A Herbal Concoction of Cinnamomum cassia and Artemisa annua Extracts Ameliorates Allergic Rhinitis in OVA-Induced Balb/C Mice by Inhibiting Th2 Signaling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
2.2. Animals
2.3. Mouse Model of Ovalbumin-Induced Allergic Rhinitis
2.4. Cell Cultures
2.5. Analysis of Cytokines in Blood and Nasal Fluid Samples
2.6. Histological Examination of Nasal Epithelial Thickness
2.7. HPLC Analysis of Organic Acids and Flavonoids
2.8. The Condition of the Automated Amino Acid Analyzer
2.9. Statistical Analysis
3. Results
3.1. Reduced Immunocyte Counts and Eosinophil Cationic Protein (ECP) Levels in the Mouse Model of OVA-Induced Allergic Rhinitis after CIAR Administration
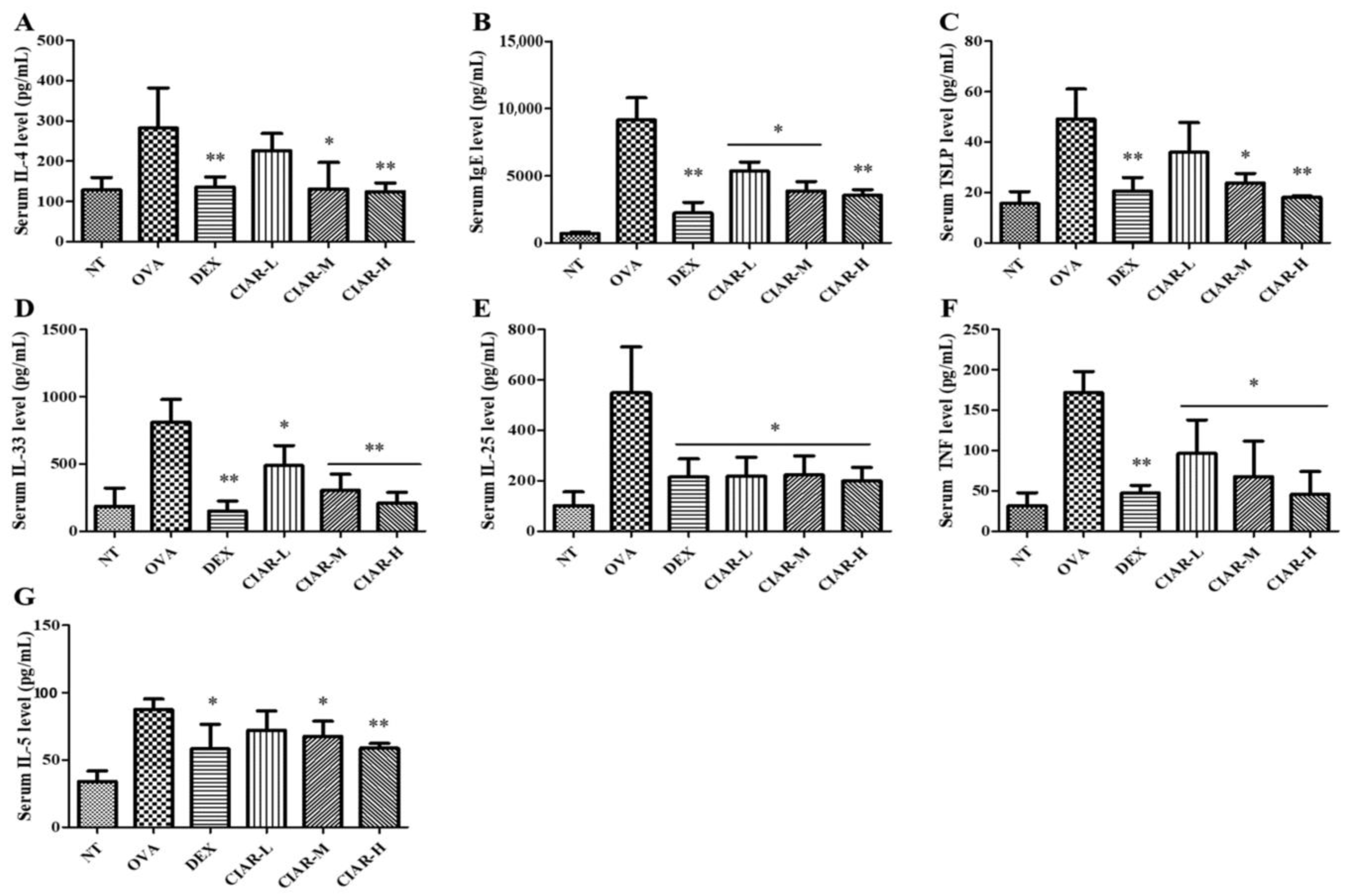
3.2. Inhibition of Th-2 Cytokine Production in Serum of Mice with OVA Induced Allergic Rhinitis
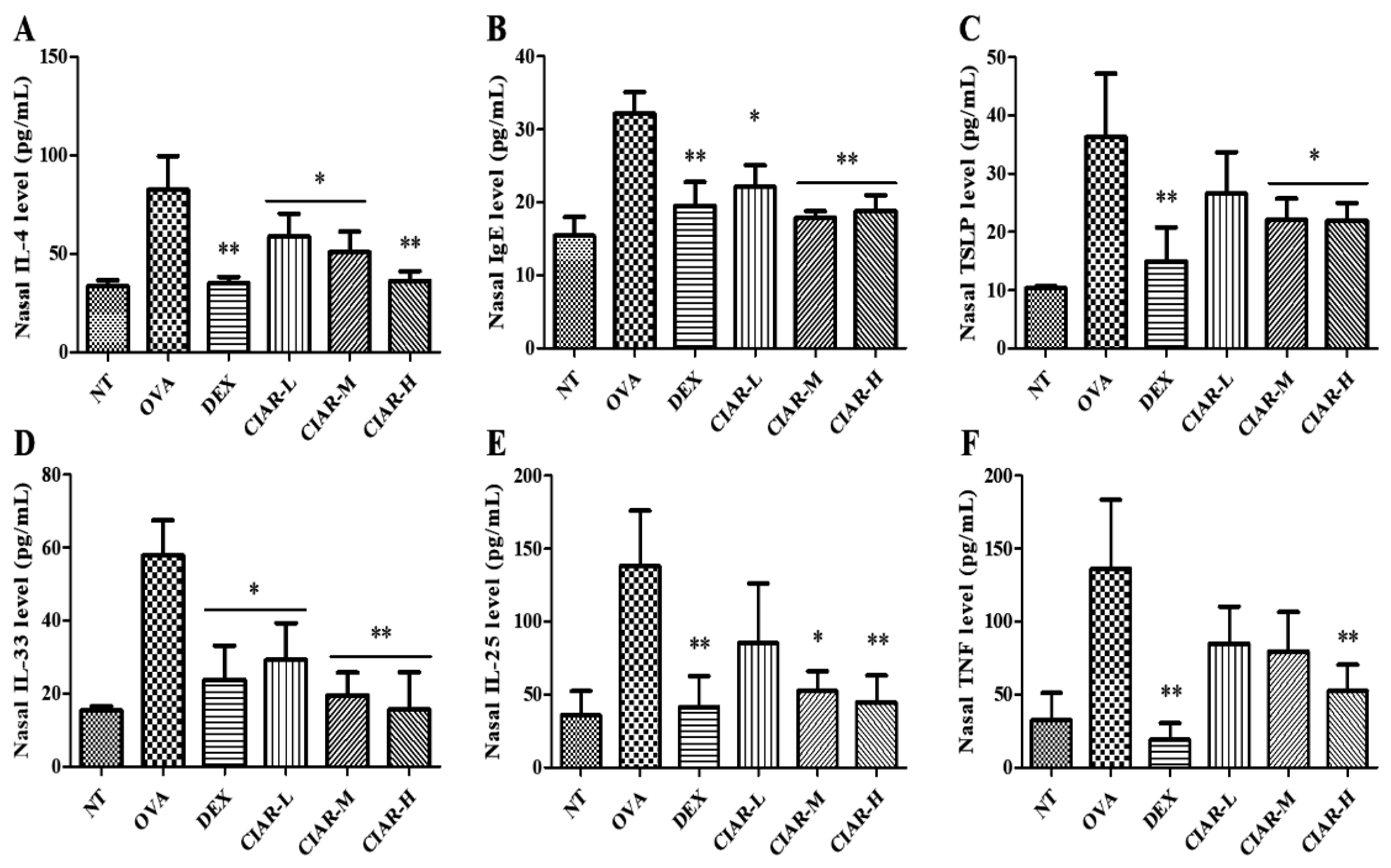
3.3. Effects of CIAR on Th2 Cytokine Production in OVA Induced Mouse Nasal Fluid
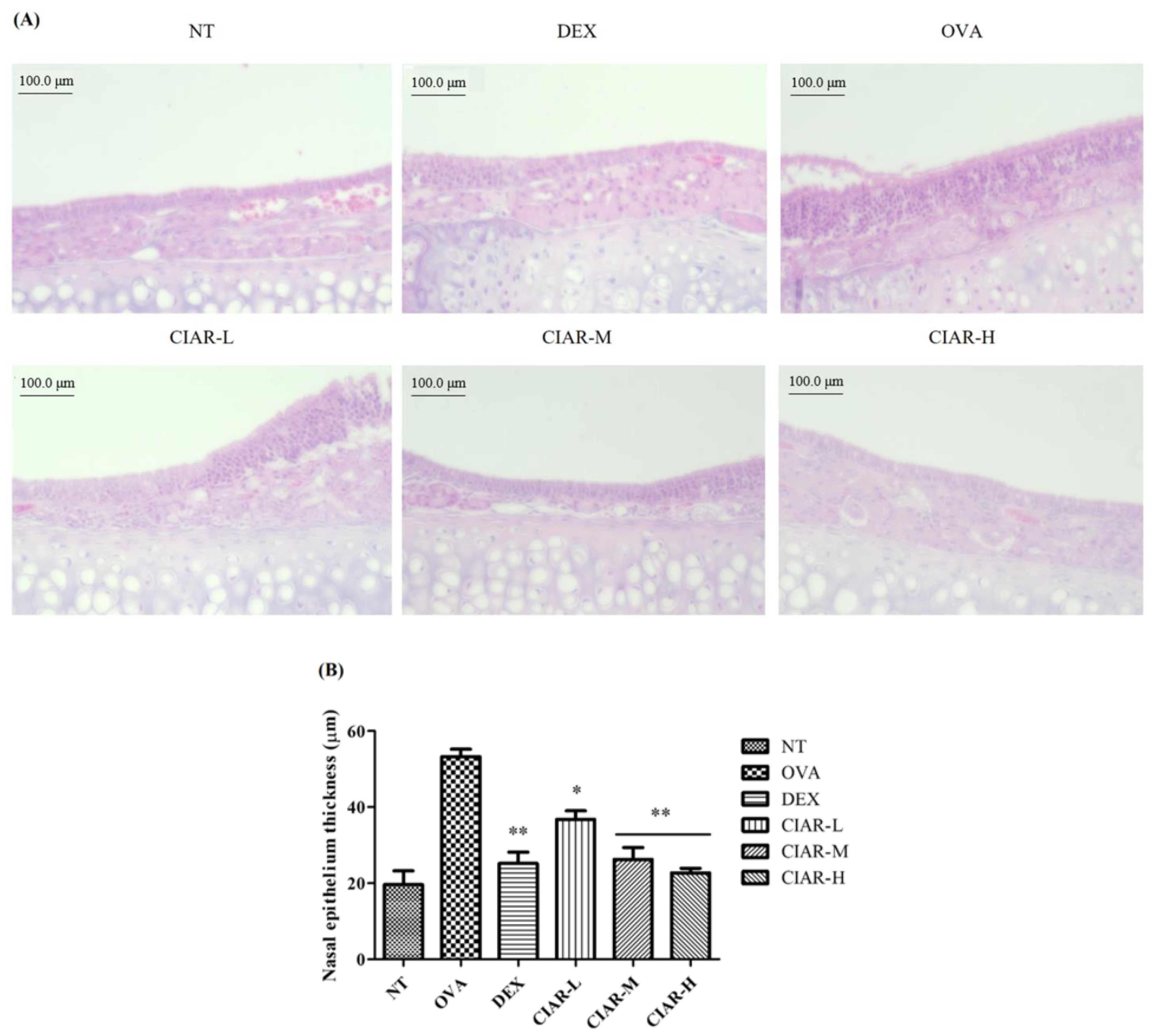
3.4. CIAR Ameliorates Nasal Respiratory Epithelial Thickness Damaged by OVA Inhalation in Mice with Allergic Rhinitis
3.5. Synergistic Effect of CIAR in In Vitro Assays on Levels of Inflammatory Cytokines
3.6. The Analyzed Chemical Components in CIAR, Organic Acids, Amino Acids, and Flavonoids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eifan, A.O.; Durham, S.R. Pathogenesis of rhinitis. Clin. Exp. Allergy 2007, 46, 1139–1151. [Google Scholar] [CrossRef]
- Skoner, D.P. Allergic thinitis: Definition, epidemiology, pathophysiology, decetion, and diagnosis. J. Allergy Clin. Immunol. 2001, 108, S2–S8. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Shin, H.J.; Lee, J.H.; Lee, J. Antiallergic effect of hizikia fusiformis in an ovalbumin-induced allergic rhinitis mouse model. Clin. Exp. Otorhinolaryngol. 2019, 12, 196–205. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.I.; Kim, J.K.; Kim, J.Y.; Ham, M.J.; Kim, D.H. Fermented red ginseng and ginsenoside Rd alleviate ovalbumin-induced allergic rhinitis in mice by suppressing IgE, interleukin-4, and interleukin-5 expression. J. Ginseng Res. 2019, 43, 635–644. