 293–299. [Google Scholar] [CrossRef]
- Helmig, D.; Klinger, L.F.; Guenther, A.; Vierling, L.; Geron, C.; Zimmerman, P. Biogenic volatile organic compound emissions (BVOCs) I. Identifications from three continental sites in the U.S. Chemosphere 1999, 38, 2163–2187. [Google Scholar] [CrossRef] [Green Version]
- Ziegenbein, F.C.; Hanssen, H.-P.; König, W.A. Secondary metabolites from Ganoderma lucidum and Spongiporus leucomallellus. Phytochemistry 2006, 67, 202–211. [Google Scholar] [CrossRef]
- Díaz-Maroto, M.C.; Castillo, N.; Castro-Vázquez, L.; González-Viñas, M.Á.; Pérez-Coello, M.S. Volatile composition and olfactory profile of pennyroyal (Mentha pulegium L.) plants. Flavour Fragr. J. 2006, 22, 114–118. [Google Scholar] [CrossRef]
- Bendahou, M.; Muselli, A.; Grignon-Dubois, M.; Benyoucef, M.; Desjobert, J.-M.; Bernardini, A.-F.; Costa, J. Antimicrobial activity and chemical composition of Origanum glandulosum Desf. essential oil and extract obtained by microwave extraction: Comparison with hydrodistillation. Food Chem. 2008, 106, 132–139. [Google Scholar] [CrossRef]
- Chen, S.-H.; Huang, T.-C.; Ho, C.-T.; Tsai, P.-J. Extraction, Analysis, and Study on the Volatiles in Roselle Tea. J. Agric. Food Chem. 1998, 46, 1101–1105. [Google Scholar] [CrossRef]
- Oruna-Concha, M.; Bakker, J.; Ames, J. Comparison of the Volatile Components of Eight Cultivars of Potato after Microwave Baking. Lebensm. Wiss. Technol. 2002, 35, 80–86. [Google Scholar] [CrossRef]
- Rezazadeh, S.; Hamedani, M.P.; Dowlatabadi, R.; Yazdani, D.; Shafiee, A. Chemical composition of the essential oils ofStachys schtschegleevii Sosn. andStachys balansae Boiss & Kotschy from Iran. Flavour Fragr. J. 2005, 21, 290–293. [Google Scholar] [CrossRef]
- Martos, P.A.; Saraullo, A.; Pawliszyn, J. Estimation of Air/Coating Distribution Coefficients for Solid Phase Microextraction Using Retention Indexes from Linear Temperature-Programmed Capillary Gas Chromatography. Application to the Sampling and Analysis of Total Petroleum Hydrocarbons in Air. Anal. Chem. 1997, 69, 402–408. [Google Scholar] [CrossRef]
- Peng, C. Prediction of retention indices: V. Influence of electronic effects and column polarity on retention index. J. Chromatogr. A 2000, 903, 117–143. [Google Scholar] [CrossRef]
- Cunico, M.M.; Lopes, A.R.; Côcco, L.C.; Yamamoto, C.I.; Plocharski, R.C.B.; Miguel, M.D.; Junior, A.G.; Auer, C.G.; Miguel, O.G. Phytochemical and antibacterial evaluation of essential oils from Ottonia martiana miq. (Piperaceae). J. Braz. Chem. Soc. 2007, 18, 184–188. [Google Scholar] [CrossRef] [Green Version]
- Füssel, U.; Dötterl, S.; Jürgens, A.; Aas, G. Inter- and Intraspecific Variation in Floral Scent in the Genus Salix and its Implication for Pollination. J. Chem. Ecol. 2007, 33, 749–765. [Google Scholar] [CrossRef]
- Vérité, P.; Nacer, A.; Kabouche, Z.; Seguin, E. Composition of seeds and stems essential oils of Pituranthos scoparius (Coss. & Dur.) Schinz. Flavour Fragr. J. 2004, 19, 562–564. [Google Scholar]
- Flamini, G.; Cioni, P.L.; Morelli, I. Analysis of the essential oil of the aerial parts of Viola etrusca from Monte Labbro (South Tuscany, Italy) andin vivo analysis of flower volatiles using SPME. Flavour Fragr. J. 2002, 17, 147–149. [Google Scholar] [CrossRef]
- Paolini, J.; Muselli, A.; Bernardini, A.-F.; Bighelli, A.; Casanova, J.; Costa, J. Thymol derivatives from essential oil of Doronicum corsicum L. Flavour Fragr. J. 2007, 22, 479–487. [Google Scholar] [CrossRef]
- Vendeuvre, C.; Bertoncini, F.; Thiιbaut, D.; Martin, M.; Hennion, M.C. Evluation of a retention model in comprehensive two-dimensional gas chromatography. J. Sep. Sci. 2005, 28, 1129–1136. [Google Scholar] [CrossRef]
