Synthesis and Critical View on the Structure-Activity Relationships of N-(Substituted phenyl)-/N-Diphenylmethyl-piperazine-Based Conjugates as Antimycobacterial Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
2.1.1. General Information
2.1.2. Synthesis of Compounds
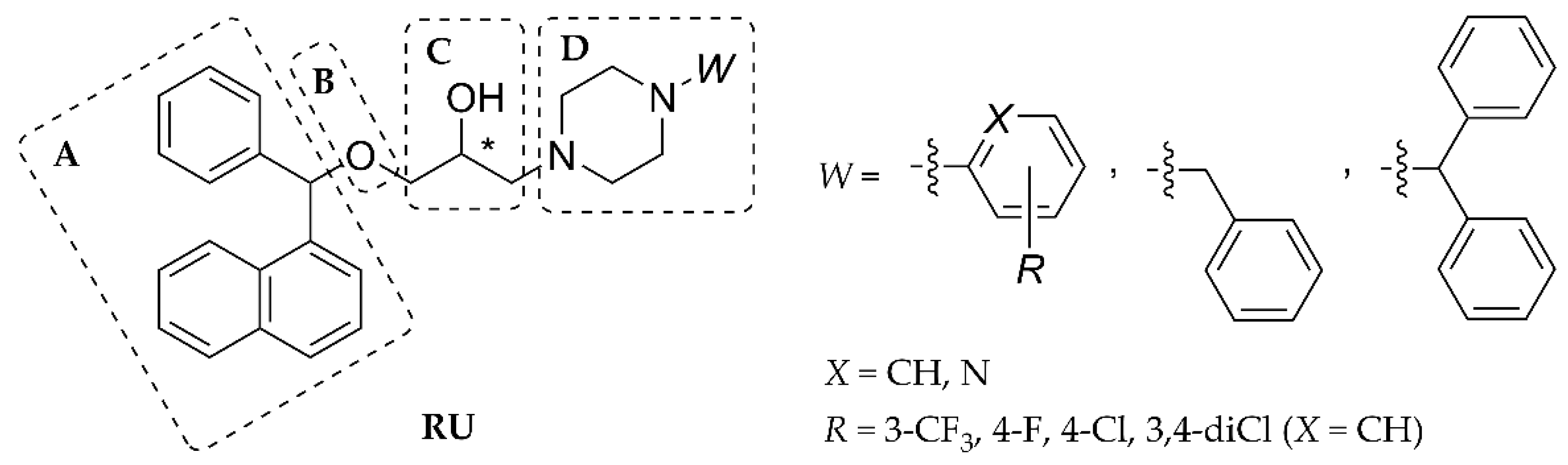
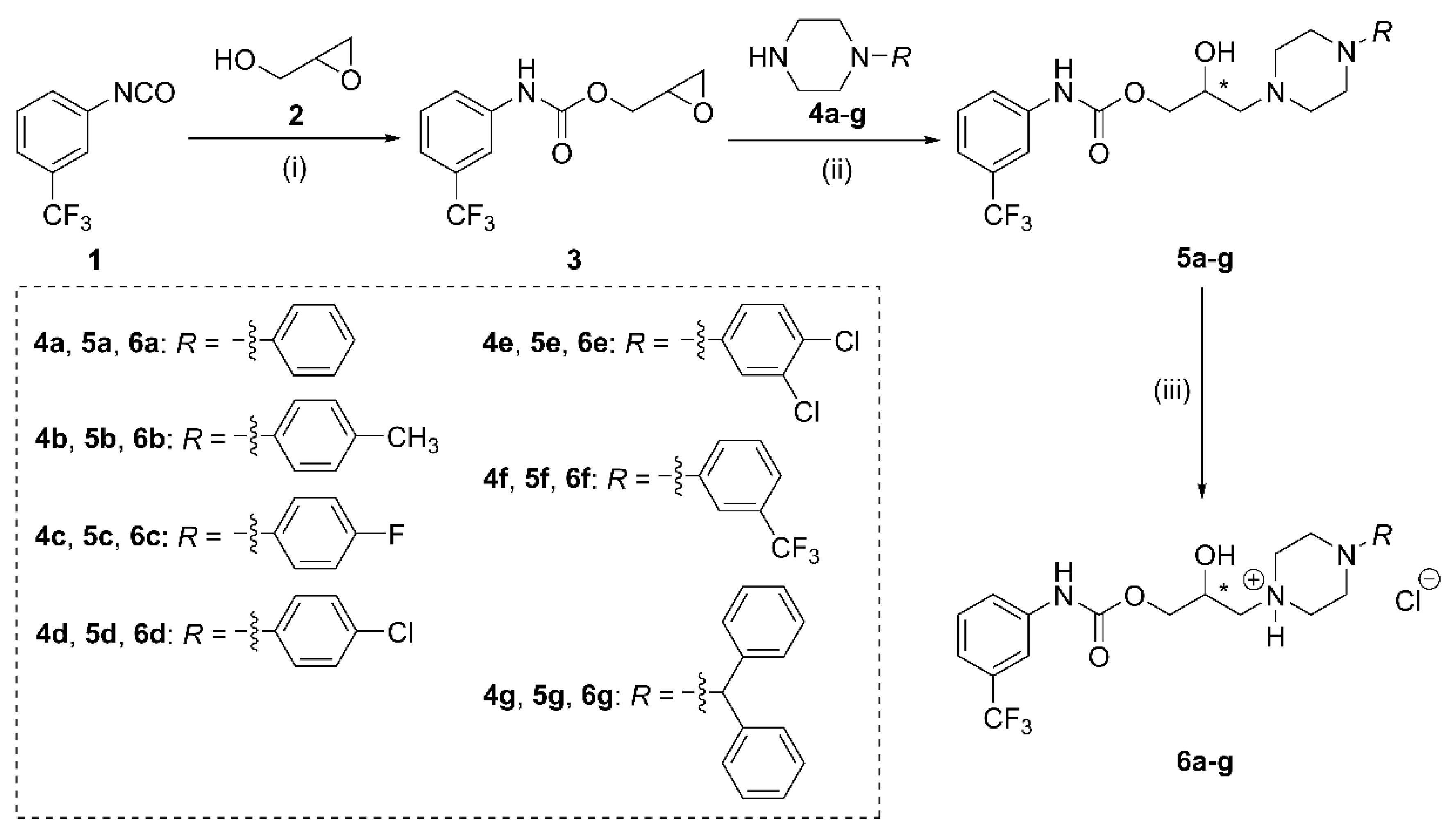
- Preparation of (±)-(oxiran-2-yl)methyl-[1-(3-trifluoromethyl)phenyl]carbamate (3)
- General preparation of carbamate compounds (5a–g)
- General preparation of piperazinium chloride salts (6a–g)
2.1.3. Determination of Some Physicochemical Properties
- Estimation of electronic properties
- Estimation of lipophilic properties
2.2. Biological Assays
2.2.1. In Vitro Antimycobacterial Evaluation
2.2.2. In Vitro Cytotoxicity Screening
2.3. In Silico Evaluation of Synthesized Bases
2.4. Calculations and Statistical Analyses
3. Results and Discussion
3.1. Chemistry
3.1.1. Synthesis of Compounds
3.1.2. Determination of Some Physicochemical Properties
- Determination of electronic properties
- Determination of lipohydrophilic properties
3.2. Biological Assays
3.2.1. In Vitro Antimycobacterial Evaluation
3.2.2. In Vitro Cytotoxicity Screening
3.3. In Silico Evaluation of Synthesized Bases
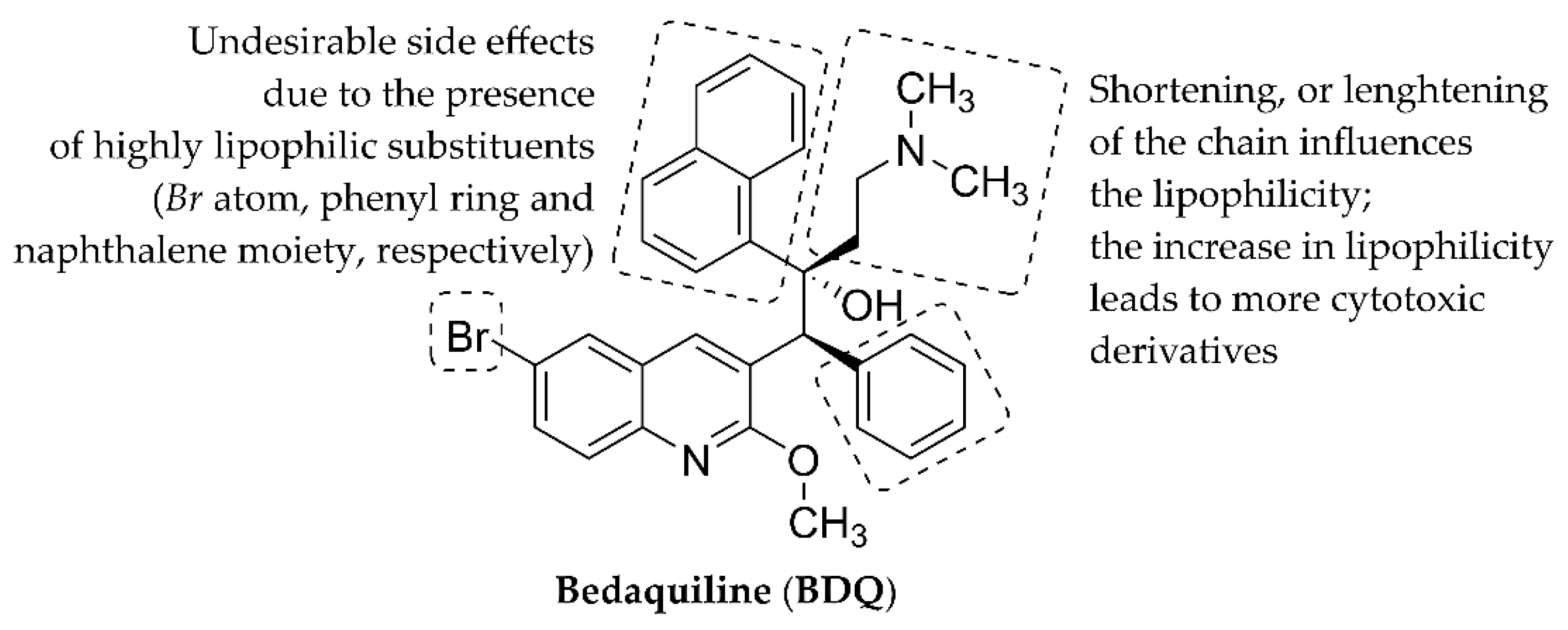
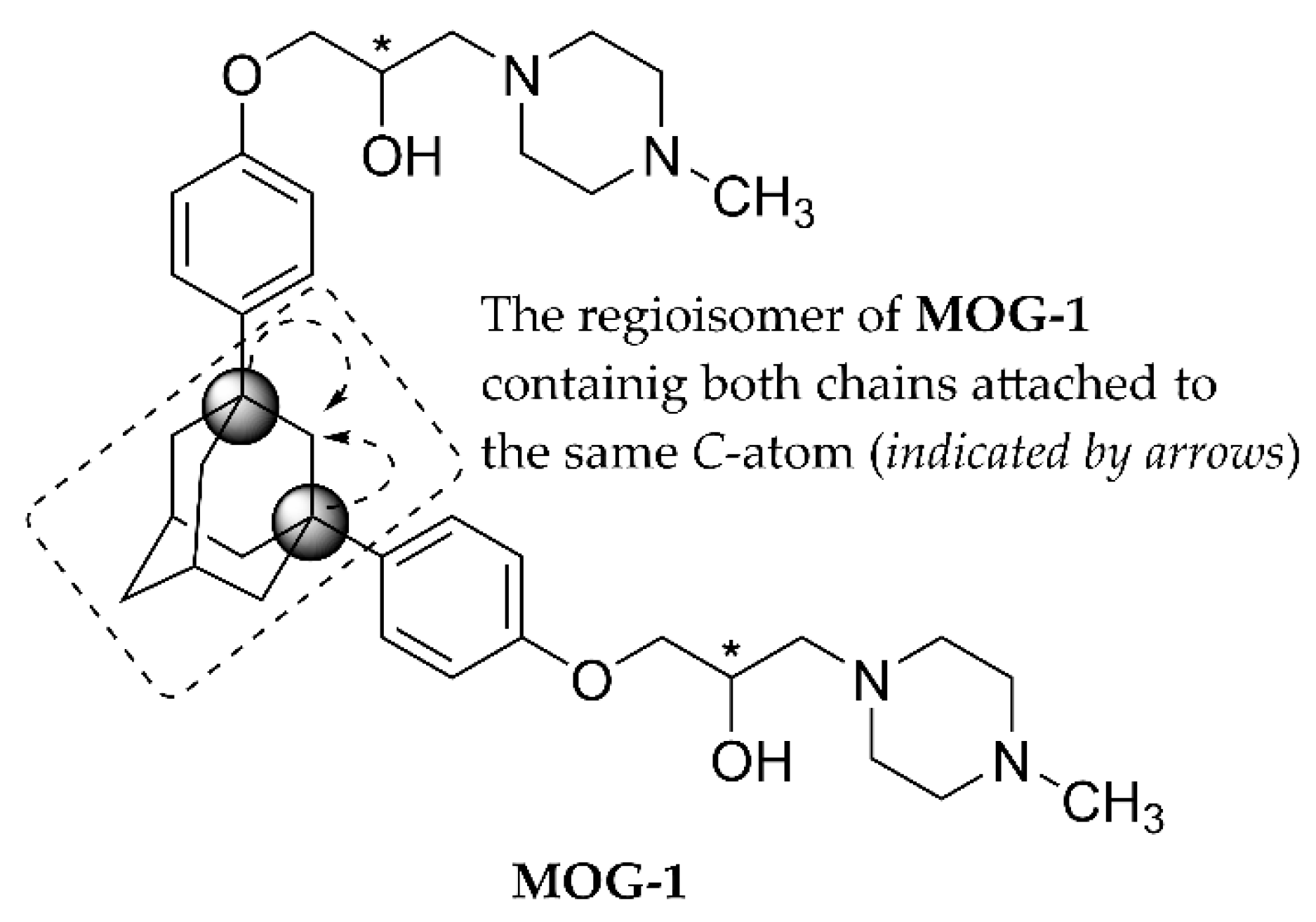
3.4. Structure-Activity Relationships
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christopher, J.A.; Brown, J.; Doré, A.S.; Errey, J.C.; Koglin, M.; Marshall, F.H.; Myszka, D.G.; Rich, R.L.; Tate, C.G.; Tehan, B.; et al. Biophysical fragment screening of the β1-adrenergic receptor: Identification of high affinity arylpiperazine leads using structure-based drug design. J. Med. Chem. 2013, 56, 3446–3455. [Google Scholar] [CrossRef]
- Soskic, V.; Sukalovic, V.; Kostic-Rajacic, S. Exploration of N-arylpiperazine binding sites of D2 dopaminergic receptor. Mini Rev. Med. Chem. 2015, 15, 988–1001. [Google Scholar] [CrossRef]
- Mazzotta, S.; Cebrero-Cangueiro, T.; Frattaruolo, L.; Vega-Holm, M.; Carretero-Ledesma, M.; Sánchez-Céspedes, J.; Cappello, A.R.; Aiello, F.; Pachón, J.; Vega-Pérez, J.M.; et al. Exploration of piperazine-derived thioureas as antibacterial and anti-inflammatory agents. In vitro evaluation against clinical isolates of colistin-resistant Acinetobacter baumannii. Bioorg. Med. Chem. Lett. 2020, 30, 127411. [Google Scholar] [CrossRef]
- Lacivita, E.; Niso, M.; Stama, M.L.; Arzuaga, A.; Altamura, C.; Costa, L.; Desaphy, J.-F.; Ragozzino, M.E.; Ciranna, L.; Leopoldo, M. Privileged scaffold-based design to identify a novel drug-like 5-HT7 receptor-preferring agonist to target Fragile X syndrome. Eur. J. Med. Chem. 2020, 199, 112395. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Hao, C.; Ma, R.; Chen, J.; Zhang, G.; Chen, Y. Synthesis and biological evaluation of a new class of multi-target heterocycle piperazine derivatives as potential antipsychotics. RSC Adv. 2021, 11, 16931–16941. [Google Scholar] [CrossRef]
- Skoreński, M.; Sieńczyk, M. The fellowship of privileged scaffolds—One structure to inhibit them all. Pharmaceuticals 2021, 14, 1164. [Google Scholar] [CrossRef]