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkarni, R.; Behboudi, S.; Sharif, S. Insights into hrole of Toll-like receptors in modulation of T cell responses. Cell Tissue Res. 2011, 343, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Singh, B.; Jaggi, A.S.; Singh, N. Mast cells: An expanding pathophysiological role from allergy to other disorders. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012, 385, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Choi, J.H.; Lee, S.N.; Cho, H.J.; Ahn, J.S.; Kim, Y.B.; Park, D.Y.; Park, S.C.; Kim, S.I.; Kang, M.J. Protein arginine methyltransferase 1 contributes to the development of allergic rhinitis by promoting the production of epithelial-derived cytokines. J. Allergy Clin. Immunol. 2021, 147, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, F.Z.; Aziz, M.; Muhammad, J.S.; Kadowaki, M. Review: Diverse pharmacological properties of Cinnamomum cassiaL A review. Pak. J. Pham. Sci. 2015, 28, 1433–1438. [Google Scholar]
- Alesaeidi, S.; Miraj, S. A syatematic review of anti-malarial properties, immunosuppressive properties, anti-inflammatory properties, and anti-cancer properties of Artemisia annua. Electron. Physician 2016, 25, 3150–3155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Cao, S.; Qiu, F.; Zhang, B. Traditional application and modern pharmacological research of Artemisia annua L. Pharm. Ther. 2020, 216, 107650. [Google Scholar] [CrossRef]
- Steels, E.; Deshpande, P.; Thakurdesai, P.; Dighe, S.; Collet, T. A randomized, double-blind placebo-controlled study of intranasal standardized cinnamon bark extract for seasonal allergic rhinitis. Complement. Med. 2019, 47, 102198. [Google Scholar] [CrossRef]