- Pérez, R.A.; Navarro, T.; de Lorenzo, C. HS–SPME analysis of the volatile compounds from spices as a source of flavour in ‘Campo Real’ table olive preparations. Flavour Fragr. J. 2007, 22, 265–273. [Google Scholar] [CrossRef]
- Riuaumatell, M.; Castellari, M.; López-Tamames, E.; Galassi, S.; Buxaderas, S. Characterisation of volatile compounds of fruit juices and nectars by HS/SPME and GC/MS. Food Chem. 2004, 87, 627–637. [Google Scholar] [CrossRef]
- Högnadóttir, A.; Rouseff, R.L. Identification of aroma active compounds in organce essence oil using gas chromatography-olfactometry and gas chromatography-mass spectrometry. J. Chromatogr. A 2003, 998, 201–211. [Google Scholar] [CrossRef]
- Carasek, E.; Pawliszyn, J. Screening of Tropical Fruit Volatile Compounds Using Solid-Phase Microextraction (SPME) Fibers and Internally Cooled SPME Fiber. J. Agric. Food Chem. 2006, 54, 8688–8696. [Google Scholar] [CrossRef]
- Zenkevich, I.G.; Chupalov, A.A. New Possibilities of Chromato Mass Pectrometric Identification of Organic Compounds Using Increments of Gas Chromatographic Retention Indices of Molecular Structural Fragments. Russ. J. Org. Chem. 1996, 32, 626–636. [Google Scholar]
- Yasuhara, A.; Shiraishi, H.; Nishikawa, M.; Yamamoto, T.; Uehiro, T.; Nakasugi, O.; Okumura, T.; Kenmotsu, K.; Fukui, H.; Nagase, M.; et al. Determination of organic components in leachates from hazardous waste disposal sites in Japan by gas chromatography–mass spectrometry. J. Chromatogr. A 1997, 774, 321–332. [Google Scholar] [CrossRef]
- Mahattanatawee, K.; Goodner, K.L.; Baldwin, E.A. Volatile constituents and character impact compounds of selected Florida’s tropical fruit. Proc. Fla. State Hort. Soc. 2005, 118, 414–418. [Google Scholar]
- Valette, L.; Fernandez, X.; Poulain, S.; Loiseau, A.-M.; Lizzani-Cuvelier, L.; Levieil, R.; Restier, L. Volatile constituents from Romanesco cauliflower. Food Chem. 2003, 80, 353–358. [Google Scholar] [CrossRef]
- Kuiate, J.R.; Bessière, J.M.; Vilarem, G.; Zollo, P.H.A. Chemical composition and antidermatophytic properties of the essential oils from leaves, flowers and fruits of Cupressus lusitanica Mill. from Cameroon. Flavour Fragr. J. 2006, 21, 693–697. [Google Scholar] [CrossRef]
- Pfeifhofer, H.W. Composition of the essential oil of Pinus canariensis Sweet ex Sprengel. Flavour Fragr. J. 2000, 15, 266–270. [Google Scholar] [CrossRef]
- Alissandrakis, E.; Tarantilis, P.A.; Harizanis, P.C.; Polissiou, M. Comparison of the Volatile Composition in Thyme Honeys from Several Origins in Greece. J. Agric. Food Chem. 2007, 55, 8152–8157. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Halatsi, E.Z.; Kontakos, S.; Karabournioti, S.; Kontominas, M.G. Volatile fraction of commercial thyme honeys produced in Mediterranean regions and key volatile compounds for geographical discrimination: A chemometric approach. Int. J. Food Prop. 2017, 20, 2699–2710. [Google Scholar] [CrossRef]
- Rodríguez-Flores, M.S.; Falcao, S.I.; Escuredo, O.; Seijo, M.C.; Vilas-Boas, M. Description of the volatile fraction of Erica honey from the northwest of the Iberian Peninsula. Food Chem. 2021, 336, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alissandrakis, E.; Tarantilis, P.; Harizanis, P.C.; Polissiou, M. Aroma investigation of unifloral Greek citrus honey using solid-phase microextraction coupled to gas chromatographic–mass spectrometric analysis. Food Chem. 2007, 100, 396–404. [Google Scholar] [CrossRef]
- Odeh, I.; Abulafi, S.; Dewik, H.; Alnajjar, I.; Imam, A.; Dembitsky, V.; Hanus, L. A variety of volatile compounds as markers in Palestinian honey from Thymus capitatus, Thymelaea hirsuta, and Tolpis virgata. Food Chem. 2007, 101, 1393–1397. [Google Scholar] [CrossRef]
- Tan, S.T.; Wilkins, A.L.; Holland, P.T.; McGhie, T.K. Extractives from New Zealand unifloral honeys. Degraded carotenoids and other substances from heather honey. J. Agric. Food Chem. 1989, 37, 1217–1221. [Google Scholar] [CrossRef]
