Effect of a Hypoxia-Controlled Atmosphere Box on Egg Respiration Intensity and Quality
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Measurement of the Breathing Intensity of Eggs
2.2.2. Measurement of Egg Quality Index
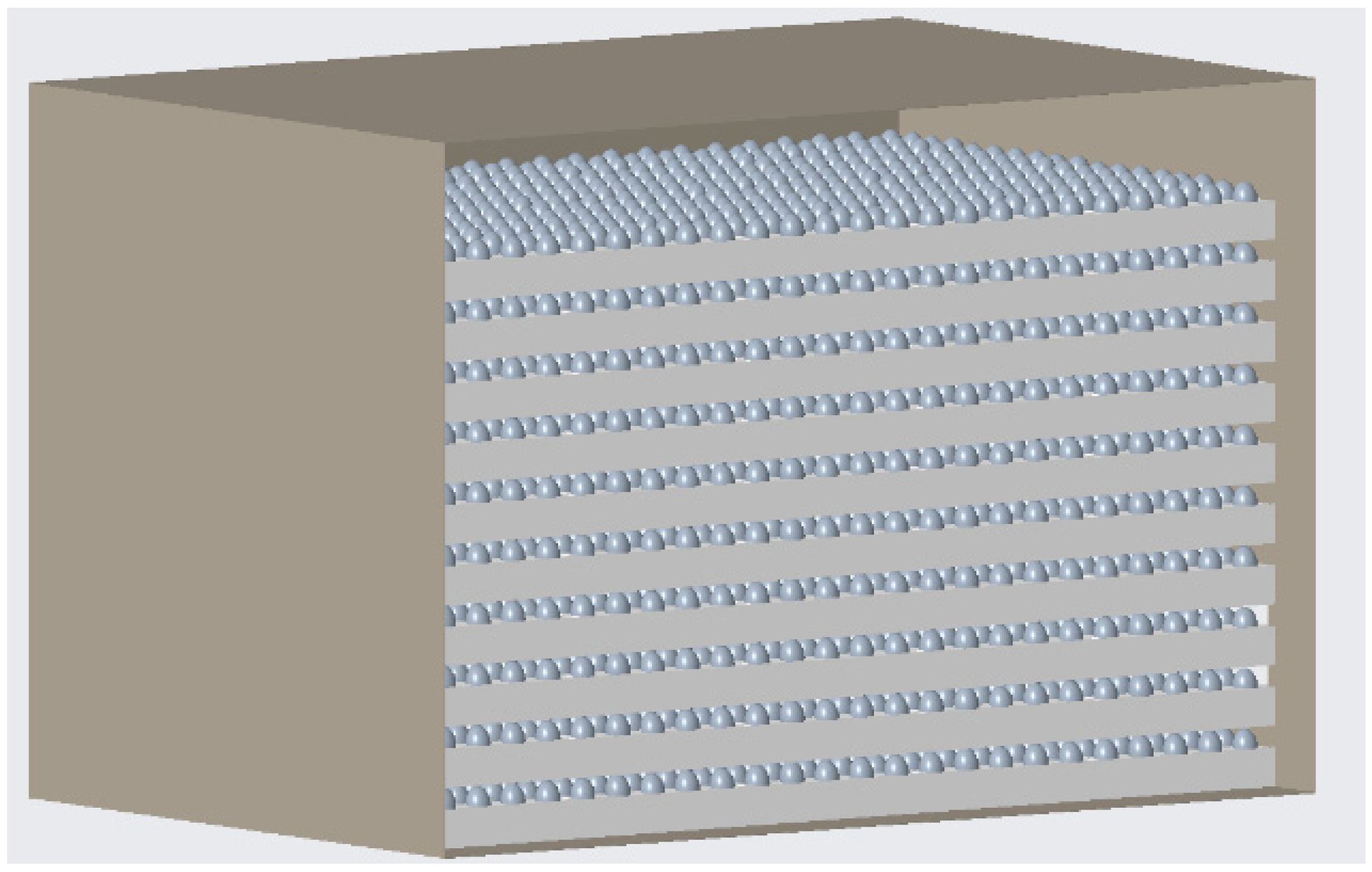
2.3. Design of the Air-Conditioning Box
2.4. Methods of Processing Data
3. Results
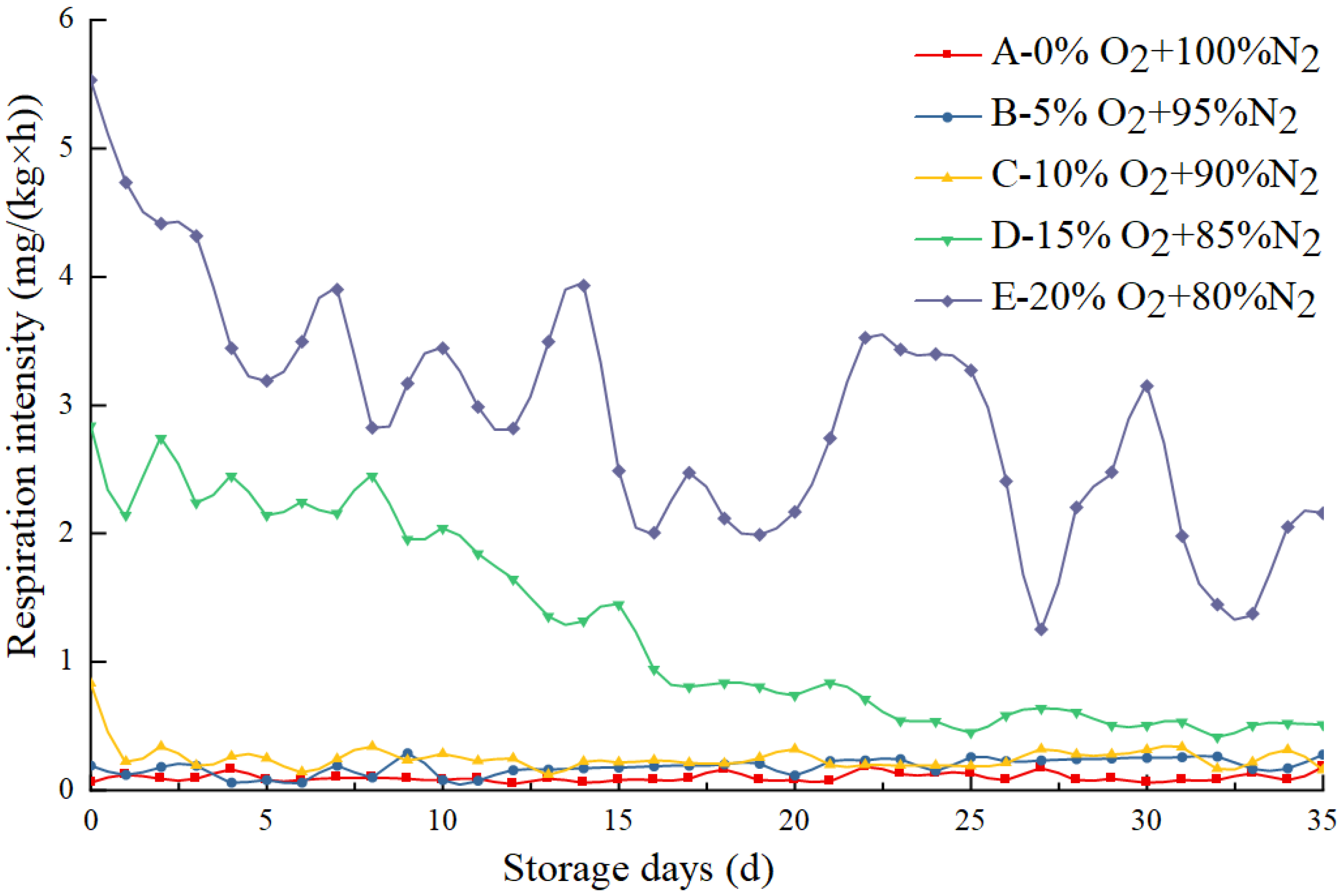
3.1. Effect of Different O2 Concentrations on the Respiration Intensity of Eggs
3.2. Changes in the Quality Indicators of Eggs under Different O2 Concentrations
3.2.1. Changes in the Weight Loss Rate of Eggs under Different O2 Concentrations during Storage
| Storage Days | A (0% O2) | B (5% O2) | C (10% O2) | D (15% O2) | E (20% O2) |
|---|---|---|---|---|---|
| 5 | 0.43 ± 0.05 b | 0.39 ± 0.03 b | 0.35 ± 0.08 b | 0.28 ± 0.04 b | 0.76 ± 0.29 a |