- Atukuri, D.; Gunjal, R.; Holagundi, N.; Korlahalli, B.; Gangannavar, S.; Akkasali, K. Contribution of N-heterocycles towards anti-tubercular drug discovery (2014–2019); predicted and reengineered molecular frameworks. Drug Dev. Res. 2021, 82, 767–783. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Dietrich, J. Privileged scaffolds in lead generation. Expert Opin. Drug Discov. 2015, 10, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Daley, C.L. The global fight against tuberculosis. Thorac. Surg. Clin. 2019, 29, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Girase, P.S.; Dhawan, S.; Kumar, V.; Shinde, S.R.; Palkar, M.B.; Karpoormath, R. An appraisal of anti-mycobacterial activity with structure-activity relationship of piperazine and its analogues: A review. Eur. J. Med. Chem. 2021, 210, 112967. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Verma, R.; Verma, S.; Vaishnav, Y.; Tiwari, S.P.; Rakesh, K.P. Anti-tuberculosis activity and its structure-activity relationship (SAR) studies of oxadiazole derivatives: A key review. Eur. J. Med. Chem. 2021, 209, 112886. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.S.; Tamboli, Y.; Krishna, V.S.; Sriram, D.; Ansari, S.A.; Alarfaj, A.A.; Hirad, A.H.; Vijayakumar, V. Design and characterisation of piperazine-benzofuran integrated dinitrobenzenesulfonamide as Mycobacterium tuberculosis H37Rv strain inhibitors. J. Enzyme Inhib. Med. Chem. 2021, 36, 1751–1759. [Google Scholar] [CrossRef]
- Singh, P.; Jaiyeola, B.; Kerru, N.; Ebenezer, O.; Bissessur, A. A review of recent advancements in anti-tubercular molecular hybrids. Curr. Med. Chem. 2017, 24, 4180–4212. [Google Scholar] [CrossRef] [PubMed]
- Alsayed, S.S.R.; Lun, S.; Payne, A.; Bishai, W.R.; Gunosewoyo, H. Facile synthesis and antimycobacterial activity of isoniazid, pyrazinamide and ciprofloxacin derivatives. Chem. Biol. Drug. Des. 2021, 97, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Upadhayaya, R.S.; Vandavasi, J.K.; Kardile, R.A.; Lahore, S.V.; Dixit, S.S.; Deokar, H.S.; Shinde, P.D.; Sarman, M.P.; Chattopadhyaya, J. Novel quinoline and naphthalene derivatives as potent antimycobacterial agents. Eur. J. Med. Chem. 2010, 45, 1854–1867. [Google Scholar] [CrossRef]
- Urban, M.; Šlachtová, V.; Brulíková, M. Small organic molecules targeting the energy metabolism of Mycobacterium tuberculosis. Eur. J. Med. Chem. 2021, 212, 113139. [Google Scholar] [CrossRef]
- Koul, A.; Vranckx, L.; Dendouga, N.; Balemans, W.; Van den Wyngaert, I.; Vergauwen, K.; Göhlmann, H.W.H.; Willebrords, R.; Poncelet, A.; Guillemont, J.; et al. Diarylquinolines are bactericidal for dormant mycobacteria as a result of disturbed ATP homeostasis. J. Biol. Chem. 2008, 283, 25273–25280. [Google Scholar] [CrossRef] [Green Version]
- Waisser, K.; Doležal, R.; Čižmárik, J.; Malík, I.; Kaustová, J. The potential antituberculotics of the series of 2-hydroxy-3-(4-phenylpiperazin-1-yl)-propylphenylcarbamates. Folia Pharm. Univ. Carol. 2007, 35–36, 45–48. [Google Scholar]
- Malík, I.; Csöllei, J.; Jampílek, J.; Stanzel, L.; Zadražilová, I.; Hošek, J.; Pospíšilová, Š.; Čížek, A.; Coffey, A.; O’Mahony, J. The structure–antimicrobial activity relationships of a promising class of the compounds containing the N-arylpiperazine scaffold. Molecules 2016, 21, 1274. [Google Scholar] [CrossRef]