- Odeh, I.; Abu-Lafi, S.; Al-Najjar, I. Determination of potential volatiles markers from citrus, eucalyptus, cotton, and wild flower Palestinian honeys using SPME followed by GCMS analysis. Int. Food Res. J. 2013, 20, 1243–1247. [Google Scholar]
- Senyuva, H.Z.; Gilbert, J.; Silici, S.; Charlton, A.; Dal, C.; Gürel, N.; Cimen, D. Profiling Turkish Honeys to Determine Authenticity Using Physical and Chemical Characteristics. J. Agric. Food Chem. 2009, 57, 3911–3919. [Google Scholar] [CrossRef]
- Izco, J.M.; Torre, P. Characterisation of volatile flavour compounds in Roncal cheese extracted by the ‘purge and trap’ method and analysed by GC-MS. Food Chem. 2000, 70, 409–417. [Google Scholar] [CrossRef]
- Hakala, M.A.; Lapveteläinen, A.T.; Kallio, H.P. Volatile Compounds of Selected Strawberry Varieties Analyzed by Purge-and-Trap Headspace GC-MS. J. Agric. Food Chem. 2002, 50, 1133–1142. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Díaz-Maroto, M.; Pérez-Coello, M. Aroma composition and new chemical markers of Spanish citrus honeys. Food Chem. 2007, 103, 601–606. [Google Scholar] [CrossRef]
- Juan-Borrás, M.; Doménech, E.; Hellebrandova, M.; Escriche, I. Effect of country origin on physicochemical, sugar and volatile composition of acacia, sunflower and tilia honeys. Food Res. Int. 2014, 60, 86–94. [Google Scholar] [CrossRef]
- Nayik, G.A.; Nanda, V. Characterization of the volatile profile of unifloral honey from Kashmir Valley of India by using solid-phase microextraction and gas chromatography–mass spectrometry. Eur. Food Res. Technol. 2015, 240, 1091–1100. [Google Scholar] [CrossRef]

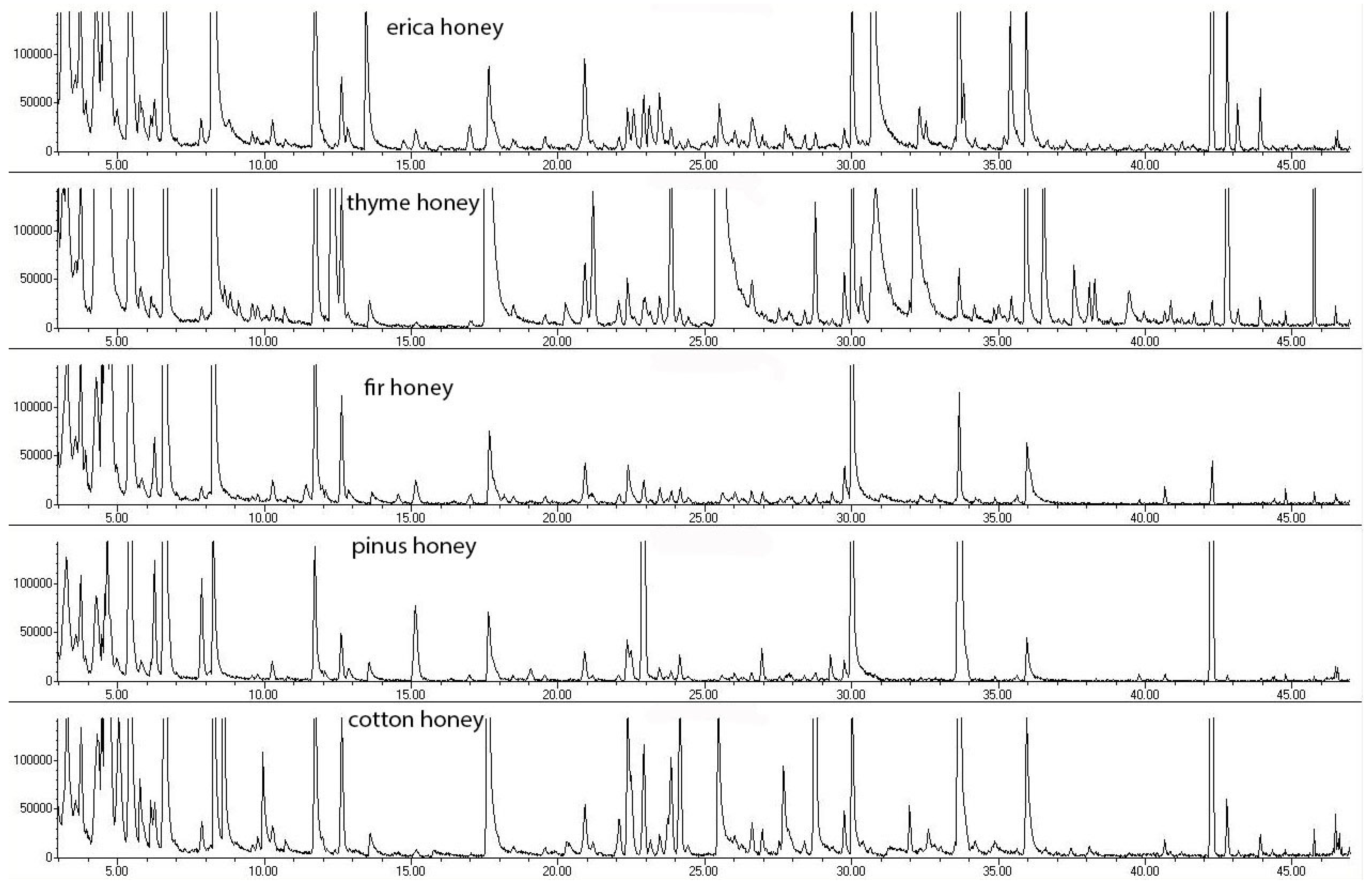
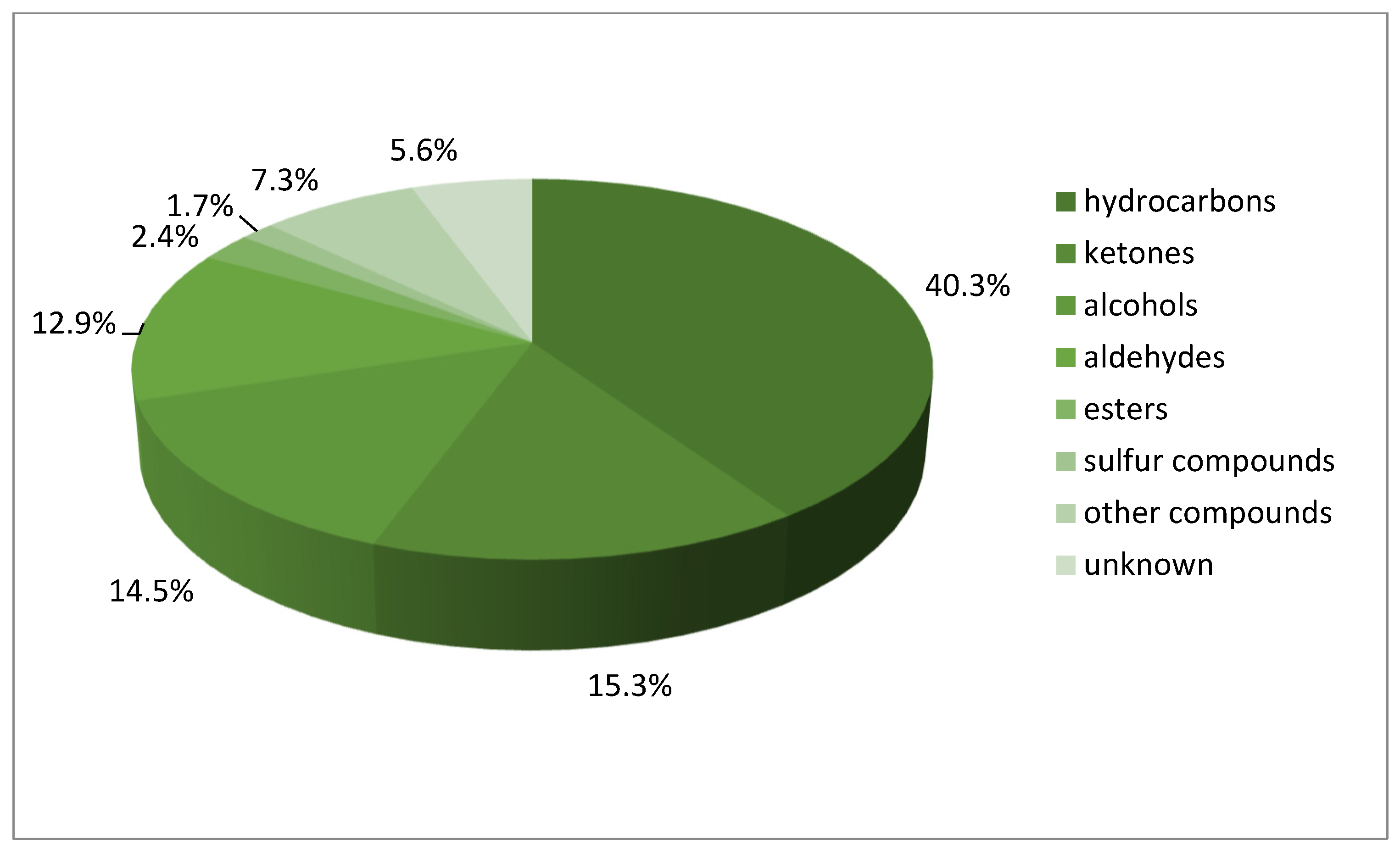
| a/a | R.T. (min) | Volatile Compound (Mass Fractions) | R.I.exp * | R.I.lit * | Percentage Participation (%) (Average ± Standard Deviation) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Fir (n = 6) | Pine (n = 19) | Erica (n = 9) | Thyme (n = 13) | Cotton (n = 5) | |||||
| C1 | 3.25 | Methyl butanal (isomer) (58, 71, 86) | 643 | 632 [42] | 5.32 ± 1.48 ab** | 6.39 ± 3.78 a | 2.90 ± 0.86 bc | 2.27 ± 1.08 c | 4.38 ± 0.78 abc |
| C2 | 3.56 | Cyclopentane, 1,3-dimethyl-(isomer) (58, 70, 83, 98) | 680 | 682 [43] | 1.62 ± 1.43 b | 1.52 ± 0.56 b | 0.94 ± 0.86 bc | 0.66 ± 0.49 | 3.21 ± 1.20 a |
| C3 | 3.73 | Heptane (57,71,100) | 700 | 700 [44] | 2.83 ± 0.41 a | 2.71 ± 0.88 a | 2.70 ± 0.69 a | 1.69 ± 0.89 b | 2.89 ± 0.52 a |
| C4 | 3.91 | Furan, 2,5-dimethyl-(57, 81, 96) | 706 | 696 [45] | 0.95 ± 0.55 a | 1.00 ± 0.61 a | 0.50 ± 0.59 ab | 0.02 ± 0.0 b | nd *** |
| C5 | 4.25 | Cyclohexane, methyl-(55, 83, 98) | 718 | 720 [46] | 3.41 ± 1.19 a | 4.07 ±2.08 a | 2.50 ± 1.18 a | 2.95 ± 3.76 a | 3.58 ± 0.80 a |
| C6 | 4.44 | 3-Buten-1-ol, 2-methyl-(56, 68, 83, 86, 98) | 724 | 716 [47] | 1.92 ± 1.38 a | 0.63 ± 0.56 a | 0.35 ± 0.37 a | 1.14 ± 3.37 a | 1.87 ± 1.05 a |
| C7 | 4.55 | 1-Pentanol (55, 70) | 728 | 735 [48] | 1.24 ± 1.69 b | 2.60 ± 1.99 b | 1.15 ± 1.02 b | 5.15 ±3.67 a | 0.76 ± 0.70 b |
| C8 | 4.6 | 1-Butanol, 2-methyl (57, 70, 85, 100) | 730 | 726 [47] | 1.52 ± 2.37 | nd | nd | 1.04 ± 2.85 | 5.87 ± 6.08 |
| C9 | 4.62 | unknown (55, 64, 68, 82) | 731 | nd | nd | nd | 4.49 ± 5.87 | nd | |
| C10 | 4.64 | 1-Butanol, 3-methyl-(56, 68, 73, 86, 98) | 731 | 718 [49] | nd | nd | nd | 0.89 ± 2.51 | nd |
| C11 | 4,68 | Methyl isobutyl ketone (58, 70, 85,100) | 733 | 729 [46] | 3.23 ± 0.83 ab | 4.86 ± 2.06 a | 3.22 ± 1.67 ab | 1.85 ± 0.85 b | 2.28 ± 3.31 b |
| C12 | 4,77 | Disulfide, dimethyl (64, 79, 94) | 736 | 723 [50] | 2.84 ± 1.76 a | 2.22 ± 1.51 ab | 1.32 ± 1.16 bc | 1.30 ± 1.05 bc | 0.80 ± 0.28 c |