| 10 | 0.71 ± 0.08 b | 0.61 ± 0.04 b | 0.67 ± 0.13 b | 0.79 ± 0.09 c | 1.06 ± 0.06 a |
| 15 | 0.84 ± 0.09 b | 0.81 ± 0.17 b | 0.83 ± 0.14 b | 0.93 ± 0.09 b | 1.36 ± 0.16 a |
| 20 | 1.01 ± 0.11 bc | 0.87 ± 0.08 c | 0.82 ± 0.03 c | 1.28 ± 0.14 b | 1.62 ± 0.31 a |
| 25 | 1.18 ± 0.14 c | 1.14 ± 0.20 c | 1.10 ± 0.14 c | 1.50 ± 0.18 b | 1.77 ± 0.30 a |
| 30 | 1.51 ± 0.20 bc | 1.49 ± 0.23 c | 1.34 ± 0.15 c | 1.87 ± 0.10 b | 2.06 ± 0.24 a |
| 35 | 1.79 ± 0.16 c | 1.67 ± 0.10 c | 1.68 ± 0.33 c | 2.08 ± 0.23 b | 2.35 ± 0.28 a |
3.2.2. Changes in the Haugh Unit of Eggs under Different O2 Concentrations during Storage
| Storage Days | A (0% O2) | B (5% O2) | C (10% O2) | D (15% O2) | E (20% O2) |
|---|---|---|---|---|---|
| 0 | 84.31 ± 3.65 a | 84.50 ± 2.98 a | 83.29 ± 2.45 a | 84.41 ± 3.17 a | 83.49 ± 2.54 a |