- Waisser, K.; Dražková, K.; Čižmárik, J.; Kaustová, J. Influence of lipophilicity on the antimycobacterial activity of the hydrochlorides of piperidinylethyl esters of ortho-substituted phenylcarbamic acids. Sci. Pharm. 2004, 72, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Waisser, K.; Doležal, R.; Palát, K., Jr.; Čižmárik, J.; Kaustová, J. QSAR Study of antimycobacterial activity of quaternary ammonium salts of piperidinylethyl esters of alkoxysubstituted phenylcarbamic acids. Folia Microbiol. 2006, 51, 21–24. [Google Scholar] [CrossRef]
- Waisser, K.; Dražková, K.; Čižmárik, J.; Kaustová, J. Antimycobacterial activity of basic ethyl esters of alkoxy-substituted phenylcarbamic acids. Folia Microbiol. 2003, 48, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Scherman, M.S.; Jones, V.; Hurdle, J.G.; Woolhiser, L.K.; Knudson, S.E.; Lenaerts, A.J.; Slayden, R.A.; McNeil, M.R.; Lee, R.E. Discovery, synthesis, and biological evaluation of piperidinol analogs with anti-tuberculosis activity. Bioorg. Med. Chem. 2009, 17, 3588–3594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, V.; Lingl, A.; Lewalter, K.; Fritz, M. ATP Synthases with novel rotor subunits: New insights into structure, function and evolution of ATPases. J. Bioenerg. Biomembr. 2005, 37, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Munnaluri, R.K.; Manga, V. In silico quest guided by physico-chemical descriptors of bedaquiline for new scaffolds with potential inhibitory capacity against homology model of Mycobacterium F1F0 ATP synthase. Asian J. Chem. 2018, 30, 904–912. [Google Scholar] [CrossRef]
- Nair, V.; Okello, M.O.; Mangu, N.K.; Seo, B.Y.; Gund, M.G. A novel molecule with notable activity against multi-drug resistant tuberculosis. Bioorg. Med. Chem. Lett. 2015, 25, 1269–1273. [Google Scholar] [CrossRef] [Green Version]
- Donato, M.T.; Lahoz, A.; Castell, J.V.; Gómez-Lechón, M.J. Cell lines: A tool for in vitro drug metabolism studies. Curr. Drug Metab. 2008, 9, 1–11. [Google Scholar]
- Parai, M.K.; Panda, G.; Chaturvedi, V.; Manju, Y.K.; Sinha, S. Thiophene containing triarylmethanes as antitubercular agents. Bioorg. Med. Chem. Lett. 2008, 18, 289–292. [Google Scholar] [CrossRef]
- Pajouhesh, H.; Lenz, G.R. Medicinal chemical properties of successful central nervous system drugs. NeuroRx 2005, 2, 541–553. [Google Scholar] [CrossRef] [Green Version]
- Pospisilova, S.; Malik, I.; Curillova, J.; Michnova, H.; Cerna, L.; Padrtova, T.; Hosek, J.; Pecher, D.; Cizek, A.; Jampilek, J. Insight into antimicrobial activity of substituted phenylcarbamoyloxypiperazinylpropanols. Bioorg. Chem. 2020, 102, 104060. [Google Scholar] [CrossRef]
- Malík, I.; Csöllei, J.; Solovič, I.; Pospíšilová, Š.; Michnová, H.; Jampílek, J.; Čížek, A.; Kapustíková, I.; Čurillová, J.; Pecháčová, M.; et al. Dibasic derivatives of phenylcarbamic acid against mycobacterial strains: Old drugs and new tricks? Molecules 2018, 23, 2493. [Google Scholar] [CrossRef] [Green Version]
- Rodger, A.; Sanders, K. Biomacromolecular applications of UV-visible absorption spectroscopy. In Encyclopedia of Spectroscopy and Spectrometry, 2nd ed.; Lindon, J.C., Tranter, G.E., Koppenaal, D.W., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2010; pp. 166–173. [Google Scholar]
- Valkó, K.; Snyder, L.R.; Glajch, J.L. Retention in reversed-phase liquid chromatography as a function of mobile-phase composition. J. Chromatogr. A 1993, 656, 501–520. [Google Scholar] [CrossRef]
- Soczewiński, E. Mechanistic molecular model of liquid–solid chromatography: Retention–eluent composition relationships. J. Chromatogr. A 2002, 965, 109–116. [Google Scholar] [CrossRef]