| C13 | 4.8 | 2-Butenal, 2-methyl-(55, 79, 84, 94, 100) | 737 | 730 [51] | nd | 0.29 ±0.63 | 0.24 ± 0.71 | 0.58 ± 0.80 | 3.12 ± 3.78 |
| C14 | 4.9 | 3-Pentanone, 2-methyl-(57, 71, 100) | 740 | 722 [52] | 0.35 ± 0.33 | 0.19 ± 0.46 | 0.26 ± 0.39 | nd | nd |
| C15 | 5.1 | unknown (58, 73, 86, 94, 101, 115) | 747 | nd | 0.52 ± 0.84 | 0.13 ± 0.29 | 0.16 ± 0.32 | 2.66 ± 1.79 | |
| C16 | 5.41 | Toluene (65, 91) | 758 | 755 [46] | 16.31 ± 2.49 a | 14.25 ± 5.69 ab | 12.69 ± 3.34 ab | 10.26 ± 4.17 b | 14.64 ± 1.97 ab |
| C17 | 5.75 | 2-Buten-1-ol, methyl-(isomer) (55, 71, 86, 97, 112) | 769 | 766 [53] | nd | 0.55 ± 1.78 ab | 0.83 ± 1.32 ab | 0.04 ± 0.09 b | 1.41 ± 0.50 a |
| C18 | 5.82 | unknown (55, 71, 77, 86, 97, 112) | 772 | 0.15 ± 0.23 | 0.17 ± 0.45 | 0.15 ± 0.22 | 0.06 ± 0.08 | nd | |
| C19 | 6.08 | 2-Butenal, 3-methyl-(55, 84) | 781 | 769 [54] | 0.26 ± 0.49 b | 0.13 ± 0.18 b | 0.08 ± 0.12 b | 0.12 ± 0.19 b | 0.95 ± 0.88 a |
| C20 | 6.25 | 1-Octene (55, 70, 83, 97, 112) | 787 | 782 [43] | 2.53 ± 0.92 a | 1.45 ± 0.76 b | 0.72 ± 0.43 c | 0.32 ± 0.19 c | 0.66 ± 0.2 ac |
| C21 | 6.61 | Octane (57, 71, 85, 114) | 799 | 800 [44] | 20.91 ± 4.49 a | 21.64 ± 11.28 a | 7.16 ± 4.49 b | 5.35 ± 1.85 b | 10.78 ± 3.62 b |
| C22 | 8.22 | Furfural (67, 96) | 826 | 831 [55] | 8.85 ± 2.75 b | 3.66 ± 3.23 b | 21.72 ± 13.95 a | 5.62 ± 3.73 b | 6.60 ± 3.31 b |
| C23 | 8.6 | 1-Pentanol, 4-methyl-(56, 69, 84, 96) | 832 | 851 [56] | nd | 0.04 ± 0.18 b | nd | nd | 2.18 ± 1.40 a |
| C24 | 9.6 | 2-Cyclopenten-1-one, 3,5,5-trimethyl-(60, 96, 109, 124) | 848 | nd | nd | 0.05 ± 0.06 | 0.51 ± 1.51 | nd | |
| C25 | 9.66 | 3-Hexen-1-ol (55, 67, 82, 91) | 849 | 838 [57] | nd | nd | nd | 0.02 ± 0.03 | 1.80 ± 2.22 |
| C26 | 9.77 | Ethylbenzene (60, 67, 82, 91, 106) | 851 | 850 [46] | 0.10 ± 0.08 a | 0.04 ± 0.09 a | 0.07 ± 0.09 a | 0.03 ± 0.05 a | 0.08 ± 0.11 a |
| C27 | 9.91 | 3-Penten-1-ol, methyl-(isomer) (56, 69, 84, 96, 100) | 854 | 845 [55] | nd | nd | nd | nd | 0.82 ± 0.57 |
| C28 | 10.27 | p-Xylene (91, 106) | 859 | 855 [42] | 0.48 ± 0.21 ab | 0.57 ± 0.31 a | 0.29 ± 0.15 bc | 0.22 ± 0.13 c | 0.48 ± 0.06 ab |
| C29 | 10.64 | Hexanol (56, 69, 84, 95) | 865 | 852 [49] | nd | 0.04 ± 0.11 | 0.01 ± 0.02 | 0.02 ± 0.04 | 0.04 ± 0.08 |
| C30 | 11.4 | Bicyclo [2.2.1]hept-2-ene, 2,3-dimethyl- {Santene} (79, 94, 122) | 878 | 876 [58] | 1.25 ± 1.80 | nd | nd | nd | nd |
| C31 | 11.97 | 1-Nonene (56, 69, 84, 91, 97, 109, 126) | 887 | 895 [59] | 0.07 ± 0.11 | 0.01 ± 0.02 | 0.09 ± 0.10 | nd | 0.03 ± 0.07 |
| C32 | 12.07 | 2-Heptanone (58, 71, 99, 114) | 889 | 871 [60] | 0.08 ± 0.03 a | 0.12 ± 0.20 a | nd | 0.03 ± 0.04 a | nd |
| C33 | 12.3 | Furan, 2,5-diethyltetrahydro-(55, 70, 88, 99, 104) | 893 | 884 [61] | nd | 1.04 ± 2.15 b | 0.30 ± 0.38 b | 6.09 ± 2.22 a | nd |
| C34 | 12.62 | Nonane (57, 71, 85, 128) | 898 | 900 [44] | 1.95 ± 0.42 a | 1.47 ± 0.43 b | 0.99 ± 0.23 c | 1.25 ± 0.53 bc | 2.49 ± 0.64 c |
| C35 | 12.84 | Heptanal (55, 70, 81, 96) | 901 | 882 [60] | 0.13 ± 0.16 | 0.03 ± 0.08 | 0.27 ± 0.18 | 0.02 ± 0.06 | nd |
| C36 | 13.58 | Ethanone, 1-(2-furanyl)-(51, 67, 95, 110) | 909 | 887 [48] | 0.13 ± 0.20 b | 0.29 ± 0.24 b | 2.87 ± 0.80 a | 0.18 ± 0.15 b | 0.35 ± 0.25 b |
| C37 | 14.67 | Cyclopentanone, 2,4,4-trimethyl-(56, 69, 83, 111, 126) | 920 | nd | nd | 0.29 ± 0.37 | nd | nd | |
| C38 | 15.16 | .alpha.-Pinene (53, 77, 93, 105, 121, 136) | 925 | 931 [62] | 1.43 ± 2.02 ab | 3.78 ± 3.71 a | 0.43 ± 0.18 b | 0.14 ± 0.22 b | 0.18 ± 0.26 b |
| C39 | 16.35 | Camphene (58, 67, 79, 93, 107, 121, 136) | 938 | 943 [62] | nd | 0.04 ± 0.12 | nd | nd | nd |
| C40 | 17.66 | Benzaldehyde (51, 77, 106) | 952 | 937 [54] | 0.91 ± 1.00 c | 4.09 ± 3.99 bc | 1.96 ± 1.38 bc | 11.01 ± 4.86 a | 5.08 ± 2.51 b |
| C41 | 18.16 | Dimethyl trisulfide (56, 64, 79, 94, 105, 126) | 957 | 943 [50] | 0.07 ± 0.18 | nd | nd | 0.03 ± 0.09 | nd |