- Lou, H.; Wang, X.; Wei, Q.; Zhao, C.; Xing, Z.; Zhang, Q.; Meng, J.; Zhang, S.; Zhou, H.; Ma, R. Artemisia Annua sublingual immunotherapy for seasonal allergic rhinitis: A multicenter, randomized trial. World Allergy Organ J. 2020, 13, 100458. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.Y.; Jeong, J.W.; Choi, I.D.; Park, S.D.; Lee, J.L.; Sim, J.H. Potential of Lilium lancifolium in alleviating pain and inflammation in a rat model of monosodium iodoacetate-induced osteoarthritis. Korean J. Plant Res. 2020, 33, 638–644. [Google Scholar]
- Kim, W.G.; Kang, G.D.; Kim, H.I.; Han, M.J.; Kim, D.H. Bifidobacterium longum IM55 and Lactobacillus plantarum IM76 alleviate allergic rhinitis in mice by restoring Th2/Treg imbalance and gut microbiota disturbance. Benef. Microbes 2019, 10, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Bae, C.H.; Kim, J.S.; Nam, W.; Kim, H.; Kim, J.; Nam, B.; Park, S.; Lee, J.; Sim, J. Fermented red ginseng alleviates ovalbumin induced inflammation in mice by suppressing interleukin-4 and immunoglobulin E expression. J. Med. Food 2021, 24, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Wikstén, J.; Toppila-Salmi, S.; Mäkelä, M. Primary prevention of airway allergy. Curr. Treat. Options Allergy 2018, 5, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.H.; Roh, D.; Lee, D.H.; Kim, S.W.; Kim, S.W.; Cho, J.H.; Kim, B.H.; Kim, B.Y. Allergic rhinitis and rhinosinusitis synergistically compromise the mental health and health-related quality of life of Korean adults: A nationwide population-based survey. PLoS ONE 2018, 13, e0191115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Lan, F.; Zhang, Y.; Zhang, L. Chinese herbal medicine to treat allergic rhinitis: Evidence from a meta-analysis. Allergy Asthma Immunol. Res 2018, 10, 34–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degirmenci, P.B.; Aksun, S.; Altin, Z.; Bilgir, F.; Arslan, I.B.; Colak, H.; Ural, B.; Kahraman, D.S.; Diniz, G.; Ozdemir, B.; et al. Allergic rhinigit and its relationship with IL-10, IL-17, TGF-β, IFN-γ, IL 22, and IL-35. Dis. Mark. 2018, 6, 9131432. [Google Scholar]
- Holgate, S.T.; Broide, D. New targets for allergic rhinitis-a disease of civilization. Nat. Rev. Drug Discov. 2003, 2, 902–914. [Google Scholar] [CrossRef]