- De Voss, J.J.; Rutter, K.; Schroeder, B.G.; Su, H.; Zhu, Y.; Barry, C.E., III. The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages. Proc. Natl. Acad. Sci. USA 2000, 97, 1252–1257. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, B.D.; De Voss, J.J. Structure and absolute configuration of mycobactin J. Tetrahedron Lett. 2001, 42, 3653–3655. [Google Scholar] [CrossRef]
- Atlas, R.; Snyder, J. Reagents, stains, and media: Bacteriology. In Manual of Clinical Microbiology (2-Volume Set), 11th ed.; Jorgensen, J.H., Pfaller, M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S., Warnock, D.W., Eds.; ASM Press: Washington, DC, USA, 2015; Volume 1, pp. 316–349. [Google Scholar]
- Franzblau, S.G.; Witzig, R.S.; McLaughlin, J.C.; Torres, P.; Madico, G.; Hernandez, A.; Degnan, M.T.; Cook, M.B.; Quenzer, V.K.; Ferguson, R.M.; et al. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J. Clin. Microbiol. 1998, 36, 362–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard, 8th ed.; CLSI Document M11-A8; CLSI: Wayne, NJ, USA, 2012; pp. 10–56. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 24th ed.; Informational Supplement M100-S24; CLSI: Wayne, NJ, USA, 2014; pp. 106–211. [Google Scholar]
- Long, J.E.; DeJesus, M.; Ward, D.; Baker, R.E.; Ioerger, T.; Sassetti, C.M. Identifying essential genes in Mycobacterium tuberculosis by global phenotypic profiling. In Gene Essentiality. Methods and Protocols; Lu, L.J., Ed.; Humana Press (Springer): New York, NY, USA, 2015; pp. 79–98. [Google Scholar]
- Brown-Elliott, B.A.; Cohen, S.; Wallace, R.J., Jr. Susceptibility testing of mycobacteria. In Antimicrobial Susceptibility Testing Protocols; Schwalbe, R., Steele-Moore, L., Goodwin, A.C., Eds.; CRC Press (Taylor and Francis Group): Boca Raton, FL, USA, 2007; pp. 243–274. [Google Scholar]
- Raspotnig, G.; Fauler, G.; Jantscher, A.; Windischhofer, W.; Schachl, K.; Leis, H.J. Colorimetric determination of cell numbers by Janus green staining. Anal. Biochem. 1999, 275, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Habala, L.; Pašková, L.; Bilková, A.; Bilka, F.; Oboňová, B.; Valentová, J. Antimicrobial activity and cytotoxicity of transition metal carboxylates derived from agaric acid. Eur. Pharm. J. 2021, 68, 46–53. [Google Scholar] [CrossRef]
- Poroikov, V.V.; Filimonov, D.A.; Ihlenfeldt, W.-D.; Gloriozova, T.A.; Lagunin, A.A.; Borodina, Y.V.; Stepanchikova, A.V.; Nicklaus, M.C. PASS biological activity spectrum predictions in the enhanced open NCI database browser. J. Chem. Inf. Comput. Sci. 2003, 43, 228–236. [Google Scholar] [CrossRef]
- Poroikov, V.; Filimonov, D.; Lagunin, A.; Gloriozova, T.; Zakharov, A. PASS: Identification of probable targets and mechanisms of toxicity. SAR QSAR Environ. Res. 2007, 18, 101–110. [Google Scholar] [CrossRef]
- Goněc, T.; Malík, I.; Csöllei, J.; Jampílek, J.; Stolaříková, J.; Solovič, I.; Mikuš, P.; Keltošová, S.; Kollár, P.; O’Mahony, J.; et al. Synthesis and in vitro antimycobacterial activity of novel N-arylpiperazines containing an ethane-1,2-diyl connecting chain. Molecules 2017, 22, 2100. [Google Scholar] [CrossRef] [Green Version]
- Granato, D.; Santos, J.S.; Escher, G.B.; Ferreira, B.L.; Maggio, R.M. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: A critical perspective. Trends Food Sci. Technol. 2018, 72, 83–90. [Google Scholar] [CrossRef]