| C42 | 18.46 | 2-Furancarboxaldehyde, 5-methyl-(53, 81, 110) | 960 | 926 [63] | nd | nd | 0.09 ± 0.12 | 0.04 ± 0.09 | nd |
| C43 | 18.54 | Benzene, 1-ethyl-4-methyl-(105, 120) | 961 | 954 [46] | 0.14 ± 0.19 | 0.02 ± 0.06 | 0.04 ± 0.12 | 0.01 ± 0.03 | 0.06 ± 0.13 |
| C44 | 19.06 | .beta.-Pinene (53, 69, 77, 93, 106, 121) | 967 | 970 [62] | nd | 0.30 ± 0.40 | nd | nd | nd |
| C45 | 19.56 | Benzene, 1,2,3-trimethyl-(91, 105, 120) | 972 | 1005 [54] | 0.03 ± 0.05 | 0.04 ± 0.08 | 0.02 ± 0.05 | nd | 0.09 ± 0.05 |
| C46 | 20.16 | Benzonitrile (55, 70, 76, 103) | 978 | 958 [55] | nd | nd | nd | 0.31 ± 0.80 | nd |
| C47 | 20.23 | 1-Octen-3-ol (57, 67, 72, 85, 100) | 979 | 963 [57] | nd | 0.04 ± 0.10 b | 0.03 ± 0.10 b | 0.08 ± 0.21 b | 0.49 ± 0.47 a |
| C48 | 20.91 | Benzene, 1,3,5-trimethyl-(91, 105, 120) | 986 | 1020 [55] | 1.55 ± 1.92 a | 0.79 ± 0.36 ab | 1.24 ± 0.23 b | 0.60 ± 0.28 b | 0.90 ± 0.12 ab |
| C49 | 21.12 | unknown (55, 67, 82, 96, 110, 137) | 988 | 0.39 ± 0.50 | nd | nd | nd | nd | |
| C50 | 21.18 | Furan, 2-pentyl-(53, 69, 81, 93, 105, 138) | 989 | 977 [54] | nd | 0.09 ± 0.32 | 0.06 ± 0.09 | 1.17 ± 0.64 | 0.15 ± 0.23 |
| C51 | 22.05 | Decane (57, 71, 91, 105, 117, 142) | 998 | 1000 [44] | 0.11 ± 0.11 | 0.06 ± 0.14 | nd | 0.19 ± 0.13 | 0.23 ± 0.26 |
| C52 | 22.1 | 2,6-Dimethyl-1,3,5,7-octatetraene, E,E-(57, 77, 91, 105, 119, 134) | 999 | 966 [55] | 0.07 ± 0.18 | 0.10 ± 0.16 | 0.05 ± 0.11 | 0.03 ± 0.10 | 0.09 ± 0.19 |
| C53 | 22.36 | °Ctanal (57, 69, 84, 100, 110) | 1002 | 1005 [55] | 1.36 ± 1.61 a | 0.61 ± 0.67 b | 0.42 ± 0.22 b | 0.46 ± 0.18 b | 0.47 ± 0.28 b |
| C54 | 22.4 | unknown (55, 69, 84, 95, 109, 137, 152) | 1003 | nd | 0.19 ± 0.83 | nd | nd | 0.17 ± 0.37 | |
| C55 | 22.56 | unknown (55, 67, 82, 96, 110, 137) | 1005 | 0.51 ± 0.69 | nd | 0.01 ± 0.02 | 0.07 ± 0.16 | nd | |
| C56 | 22.58 | 2,4-Hexadiene, 2,5-dimethyl-(55, 70, 82, 95, 110) | 1006 | nd | nd | 0.20 ± 0.27 | 0.01 ± 0.03 | nd | |
| C57 | 23.05 | 2-Cyclohexen-1-one (68, 81, 124) | 1011 | nd | nd | 0.38 ± 0.23 | nd | nd | |
| C58 | 23.14 | 3-Carene (77, 93, 105, 121, 136) | 1012 | 1015 [62] | nd | 0.85 ± 1.42 | 0.11 ± 0.32 | 0.20 ± 0.63 | 0.30 ± 0.64 |
| C59 | 23.47 | Benzene, 1,2,4-trimethyl-(68, 77, 91, 105, 120) | 1017 | 1020 [55] | 0.34 ± 0.13 b | 0.11 ± 0.13 b | 0.96 ± 0.61 a | 0.22 ± 0.10 b | 0.32 ± 0.10 b |
| C61 | 23.86 | Benzene, 1-methyl-4-(1-methylethyl)- {p-Cymene} (77, 91, 119, 134) | 1022 | 1011 [62] | 0.28 ± 0.17 b | 0.62 ± 0.56 b | 0.24 ± 0.15 b | 1.46 ± 0.37 a | 0.51 ± 0.53 b |
| C62 | 24.15 | D-Limonene (53, 68, 93, 107, 121, 136) | 1026 | 1020 [62] | 0.29 ± 0.30 a | 3.87 ± 7.34 a | 0.04 ± 0.08 a | 0.28 ± 0.22 a | 2.64 ± 4.01 a |
| C63 | 25.3 | 3-Cyclohexen-1-one, 3,5,5-trimethyl- {b-isophorone} (55, 67, 81, 96, 123, 138) | 1041 | 1044 [64] | nd | nd | 0.08 ± 0.13 | nd | nd |
| C64 | 25.58 | Benzeneacetaldehyde (65, 91, 120) | 1044 | 1048 [65] | 0.14 ± 0.20 b | 0.34 ± 0.55 b | 1.53 ± 1.34 b | 12.18 ± 9.38 a | 1.09 ± 0.78 b |
| C65 | 26.02 | Benzene, 1-methyl-3-propyl-(65, 91, 105, 120, 134) | 1050 | 1042 [46] | nd | 0.05 ± 0.09 | 0.08 ± 0.12 | nd | 0.01 ± 0.02 |
| C66 | 26.5 | 2-Cyclohexen-1-one, 3,5,5-trimethyl- {a-isophorone} (54, 82, 91, 138) | 1056 | 1086 [48] | nd | nd | 0.13 ± 0.20 | 0.02 ± 0.07 | nd |
| C67 | 26.57 | Benzene, 1-ethyl-2,3-dimethyl-(91, 119, 134) | 1057 | 1085 [66] | 0.12 ± 0.14 ab | 0.06 ± 0.15 b | 0.25 ± 0.16 a | 0.11 ± 0.12 ab | 0.21 ± 0.18 ab |
| C68 | 26.61 | 1,4-Cyclohexadiene,1-methyl-4-(1-methylethyl)- {γ-terpinene} (77, 93, 105, 119, 136) | 1058 | 1047 [62] | nd | 0.10 ± 0.18 | nd | 0.11 ± 0.22 | nd |
| C69 | 26.96 | Octane, 1-chloro (55, 69, 83, 94, 105) | 1063 | 1051 [67] | 0.30 ± 0.27 a | 0.22 ± 0.25 ab | 0.12 ± 0.14 ab | 0.03 ± 0.07 b | 0.23 ± 0.08 ab |