| 5 | 74.30 ± 4.45 a | 75.40 ± 5.75 a | 73.22 ± 4.75 a | 73.18 ± 6.25 a | 72.02 ± 2.06 a |
| 10 | 68.60 ± 4.89 a | 69.61 ± 4.87 a | 70.67 ± 8.54 a | 66.49 ± 6.77 a | 67.06 ± 5.87 a |
| 15 | 68.03 ± 3.82 a | 66.27 ± 4.76 ab | 65.30 ± 5.45 b | 64.27 ± 4.23 b | 65.90 ± 3.32 b |
| 20 | 67.15 ± 3.98 a | 65.87 ± 3.82 a | 63.75 ± 4.98 b | 64.28 ± 2.89 b | 63.85 ± 5.32 ab |
| 25 | 66.10 ± 5.66 a | 64.07 ± 2.45 a | 62.53 ± 3.06 ab | 62.88 ± 4.49 b | 61.15 ± 2.89 b |
| 30 | 64.77 ± 5.74 a | 65.90 ± 5.85 a | 63.47 ± 4.01 a | 60.15 ± 5.76 b | 58.35 ± 4.93 b |
| 35 | 63.87 ± 3.39 a | 65.66 ± 3.39 a | 62.28 ± 5.32 a | 59.28 ± 3.83 b | 56.72 ± 3.19 b |
3.2.3. Changes in the pH of Eggs Subjected to Different O2 Concentrations during Storage
| Storage Days | A (0% O2) | B (5% O2) | C (10% O2) | D (15% O2) | E (20% O2) |
|---|---|---|---|---|---|