- Pospíšilová, Š.; Malík, I.; Bezouskova, K.; Kauerova, T.; Kollar, P.; Csöllei, J.; Oravec, M.; Cizek, A.; Jampilek, J. Dibasic derivatives of phenylcarbamic acid as prospective antibacterial agents interacting with cytoplasmic membrane. Antibiotics 2020, 9, 64. [Google Scholar] [CrossRef] [Green Version]
- Jackson, D.A. Stopping rules in principal components analysis: A comparison of heuristical and statistical approaches. Ecology 1993, 74, 2204–2214. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal component analysis. WIREs Comp. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Morini, G.; Pozzoli, C.; Menozzi, A.; Comini, M.; Poli, E. Synthesis of 1,2-benzisothiazolyloxypropanolamine derivatives and investigation of their activity at β-adrenoceptors. Farmaco 2005, 60, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Čižmárik, J.; Borovanský, A. Synthesis, u.v. and i.r. spectra of 2-piperidnoethyl esters of alkyloxyphenylcarbamic acids. Chem. Zvesti 1975, 29, 119–123. [Google Scholar]
- Ušćumlić, G.S.; Petrović, S.D. Reversal of substituent effect on electronic absorption spectra of N-(4-substituted phenyl)-benzamides in different solvents. Indian J. Chem. 2002, 41B, 206–210. [Google Scholar]
- Fleming, I.; Williams, D. Ultraviolet and visible spectra. In Spectroscopic Methods in Organic Chemistry, 7th ed.; Fleming, I., Williams, D., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 55–84. [Google Scholar]
- Vilchèze, C. Mycobacterial cell wall: A source of successful targets for old and new drugs. Appl. Sci. 2020, 10, 2278. [Google Scholar] [CrossRef] [Green Version]
- Batt, S.M.; Minnikin, D.E.; Besra, G.S. The thick waxy coat of mycobacteria, a protective layer against antibiotics and the host’s immune system. Biochem. J. 2020, 477, 1983–2006. [Google Scholar] [CrossRef]
- Dulberger, C.L.; Rubin, E.J.; Boutte, C.C. The mycobacterial cell envelope—A moving target. Nat. Rev. Microbiol. 2020, 18, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Alsayed, S.R.R.; Lun, S.; Payne, A.; Bishai, W.R.; Gunosewoyo, H. Design, synthesis and antimycobacterial evaluation of novel adamantane and adamantanol analogues effective against drug-resistant tuberculosis. Bioorg. Chem. 2021, 106, 104486. [Google Scholar] [CrossRef]
- Marvadi, S.K.; Krishna, V.S.; Sriram, D.; Kantevari, S. Synthesis of novel morpholine, thiomorpholine and N-substituted piperazine coupled 2-(thiophen-2-yl)dihydroquinolines as potent inhibitors of Mycobacterium tuberculosis. Eur. J. Med. Chem. 2019, 164, 171–178. [Google Scholar] [CrossRef]
- Bvumbi, M.V.; van der Westhuyzen, C.; Mmutlane, E.M.; Ngwane, A. Riminophenazine derivatives as potential antituberculosis agents: Synthesis, biological, and electrochemical evaluations. Molecules 2021, 26, 4200. [Google Scholar] [CrossRef]
- Baptista, R.; Bhowmick, S.; Shen, J.; Mur, L.A.J. Molecular docking suggests the targets of anti-mycobacterial natural products. Molecules 2021, 26, 475. [Google Scholar] [CrossRef]
- Bouz, G.; Bouz, S.; Janďourek, O.; Konečná, K.; Bárta, P.; Vinšová, J.; Doležal, M.; Zitko, J. Synthesis, biological evaluation, and in silico modeling of N-substituted quinoxaline-2-carboxamides. Pharmaceuticals 2021, 14, 768. [Google Scholar] [CrossRef] [PubMed]