- Hong, H.; Liao, S.; Chen, F.; Yang, Q.; Wang, D.Y. Role of IL-25, IL-33, and TSLP in triggering united airway diseases toward type 2 inflammation. Allergy 2020, 75, 2794–2804. [Google Scholar] [CrossRef]
- Yao, X.J.; Liu, X.F.; Wang, X.D. Potential role of interleukin-25/interleukin-33/thymic stromal lymphopoietin-fibrocyte axis in the pathogenesis of allergic airway diseases. Chin. Med. J. 2018, 131, 1983–1989. [Google Scholar] [CrossRef]
- Patel, N.N.; Kohanski, M.A.; Maina, I.W.; Workman, A.D.; Herbert, D.R.; Cohen, N.A. Sentinels at the wall: Epithelial-derived cytokines serve as triggers of upper airway type 2 inflammation. Int. Forum Allergy Rhinol. 2019, 9, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.R.; Choi, H.S.; Seo, H.S.; Choi, Y.K.; Shin, Y.C.; Ko, S.G. Topical application of herbal mixture extract inhibits ovalbumin-or 2,4-dinitrochlorobenzene-induced atopic dermatitis. Evid. Based Complement. Altern. Med. 2012, 2012, 545497. [Google Scholar] [CrossRef]
- Jung, H.W.; Jung, J.K.; Kim, Y.H.; Kang, J.S.; Park, Y.K. Effect of KOBO3, a polyherbal medicine, on ovalbumin-induced allergic rhinitis in guinea pigs. Chin. Med. 2012, 7, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.J.; Song, M.J.; Kwon, H.S.; Ji, G.E.; Sung, M.K. Oral administration of fermented red ginseng suppressed ovalbumin-induced allergic responses in female BALB/c mice. Phytomedicine 2012, 19, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Okano, M.; Nishizaki, K.; Abe, M.; Wang, M.M.; Yoshino, T.; Satoskar, A.R.; Masuda, Y.; Han, D.A. Strain-dependent induction of allergic rhinitis without adjuvant in mice. Allergy 1999, 6, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Numata, K.; Ito, T.; Takagi, K.; Matsukawa, A. Innate immune response in th1- and th2-dominant mouse strain. Shock 2004, 5, 460–466. [Google Scholar] [CrossRef]