| 0 | 8.66 ± 0.02 a | 8.65 ± 0.03 a | 8.58 ± 0.05 a | 8.59 ± 0.01 a | 8.64 ± 0.03 a |
| 5 | 8.73 ± 0.03 a | 8.68 ± 0.04 a | 8.69 ± 0.02 a | 8.65 ± 0.02 a | 8.70 ± 0.12 a |
| 10 | 8.88 ± 0.04 a | 8.71 ± 0.06 b | 8.73 ± 0.09 b | 8.70 ± 0.03 b | 8.75 ± 0.04 b |
| 15 | 9.04 ± 0.02 a | 8.94 ± 0.02 b | 8.87 ± 0.04 b | 8.84 ± 0.05 b | 8.88 ± 0.01 b |
| 20 | 9.13 ± 0.04 a | 9.07 ± 0.03 ab | 9.04 ± 0.04 b | 8.90 ± 0.02 c | 8.98 ± 0.02 b |
| 25 | 9.14 ± 0.03 a | 9.13 ± 0.03 a | 9.10 ± 0.06 a | 8.94 ± 0.01 c | 9.03 ± 0.02 b |
| 30 | 9.18 ± 0.04 a | 9.16 ± 0.02 a | 9.14 ± 0.01 a | 8.97 ± 0.01 c | 9.09 ± 0.07 b |
| 35 | 9.23 ± 0.05 a | 9.21 ± 0.11 a | 9.20 ± 0.04 a | 9.09 ± 0.03 b | 9.16 ± 0.04 a |
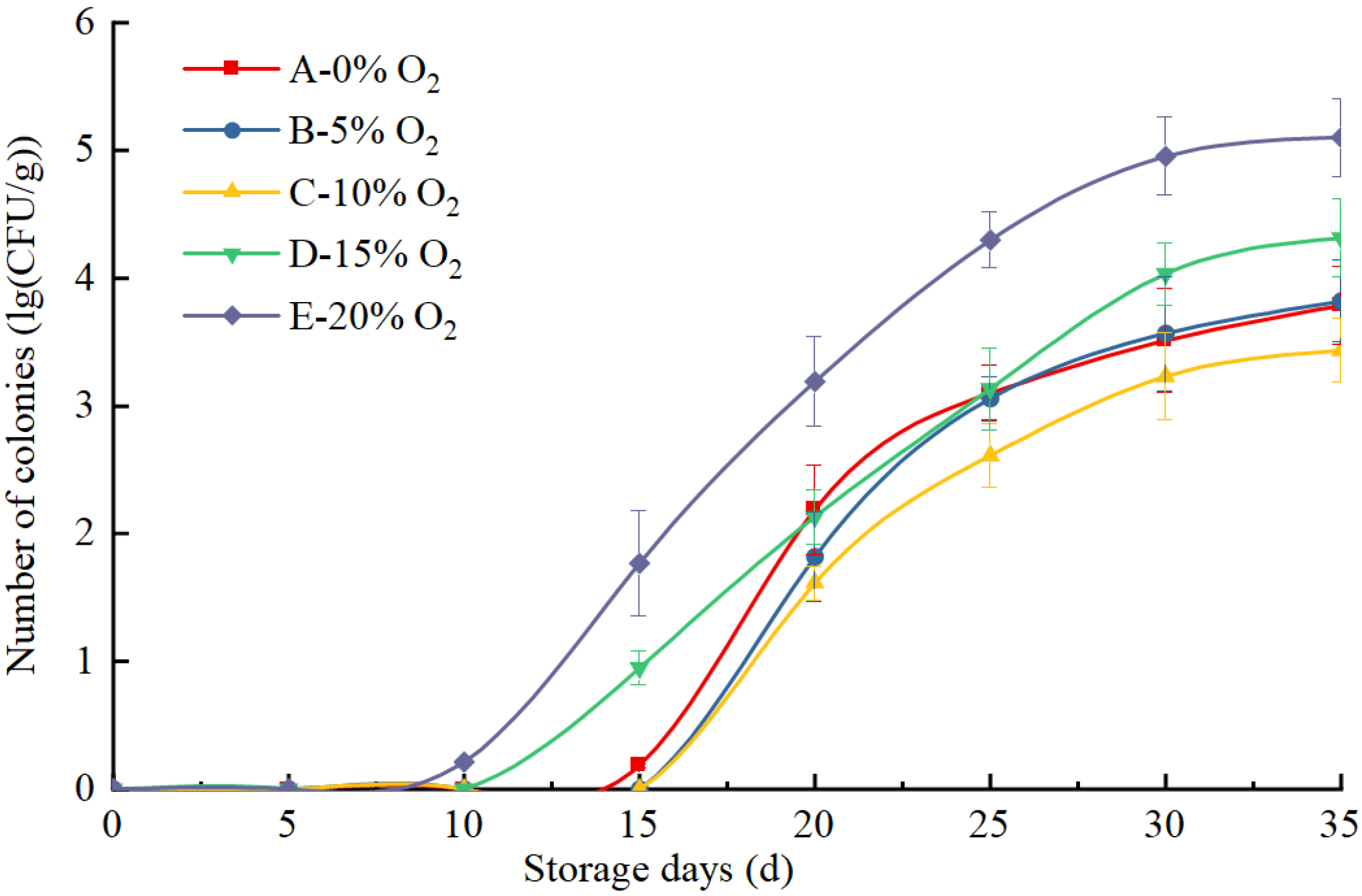
3.2.4. Changes in the Total Number of Colonies in Eggs at Different O2 Concentrations during Storage