- Nemeček, P.; Mocák, J.; Lehotay, J.; Waisser, K. Prediction of HPLC retention factor of potential antituberculotics by QSRR. J. Liq. Chromatogr. Relat.Technol. 2011, 34, 168–181. [Google Scholar] [CrossRef]
- Nemeček, P.; Mocák, J.; Lehotay, J.; Waisser, K. Prediction of anti-tuberculosis activity of 3-phenyl-2H-1,3-benzoxazine-2,4(3H)-dione derivatives. Chem. Pap. 2013, 67, 305–312. [Google Scholar] [CrossRef]
- Nasal, A.; Siluk, D.; Kaliszan, R. Chromatographic retention parameters in medicinal chemistry and molecular pharmacology. Curr. Med. Chem. 2003, 10, 381–426. [Google Scholar] [CrossRef]
- Ranušová, P.; Nemeček, P.; Lehotay, J.; Čižmárik, J. QSRR modelling aimed on the HPLC retention prediction of dimethylamino- and pyrrolidino-substitued esters of alkoxyphenylcarbamic acid. Chem. Pap. 2021, 75, 2525–2535. [Google Scholar] [CrossRef]
- Kubinyi, H. Parameters. In Methods and Principles in Medicinal Chemistry. QSAR: Hansch Analysis and Related Approaches; Mannhold, R., Krogsgaard-Larsen, P., Timmerman, H., Eds.; VCH Verlagsgesellschaft: Weinheim, Germany, 1993; Volume 1, pp. 21–56. [Google Scholar]
- Heinrichs, M.T.; May, R.J.; Heider, F.; Reimers, T.; Sy, S.K.B.; Peloquin, C.A.; Derendorf, H. Mycobacterium tuberculosis strains H37ra and H37rv have equivalent minimum inhibitory concentrations to most antituberculosis drugs. Int. J. Mycobacteriol. 2018, 7, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Malík, I.; Govender, R.; Sedlárová, E.; Csöllei, J.; Jampílek, J.; Coffey, A.; O′Mahony, J. The in vitro evaluation of ortho-/meta-/para-alkoxyphenylcarbamic acid esters bearing 4-(2-fluoro-/2-methylphenyl)piperazin-1-yl moiety against Mycobacterium tuberculosis H37Ra strain. JMBFS J. Microbiol. Biotechnol. Food Sci. 2014, 3, 286–288. [Google Scholar]
- Malík, I.; Govender, R.; Sedlárová, E.; Csöllei, J.; Jampílek, J.; Coffey, A.; O′Mahony, J. The in vitro evaluation of ortho-/meta-/para-alkoxyphenylcarbamic acid esters containing 4-(4-fluorophenyl)piperazin-1-yl moiety against Mycobacterium tuberculosis H37Ra strain. JMBFS J. Microbiol. Biotechnol. Food Sci. 2014, 3, 491–493. [Google Scholar]
- Malík, I.; Govender, R.; Sedlárová, E.; Csöllei, J.; Jampílek, J.; Coffey, A.; O′Mahony, J.; Stanzel, L. The in vitro investigation of ortho-/meta-/para-alkoxyphenylcarbamic acid esters containing substituted N-phenylpiperazin-1-yl fragment against Mycobacterium tuberculosis H37Ra strain. JMBFS J. Microbiol. Biotechnol. Food Sci. 2014, 3, 395–397. [Google Scholar]
- Rana, P.; Aleo, M.D.; Wen, X.; Kugut, S. Hepatotoxicity reports in the FDA adverse event reporting system database: A comparison of drugs that cause injury via mitochondrial or other mechanisms. Acta Pharm. Sin. B 2021. [Google Scholar] [CrossRef]
- Kakkar, A.S.; Dahiya, N. Bedaquiline for the treatment of resistant tuberculosis: Promises and pitfalls. Tuberculosis 2014, 94, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Pawara, R.; Pawara, K.; Ahmed, F.; Shirkhedkar, A.; Surana, S. A structural insight of bedaquiline for the cardiotoxicity and hepatotoxicity. Tuberculosis 2019, 117, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Ballell, L.; Bates, R.H.; Young, R.J.; Alvarez-Gomez, D.; Alvarez-Ruiz, E.; Barroso, V.; Blanco, D.; Crespo, B.; Escribano, J.; González, R.; et al. Fueling open-source drug discovery: 177 small-molecule leads against tuberculosis. ChemMedChem 2013, 8, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Ambrożkiewicz, W.; Kučerová-Chlupáčová, M.; Janďourek, O.; Konečná, K.; Paterová, P.; Bárta, P.; Vinšová, J.; Doležal, M.; Zitko, J. 5-Alkylamino-N-phenylpyrazine-2-carboxamides: Design, preparation, and antimycobacterial evaluation. Molecules 2020, 25, 1561. [Google Scholar] [CrossRef] [Green Version]