- Yang, E.J.; Lee, J.S.; Song, B.B.; Yun, C.Y.; Kim, D.H.; Kim, I.S. Anti-inflammatory effects of ethanolic extract from Lagerstroemia indica on airway inflammation in mice. J. Ethnopharmacol. 2011, 136, 422–427. [Google Scholar] [CrossRef]
- Sun, Q.; Yang, X.; Zhong, B.; Jiao, F.; Li, C.; Li, D.; Li, X.; Sun, J.; Lu, S. Upregulated protein arginine methyltransferase 1 by IL-4 increases eotaxi-1 expression I airway epithelial cells and participates in antigen-induced pulmonary inflammation in rats. J. Immunol. 2012, 188, 3506–3512. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.A.; Jung, J.H.; Choi, Y.S.; Kang, G.; Kim, S.T. Anti-inflammatory effect of wogonin on allergic responses in ovalbumin-induced allergic rhinitis in the mouse. Allergy Rhonol. 2018, 9, 2152656718764143. [Google Scholar] [CrossRef] [Green Version]
- Benson, M.; Strannegard, I.L.; Wennergren, G.; Strannegard, O. Interleukin-5 and interleukin-8 in relation to eosinophils and neutrophils in nasal fluids from school children with seasonal allergic rhinitis. Pediatr. Allergy Immunol. 1999, 10, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Tan, J.; Zheng, Y. Chlorogenic acid alleviated allergic inflammatory responses through regulation th1/th2 balance in ovalbumin-induced allergic rhinitis mice. Med. Sci. Monit. 2020, 26, e923358. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Oh, S.Y.; Zhu, Z.; Lee, J.; Lane, A.P. Spontaneous eosinophilic nasal inflammation in a genetically-mutant mouse: Comparative study with an allefgic inflammation model. PLoS ONE 2012, 7, e35114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soyka, M.B.; Veen, W.; Holzmann, D.; Akdis, M.; Akdis, C.A. Scientific foundations of allergen-specific immunotherapy for allergic disease. Transl. Basic Res. Clin. Pract. 2014, 146, 1347–1357. [Google Scholar] [CrossRef]
- Han, N.R.; Moon, P.D.; Kim, H.M.; Jeong, H.J. TSLP exacerbates septic inflammation via murine double minute 2(MDM2) signaling pathway. J. Clin. Med. 2019, 8, 1350. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.; Bae, S.; Jhun, H.; Lee, S.; Choi, J.; Kang, T.; Kwak, A.; Hong, K.; Kim, E.; Jo, S.; et al. Identification of constitutively active interleukin 33 (IL-33) splice variant. J. Biol. Chem. 2011, 286, 20078–20086. [Google Scholar] [CrossRef] [Green Version]