| C70 | 27.06 | Acetophenone (51, 77, 105, 120) | 1064 | 1052 [49] | nd | nd | nd | 0.03 ± 0.07 | nd |
| C71 | 27.52 | Cycloheptanemethanol (55, 67, 77, 82, 97, 108) | 1070 | 1143 [55] | nd | 0.08 ± 0.13 | nd | 0.02 ± 0.05 | 0.02 ± 0.04 |
| C72 | 27.53 | Beta.Farnesene (55, 69, 79, 93, 119, 137, 152) | 1071 | 0.07 ± 0.18 | nd | nd | 0.05 ± 0.09 | nd | |
| C73 | 27.7 | 2-Furanmethanol,5-ethenyltetrahydro-,alpha.,alpha.,5-trimethyl-,cis-(59, 68, 81, 94, 111) | 1072 | 1064 [68] | 0.05 ± 0.09 b | 0.13 ± 0.27 b | 0.06 ± 0.13 b | 0.30 ± 0.56 b | 1.54 ± 1.16 a |
| C74 | 28.4 | Benzene, 2-ethyl-1,4-dimethyl-(91, 105, 119, 134, 207) | 1081 | 1068 [46] | 0.07 ± 0.07 | 0.02 ± 0.07 | 0.09 ± 0.09 | 0.05 ± 0.05 | 0.03 ± 0.05 |
| C75 | 28.68 | Benzene, 1-methyl-4-(1-methylethenyl)-(91, 117, 132) | 1083 | 1081 [68] | 0.17 ± 0.20 b | 0.53 ± 1.36 b | 0.13 ± 0.10 b | 0.93 ± 0.31 ab | 1.90 ± 2.86 a |
| C76 | 28.7 | Cyclohexene,1-methyl-4-(1-methylethylidene)- {a-Terpinolene}(55, 67, 79, 93, 105, 121, 136) | 1085 | 1078 [62] | nd | 0.04 ± 0.13 | 0.01 ± 0.01 | nd | nd |
| C77 | 29.28 | 2-Nonanone (53, 58, 67, 71, 95, 142) | 1093 | 1074 [60] | 0.18 ± 0.06 a | 0.11 ± 0.17 ab | nd | 0.03 ± 0.09 b | 0.02 ± 0.03 b |
| C78 | 29.73 | 1,6-Octadien-3-ol, 3,7-dimethyl-(55, 67, 71, 80, 93, 121) | 1095 | 1081 [62] | nd | nd | nd | 0.10 ± 0.27 | nd |
| C79 | 29.75 | Undecane (57, 71, 85, 93, 119) | 1099 | 1100 [44] | 0.81 ± 0.42 ab | 031 ± 0.22 cd | 0.16 ± 0.06 d | 0.51 ± 0.37 bc | 0.99 ± 0.62 a |
| C80 | 30.01 | Nonanal (57, 70, 82, 98, 114) | 1103 | 1104 [55] | 8.49 ± 5.83 a | 7.97 ± 6.23 a | 3.03 ± 1.26 b | 2.73 ± 1.15 b | 3.43 ± 2.24 b |
| C81 | 30.82 | 2-Cyclohexen-1-one, 3,5,5-trimethyl- {isophorone} (54, 82, 138) | 1117 | 1097 [55] | nd | nd | 10.62 ± 13.79 | 0.03 ± 0.08 | nd |
| C82 | 30.85 | Phenylethyl Alcohol (65, 91, 122) | 1119 | 1136 [55] | nd | nd | nd | 0.36 ± 0.66 | nd |
| C83 | 31.97 | 1,4,8-p-Menthatriene (77, 91, 119, 134) | 1136 | 0.03 ± 0.07 | 0.01 ± 0.04 | nd | 0.05 ± 0.11 | 0.20 ± 0.22 | |
| C84 | 32.2 | Benzyl nitrile (51, 67, 90, 109, 117, 137) | 1140 | 1138 [55] | nd | 0.03 ± 0.15 | nd | 2.20 ± 2.98 | nd |
| C85 | 32.26 | 2,6,6-Trimethyl-2-cyclohexene-1,4-dione {4-Ketoisophorone} (68, 96, 152) | 1141 | 1115 [54] | nd | nd | 0.85 ± 0.64 | nd | nd |
| C86 | 32.28 | Lilac aldehyde (isomer I) (55, 67, 81, 93, 111, 121, 153) | 1144 | 1153 [69] | 0.15 ± 0.37 | nd | nd | 0.06 ± 0.20 | nd |
| C87 | 32.56 | Ethanone, 1-(1,4-dimethyl-3-cyclohexen-1-yl)-(67, 77, 90, 109, 117, 137, 152) | 1146 | 1145 [70] | nd | nd | nd | 0.05 ± 0.13 | 0.08 ± 0.12 |
| C88 | 32.59 | 2-Hydroxy-3,5,5-trimethyl-cyclohex-2-enone {2-Hydroxyisophorone} (55, 70, 83, 96, 112, 139, 154) | 1147 | nd | nd | 0.19 ± 0.37 | nd | nd | |
| C89 | 32.6 | Tetramethylbenzene (isomer) (91, 119, 134) | 1148 | 1145 [46] | nd | nd | 0.30 ± 0.27 | nd | nd |
| C90 | 32.77 | Lilac aldehyde (isomer II) (55, 67, 81, 93, 111, 125, 153) | 1150 | 1148 [71] | 0.16 ± 0.40 | 0.01 ± 0.06 | nd | 0.11 ± 0.27 | nd |
| C91 | 33 | 2H-Pyran,3,6-dihydro-4-methyl-2-(2-methyl-1-propenyl)-(55, 68, 83, 91, 109, 119, 134) | 1154 | 1137 [72] | 0.02 ± 0.06 | nd | nd | 0.07 ± 0.14 | 0.02 ± 0.04 |
| C92 | 33.48 | Borneol (55, 69, 79, 95, 110, 139) | 1162 | 1148 [62] | nd | 0.01 ± 0.03 | 0.03 ± 0.06 | nd | nd |
| C93 | 33.81 | unknown (55, 73, 91, 105, 121, 131, 149, 207) | 1167 | nd | nd | 0.13 ± 0.20 | nd | nd | |
| C94 | 34.18 | Benzoic acid, ethyl ester (51,77, 105, 122, 150) | 1173 | 1160 [55] | nd | nd | 0.14 ± 0.43 | nd | nd |
| C95 | 34.2 | 3-Cyclohexen-1-ol,4-methyl-1-(1-methylethyl)-(71, 93, 111, 119, 128, 136) | 1174 | 1161 [62] | nd | 0.01 ± 0.04 | nd | 0.06 ± 0.09 | nd |
| C96 | 34.22 | Naphthalene (63, 102, 128) | 1175 | 1170 [73] | nd | 0.01 ± 0.04 | 0.43 ± 0.79 | 0.03 ± 0.06 | nd |