- Poce, G.; Consalvi, S.; Venditti, G.; Alfonso, S.; Desideri, N.; Fernandez-Menendez, R.; Bates, R.H.; Ballell, L.; Barros Aguirre, D.; Rullas, J.; et al. Novel pyrazole-containing compounds active against Mycobacterium tuberculosis. ACS Med. Chem. Lett. 2019, 10, 1423–1429. [Google Scholar] [CrossRef]
- El-Azab, A.S.; Abdel-Aziz, A.A.-M.; Abou-Zeid, L.A.; El-Husseiny, W.M.; El Morsy, A.M.; El-Gendy, M.A.; El-Sayed, M.A.-A. Synthesis, antitumour activities and molecular docking of thiocarboxylic acid ester-based NSAID scaffolds: COX-2 inhibition and mechanistic studies. J. Enzyme Inhib. Med. Chem. 2018, 33, 989–998. [Google Scholar] [CrossRef] [PubMed]
- El-Adl, K.; Sakr, H.; El-Hddad, S.S.A.; El-Helby, A.-G.A.; Nasser, M.; Abulkhair, H.S. Design, synthesis, docking, ADMET profile, and anticancer evaluations of novel thiazolidine-2,4-dione derivatives as VEGFR-2 inhibitors. Arch. Pharm. 2021, 354, e2000491. [Google Scholar] [CrossRef] [PubMed]
- Secrist, J.; Anathan, S.; Kwong, C.; Maddry, J.; Reynolds, R.; Poffenberger, A.; Michael, M.; Miller, L.; Krahenbuhl, J.; Adams, L.; et al. Search for new drugs for treatment of tuberculosis. Antimicrob. Agents Chemother. 2001, 45, 1943–1946. [Google Scholar]
- Andries, K.; Verhasselt, P.; Guillemont, J.; Göhlmann, H.W.H.; Neefs, J.-M.; Winkler, H.; Van Gestel, J.; Timmerman, P.; Zhu, M.; Lee, E.; et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 2005, 307, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Ignatius, E.H.; Dooley, K.E. New drugs for the treatment of tuberculosis. Clin. Chest Med. 2019, 40, 811–827. [Google Scholar] [CrossRef]
- Stutz, M.D.; Clark, M.P.; Doerflinger, M.; Pellegrini, M. Mycobacterium tuberculosis: Rewiring host cell signaling to promote infection. J. Leukoc. Biol. 2018, 103, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Esposito, S.; Bianchini, S.; Blasi, F. Bedaquiline and delamanid in tuberculosis. Expert Opin. Pharmacother. 2015, 16, 2319–2330. [Google Scholar] [CrossRef]
- Cohen, K.; Maartens, G. A safety evaluation of bedaquiline for the treatment of multi-drug resistant tuberculosis. Expert Opin. Drug Saf. 2019, 18, 875–882. [Google Scholar] [CrossRef]
- van den Berg, R.A.; Hoefsloot, H.C.; Westerhuis, J.A.; Smilde, A.K.; van der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genom. 2006, 7, 142. [Google Scholar] [CrossRef] [Green Version]
- Bro, R.; Smilde, A.K. Principal component analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef] [Green Version]
- Boulet, J.C.; Roger, J.M. Pretreatments by means of orthogonal projections. Chemometr. Intell. Lab. Syst. 2012, 117, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Georgiadis, M.-O.; Kourbeli, V.; Ioannidou, V.; Karakitsios, E.; Papanastasiou, I.; Tsotinis, A.; Komiotis, D.; Vocat, A.; Cole, S.T.; Taylor, M.C.; et al. Synthesis of diphenoxyadamantane alkylamines with pharmacological interest. Bioorg. Med. Chem. Lett. 2019, 29, 1278–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Entry | λ1 (nm) | log ε1 | λ2 (Ch–T) (nm) | log ε2 (Ch–T) | λ3 (nm) | log ε3 |