- Eliopoulos, A.G.; Dumitru, C.D.; Wang, C.C.; Cho, J.; Tsichlis, P.N. Induction of cox-2 by lps in macrophages is regulated by tpl2-dependent creb activation signals. EMBO J. 2002, 21, 4831–4840. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Wu, C.; Li, P.; Li, N.; Zhang, D.; Zhu, Q.; Ren, W.; Peng, Y. Functions, and signaling pathways of amino acids in intestinal inflammation. BioMed Res. Int. 2018, 2018, 9171905. [Google Scholar] [CrossRef]
- Grilli, E.; Tugnoli, B.; Passey, J.L.; Stahl, C.H.; Piva, A.; Moeser, A.J. Impact of dietary organic acids and botanicals on intestinal integrity and inflammation in weaned pigs. BMC Vet. Res. 2015, 11, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicastro, H.; Carvalho, M.; Barquilha, G.; Ferreira, L.S. Leucine supplementation: A possible anti-inflammatory strategy evidences from a pilot study. SL Nutr. Metab. 2017, 1, 114. [Google Scholar]
- Gu, C.; Mao, X.; Chen, D.; Yu, B.; Yang, Q. Isoleucine plays an important role for maintaining immune function. Curr. Protein Pept. Sci. 2019, 7, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Sturm, E.M.; Knuplez, E.; Marsche, G. Role of short chain fatty acids and apolipoproteins in the regulation of eosinophilia-associated diseases. Int. J. Mol. Sci. 2021, 22, 4377. [Google Scholar] [CrossRef]
- Liu, P.; Wang, L.; Li, H.; Tan, L.; Ying, X.; Ju, B. Two new organic acids from Portulaca oleracea L. and their anti-inflammatory and anticholinesterase activities. Nat. Prod. Res. 2021, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]


| Group | NT | OVA | Dex | CIAL-L | CIAL-M | CIAR-H |
|---|---|---|---|---|---|---|
| Epithelium thickness | 19.64 ± 3.65 | 53.26 ± 1.96 | 25.17 ± 2.99 ** | 36.77 ± 2.25 * | 26.28 ± 3.09 ** | 22.72 ± 1.23 ** |
| CI | AR | |
|---|---|---|