| C97 | 34.62 | 3,6-Dimethyl-2,3,3a,4,5,7a-hexahydrobenzofuran-(55, 69, 91, 109, 137, 152) | 1179 | 1178 [74] | 0.10 ± 0.18 | nd | nd | 0.01 ± 0.04 | nd |
| C98 | 34.65 | 1-Nonanol (56, 70, 83, 98, 128) | 1181 | 1169 [55] | nd | 0.02 ± 0.09 | nd | nd | nd |
| C99 | 34.97 | 4-Carene (77, 93, 105, 121, 136) | 1187 | nd | nd | nd | 0.09 ± 0.16 | nd | |
| C100 | 35.2 | Methyl salicylate (92, 120, 152) | 1190 | 1176 [49] | nd | nd | 0.38 ± 0.49 a | 0.06 ± 0.13 b | nd |
| C101 | 35.46 | 1,3-Cyclohexadiene-1-carboxaldeyde, 2,6,6-trimethyl-(77, 91, 107, 121, 150) {Safranal} | 1195 | 1186 [55] | nd | 0.02 ±0.07 b | 1.63 ± 0.72 a | 0.19 ± 0.31 b | nd |
| C102 | 35.61 | Octanoic acid, ethyl ester (57, 70, 73, 88, 107, 121, 127, 150) | 1197 | 1183 [55] | nd | nd | 0.20 ± 0.54 | nd | nd |
| C103 | 35.93 | Naphthalene,1,2,3,4-tetrahydro-1,1,6-trimethyl-(77, 91,117, 131, 144, 159, 174) | 1203 | 1235 [75] | nd | nd | nd | 0.29 ± 0.70 | nd |
| C104 | 35.95 | Decanal (57, 70, 82, 95, 112, 128) | 1204 | 1204 [55] | 2.17 ± 1.65 abc | 0.92 ± 0.6 c | 3.41 ± 2.25 a | 1.79 ± 0.99 bc | 2.95 ± 0.74 ab |
| C105 | 36.27 | 3-Cyclohexene-1-acetaldehyde,.alpha.,4-dimethyl-{1-p-menthen-9-al} (55, 67, 79, 94) | 1210 | 1232 [76] | 0.63 ± 1.55 | nd | nd | 0.02 ± 0.08 | nd |
| C106 | 36.55 | Furan, 3-phenyl-(115, 144) | 1216 | 1216 [55] | nd | 0.06 ± 0.26 b | 0.01 ± 0.02 b | 1.34 ± 0.75 a | nd |
| C107 | 37.3 | Bicyclo [3.3.0]octan-2-one, 4,7,7-trimethyl-(54, 82, 91, 110, 131, 146, 151, 166) | 1231 | nd | nd | 0.18 ± 0.28 | 0.02 ± 0.06 | nd | |
| C108 | 37.56 | Benzaldehyde, 4-(1-methylethyl)-{Cuminal}(51, 77, 91, 105, 119, 133, 148) | 1236 | 1230 [55] | nd | nd | nd | 0.11 ± 0.13 | nd |
| C109 | 38.1 | Naphthalene,1,2,3,4-tetrahydro-2,5,8-trimethyl-(77, 91, 105, 115, 131, 144, 159, 174) | 1247 | 1289 [55] | nd | 0.01 ± 0.03 | nd | 0.11 ± 0.24 | 0.04 ± 0.06 |
| C110 | 38.11 | Benzofuran,2,3-dihydro-2,2,5,6-tetramethyl (91, 105, 133, 144, 161, 176) | 1249 | nd | nd | 0.04 ± 0.09 | nd | nd | |
| C111 | 38.27 | 1-Naphthalenol, 2-methyl-(91, 115, 129, 158) | 1251 | nd | nd | nd | 0.04 ± 0.08 | nd | |
| C112 | 38.43 | 2H-1-Benzopyran,3,5,6,8a-tetrahydro-2,5,5,8a-tetramethyl-, cis{Edulan II} (77, 91, 133, 177) | 1254 | 1247 [77] | nd | 0.01 ± 0.03 | 0.02 ± 0.05 | 0.02 ± 0.07 | nd |
| C113 | 39.38 | 1-Butanone, 3-methyl-1-phenyl-(51, 77, 91, 105, 115, 146, 162) | 1273 | 1273 [78] | nd | nd | nd | 0.15 ± 0.25 | nd |
| C114 | 40 | Naphthalene, 2-methyl-(115, 142) | 1286 | 1277 [79] | nd | nd | 0.35 ± 0.92 | nd | nd |
| C115 | 40.67 | Tridecane (57, 71, 85, 99, 112) | 1299 | 1300 [44] | 0.18 ± 0.04 | 0.05 ± 0.06 | 0.01 ± 0.03 | 0.05 ± 0.06 | 0.16 ± 0.05 |
| C116 | 41.24 | 2H-1-Benzopyran,3,5,6,8a-tetrahydro-2,5,5,8a-tetramethyl-, trans{Edulan I} (77, 91, 133, 177, 192) | 1315 | 1315 [80] | nd | 0.01 ± 0.05 | 0.21 ± 0.20 | 0.10 ± 0.27 | nd |
| C117 | 42.78 | Naphthalene,1,2-dihydro-1,1,6-trimethyl-(115, 128, 142, 157, 172) | 1357 | 1136 [81] | nd | 0.34 ± 0.85 a | 2.86 ± 1.90 a | 3.48 ± 6.43 a | 0.33 ± 0.15 a |
| C118 | 43.14 | 4,7-Dihydroxy-5-methylcumarin (122, 149, 164, 192) | 1367 | nd | nd | 0.55 ± 0.63 | 0.01 ± 0.04 | nd | |
| C119 | 43.92 | 2-Buten-1-one,1-(2,6,6-trimethyl-1,3-cyclohexadien-1-yl)-,(E) {Damascenone, trans-}(69, 77, 91, 105, 121, 147, 175, 190, 207) | 1388 | 1362 [72] | nd | 0.01 ± 0.03 b | 0.45 ± 0.22 a | 0.24 ± 0.48 ab | 0.05 ± 0.08 b |
| C120 | 44.78 | Caryophyllene (55, 69, 79, 93, 105, 119, 133, 147, 161, 175) | 1420 | 1424 [62] | 0.08 ± 0.08 | 0.06 ± 0.09 | nd | 0.10 ± 0.12 | 0.03 ± 0.08 |
| C121 | 45.08 | 1H-Cyclopropa[a]naphthalene,1a,2,3,5,6,7,7a,7b-octahydro-1,1,7,7a-tetramethyl-[1aR(1a.alpha.,7.alpha.7a.alpha.,7b.alpha)]—(Calarene) (79, 91, 105, 119, 133, 147, 161, 189, 204) | 1434 | 1427 [82] | nd | 0.08 ± 0.18 | 0.01 ± 0.02 | nd | nd |