|---|---|---|---|---|---|---|
| 6a | 203.50 | 4.53 | 238.50 | 4.30 | 280.00 | 3.25 |
| 6b | 203.50 | 4.52 | 239.00 | 4.28 | 280.50 | 3.21 |
| 6c | 204.00 | 4.24 | 239.00 | 4.27 | 281.00 | 3.24 |
| 6d | 204.00 | 4.48 | 241.50 | 4.28 | 280.50 | 3.25 |
| 6e | 206.00 | 4.55 | 240.00 | 4.32 | 286.50 | 3.34 |
| 6f | 205.00 | 4.51 | 241.00 | 4.31 | 286.50 | 3.57 |
| 6g | 204.00 | 4.47 | 243.00 | 4.33 | 279.50 | 3.07 |
| Entry | log kw | S | 1 χ2red | 2 RSS | 3 R | 4 Adj. R2 | 5 RMSE | 6 F | 7 Prob > F |
|---|---|---|---|---|---|---|---|---|---|
| 6a | 4.1170 | 4.7782 | 0.0036 | 0.0179 | 0.9945 | 0.9867 | 0.0598 | 447.63 | 4.37 × 10−6 |
| 6b | 4.8419 | 5.5848 | 0.0017 | 0.0066 | 0.9976 | 0.9939 | 0.0408 | 821.64 | 8.82 × 10−6 |
| 6c | 4.2672 | 4.9826 | 0.0040 | 0.0201 | 0.9943 | 0.9863 | 0.0635 | 431.66 | 4.78 × 10−6 |
| 6d | 4.8631 | 5.5062 | 0.0020 | 0.0097 | 0.9977 | 0.9945 | 0.0441 | 1089.80 | 4.79 × 10−7 |
| 6e | 5.0882 | 5.5840 | 0.0004 | 0.0016 | 0.9997 | 0.9992 | 0.0176 | 7051.83 | 4.54 × 10−9 |
| 6f | 4.9260 | 5.3504 | 0.0023 | 0.0114 | 0.9972 | 0.9932 | 0.0478 | 876.96 | 8.23 × 10−7 |
| 6g | 5.2184 | 5.9042 | 0.0011 | 0.0056 | 0.9989 | 0.9973 | 0.0335 | 2180.52 | 8.51 × 10−8 |
| Entry | MIC (μM) | IC50 (μM) HepG2 | |||
|---|---|---|---|---|---|
| Mtb H37Ra | MK DSM | MS | MM | ||
| 6a | 69.58 | 69.58 | 139.16 | 69.58 | 83.08 |
| 6b | 33.76 | 33.76 | 67.52 | 33.76 | 33.10 |
| 6c | 33.48 | 66.96 | 133.92 | 66.96 | 49.36 |
| 6d | 32.36 | 8.09 | 64.73 | 15.12 | 43.35 |
| 6e | 3.78 | 15.13 | 30.25 | 15.12 | 29.39 |
| 6f | 15.15 | 15.15 | 15.15 | 30.36 | 54.71 |
| 6g | 3.63 | 14.54 | 14.54 | 8.09 | 22.18 |
| INH | 58.33 | 29.16 | 43.75 | 29.16 | nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čurillová, J.; Pecháčová, M.; Padrtová, T.; Pecher, D.; Mascaretti, Š.; Jampílek, J.; Pašková, Ľ.; Bilka, F.; Kováč, G.; Malík, I. Synthesis and Critical View on the Structure-Activity Relationships of N-(Substituted phenyl)-/N-Diphenylmethyl-piperazine-Based Conjugates as Antimycobacterial Agents. Appl. Sci. 2022, 12, 300. https://doi.org/10.3390/app12010300
Čurillová J, Pecháčová M, Padrtová T, Pecher D, Mascaretti Š, Jampílek J, Pašková Ľ, Bilka F, Kováč G, Malík I. Synthesis and Critical View on the Structure-Activity Relationships of N-(Substituted phenyl)-/N-Diphenylmethyl-piperazine-Based Conjugates as Antimycobacterial Agents. Applied Sciences. 2022; 12(1):300. https://doi.org/10.3390/app12010300
Chicago/Turabian StyleČurillová, Jana, Mária Pecháčová, Tereza Padrtová, Daniel Pecher, Šárka Mascaretti, Josef Jampílek, Ľudmila Pašková, František Bilka, Gustáv Kováč, and Ivan Malík. 2022. "Synthesis and Critical View on the Structure-Activity Relationships of N-(Substituted phenyl)-/N-Diphenylmethyl-piperazine-Based Conjugates as Antimycobacterial Agents" Applied Sciences 12, no. 1: 300. https://doi.org/10.3390/app12010300
APA StyleČurillová, J., Pecháčová, M., Padrtová, T., Pecher, D., Mascaretti, Š., Jampílek, J., Pašková, Ľ., Bilka, F., Kováč, G., & Malík, I. (2022). Synthesis and Critical View on the Structure-Activity Relationships of N-(Substituted phenyl)-/N-Diphenylmethyl-piperazine-Based Conjugates as Antimycobacterial Agents. Applied Sciences, 12(1), 300. https://doi.org/10.3390/app12010300