| Malic acid | 6.54 ± 1.21 | 12.77 ± 2.1 |
| Acetic acid | 6.64 ± 1.24 | 1.27 ± 0.78 |
| Maleic acid | 0.37 ± 0.09 | 0.32 ± 0.94 |
| Succinic acid | 49.22 ± 3.4 | N.D. |
| Propionic acid | 5.43 ± 1.2 | 36.12 ± 2.01 |
| Tartaric acid | 3.55 ± 0.72 | 2.36 ± 0.65 |
| Butyric acid | 314.09 ± 10.62 | N.D. |
| CI | AR | |
|---|---|---|
| P-Ser | 133.5 ± 6.7 | 340.5 ± 13.6 |
| Asp | 81.56 ± 10.5 | 894.5 ± 3.4 |
| Thr | N.D. | 405.3 ± 3.4 |
| Glu | N.D. | 1079.1 ± 13.6 |
| Gly | N.D. | 131.9 ± 3.4 |
| Ala | N.D. | 1899.3 ± 6.8 |
| Val | 227.4 ± 7 | 1052.8 ± 3.4 |
| Cys | N.D. | 64.8 ± 3.4 |
| Ile | N.D. | 136.7 ± 3.4 |
| Leu | N.D. | 417.3 ± 6.8 |
| Phe | N.D. | 4125.5 ± 6.78 |
| g-ABA | 180.4 ± 3 | 1148.7 ± 3.4 |
| CI | AR | |
|---|---|---|
| Quercetin | 4.2 ± 0.67 | 7.3 ± 0.71 |
| Kaemferol | 2.5 ± 0.42 | 2.1 ± 0.78 |
| Isorhamnetic | N.D. | N.D. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, C.; Kim, J.; Park, S.; Shim, J.; Lee, J. A Herbal Concoction of Cinnamomum cassia and Artemisa annua Extracts Ameliorates Allergic Rhinitis in OVA-Induced Balb/C Mice by Inhibiting Th2 Signaling. Appl. Sci. 2022, 12, 340. https://doi.org/10.3390/app12010340
Bae C, Kim J, Park S, Shim J, Lee J. A Herbal Concoction of Cinnamomum cassia and Artemisa annua Extracts Ameliorates Allergic Rhinitis in OVA-Induced Balb/C Mice by Inhibiting Th2 Signaling. Applied Sciences. 2022; 12(1):340. https://doi.org/10.3390/app12010340
Chicago/Turabian StyleBae, Chuhyun, Jisoo Kim, Soodong Park, Jaejung Shim, and Junglyoul Lee. 2022. "A Herbal Concoction of Cinnamomum cassia and Artemisa annua Extracts Ameliorates Allergic Rhinitis in OVA-Induced Balb/C Mice by Inhibiting Th2 Signaling" Applied Sciences 12, no. 1: 340. https://doi.org/10.3390/app12010340
APA StyleBae, C., Kim, J., Park, S., Shim, J., & Lee, J. (2022). A Herbal Concoction of Cinnamomum cassia and Artemisa annua Extracts Ameliorates Allergic Rhinitis in OVA-Induced Balb/C Mice by Inhibiting Th2 Signaling. Applied Sciences, 12(1), 340. https://doi.org/10.3390/app12010340