| C122 | 45.75 | n- H/C (57, 71, 85, 99, 113, 119) | 1465 | 0.02 ± 0.06 | 0.02 ± 0.06 | 0.01 ± 0.04 | 0.58 ± 0.39 | 0.10 ± 0.06 | |
| C123 | 46.48 | Pentadecane (57, 71, 85) | 1499 | 1500 [44] | 0.08 ± 0.07 b | 0.05 ± 0.06 ab | 0.01 ± 0.02 c | 0.07 ± 0.04 b | 0.10 ± 0.09 a |
| C124 | 46.95 | Naphthalene,1,2,3,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)-, (1S-cis)-{b-Cadinene} (81, 91, 105, 119, 134, 161, 189, 204) | 1528 | 1508 [83] | 0.01 ± 0.01 | 0.01 ± 0.02 | nd | nd | nd |
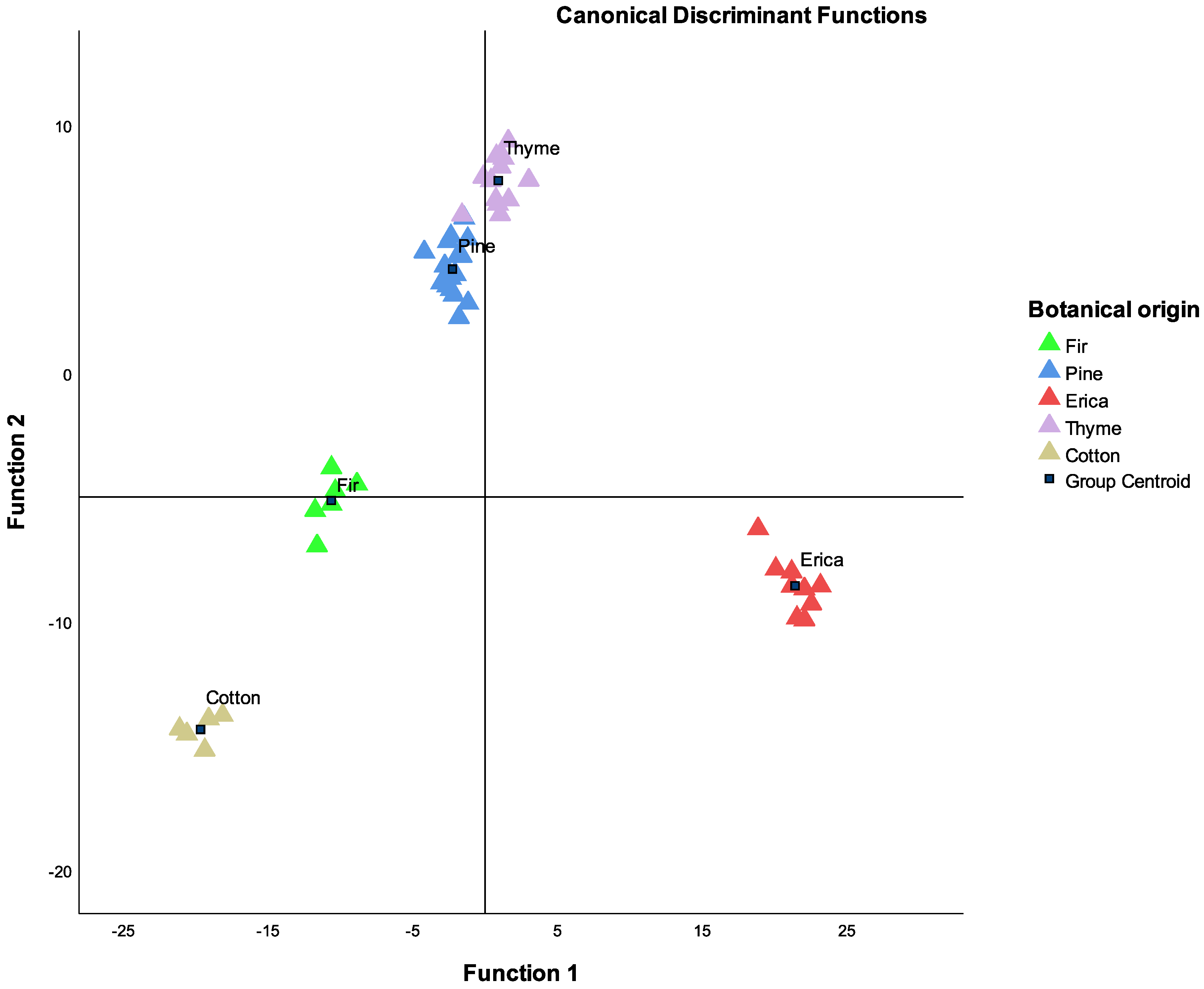
| Function at Group Centroids | ||||
|---|---|---|---|---|
| Botanical Origin | Function | |||
| 1 | 2 | 3 | 4 | |
| Fir | −10.623 | −5.151 | −11.559 | 2.967 |
| Pine | −2.250 | 4.176 | −0.549 | −3.841 |
| Erica | 21.424 | −8.579 | 0.103 | 0.008 |
| Thyme | 0.918 | 7.740 | 2.879 | 4.136 |
| Cotton | −19.655 | −14.369 | 8.287 | 0.265 |
| Eigenvalue | 574.123 | 255.991 | 42.905 | 24.068 |
| % Variation | 58.9 | 25.5 | 10.8 | 4.8 |
| % Cumulative variation | 58.9 | 84.4 | 95.2 | 100.0 |
| Botanical Origin | Predicted Group Membership | ||||||
|---|---|---|---|---|---|---|---|
| Fir | Pine | Erica | Thyme | Cotton | Total | ||
| Original (%) | Fir | 100.0 | 0 | 0 | 0 | 0 | 100.0 |
| Pine | 10.5 | 84.2 | 0 | 0 | 0 | 100.0 | |
| Erica | 0 | 0 | 100.0 | 0 | 0 | 100.0 | |
| Thyme | 0 | 0 | 0 | 100.0 | 0 | 100.0 | |
| Cotton | 0 | 0 | 0 | 0 | 100.0 | 100.0 | |
| Cross-validated (%) | Fir | 83.3 | 16.7 | 0 | 0 | 0 | 100.0 |
| Pine | 10.5 | 84.2 | 0 | 5.3 | 0 | 100.0 | |
| Erica | 0 | 0 | 100.0 | 0 | 0 | 100.0 | |
| Thyme | 0 | 0 | 0 | 100.0 | 0 | 100.0 | |
| Cotton | 0 | 0 | 0 | 0 | 100.0 | 100.0 | |
| Fir = −21.793 + 3.485 × C19 + 9.247 × C20 + 1.916 × C24 + 0.548 × C34 − 2.373 × C38 − 4.593 × C49 − 1.776 × C62 − 0.166 × C68 + 95.518 × C121 − 0.369 × C124 | |||||
| Pine = −7.115 + 2.145 × C19 + 4.959 × C20 − 0.139 × C24 + 0.187 × C34 + 0.236 × C38 + 0.777 × C49 + 4.041 × C62 + 0.063 × C68 + 14.959 × C121 + 0.020 × C124 | |||||
| Erica = −37.589 + 1.069 × C19 − 1.149 × C20 − 8.629 × C24 − 1.562 × C34 + 23.255 × C38 − 29.924 × C49 + 6.489 × C62 + 0.033 × C68 − 11.829 × C121 + 13.108 × C124 | |||||
| Thyme = −17.553 + 1.509 × C19 + 2.871 × C20 − 3.605 × C24 − 1.289 × C34 + 1.240 × C38 + 2.005 × C49 + 9.706 × C62 − 0.728 × C68 − 23.198 × C121 + 5.044 × C124 | |||||
| Cotton = −109.333 + 29.508 × C19 + 2.678 × C20 + 59.756 × C24 + 2.982 × C34 − 2.621 × C38 + 108.549 × C49 − 17.208 × C62 − 0.037 × C68 + 87.765 × 121 − 46.286 × C124 | |||||
| X1 | X2 | X3 | X4 | X5 | |
| Fscore_max | 26.05 | 38.70 | 2.62 | 19.43 | 13.58 |
| Assigned Honey Type | Thyme | Fir | Pine | Thyme | Thyme |
| Correct Honey Type | Thyme | Fir | Pine | Thyme | Thyme |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tananaki, C.; Liolios, V.; Kanelis, D.; Rodopoulou, M.A. Investigation of Volatile Compounds in Combination with Multivariate Analysis for the Characterization of Monofloral Honeys. Appl. Sci. 2022, 12, 264. https://doi.org/10.3390/app12010264
Tananaki C, Liolios V, Kanelis D, Rodopoulou MA. Investigation of Volatile Compounds in Combination with Multivariate Analysis for the Characterization of Monofloral Honeys. Applied Sciences. 2022; 12(1):264. https://doi.org/10.3390/app12010264
Chicago/Turabian StyleTananaki, Chrysoula, Vasilios Liolios, Dimitrios Kanelis, and Maria Anna Rodopoulou. 2022. "Investigation of Volatile Compounds in Combination with Multivariate Analysis for the Characterization of Monofloral Honeys" Applied Sciences 12, no. 1: 264. https://doi.org/10.3390/app12010264
APA StyleTananaki, C., Liolios, V., Kanelis, D., & Rodopoulou, M. A. (2022). Investigation of Volatile Compounds in Combination with Multivariate Analysis for the Characterization of Monofloral Honeys. Applied Sciences, 12(1), 264. https://doi.org/10.3390/app12010264