3.3. Comprehensive Evaluation of the Storage Effect in Different O2 Concentrations
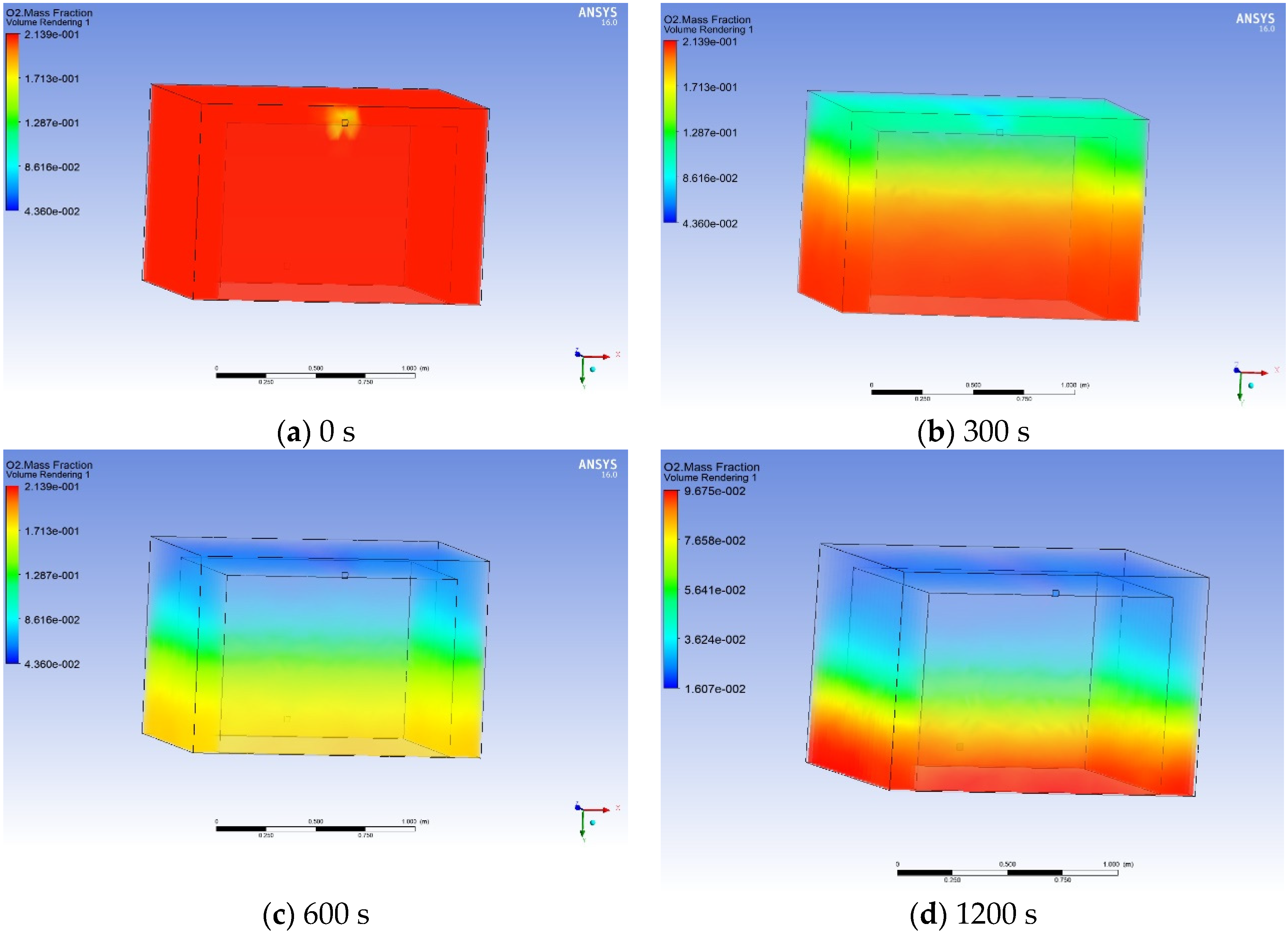
3.4. Process Simulation and Calculation of Air-Conditioning Costs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Onyenweaku, E.O.; Ene-Obong, H.N.; Oko, G.E.; Williams, I.O. Contribution of Eggs and Other Cholesterol- containing Foods to Total Dietary Cholesterol Intake, and Their Influence on Serum Lipid Profile of Adults in Calabar, Nigeria. Eur. J. Nutr. 2019, 9, 329–340. [Google Scholar] [CrossRef]
- Duan, Y.F.; Wang, Q.H. Optimization of a Predictive Model for Rapid Detection of Egg Freshness Using Visible Near-Infrared Spectra Based on Combination of Feature Selection and Feature Extraction. Food Sci. 2020, 41, 273–278. [Google Scholar]
- Fu, D.D.; Wang, Q.H.; Ma, M.H.; Xu, F. Correlation Analysis between Egg Freshness Indexes and S-Ovalbumin Content during Storage. Food Sci. 2018, 39, 1–6. [Google Scholar]
- Zheng, Y.; Ding, C.Z.; Ma, J. Price Conduction Effect of Different Market Links in China’s Egg Industry. J. Agro-For. Econ. Manag. 2018, 17, 727–737. [Google Scholar]
- Skrypec, S.; Doh, H.; Whiteside, W.S. Effect of oxygen scavenging films and modified atmosphere on the color quality of hot-filled freestone peach puree. J. Food Process Eng. 2021, 44, e13681. [Google Scholar] [CrossRef]
- Kaynas, K.; Iftci, H.N. Effect of Controlled and Modified Atmosphere on Storage Quality of Cauliflower (Brassica oleracea L. var. botrytis cv. Iglo) Heads. J. Agric. Fac. Gaziosmanpasa Univ. 2020, 37, 94–101. [Google Scholar]
- Pace, B.; Capotorto, I.; Palumbo, M.; Pelosi, S.; Cefola, M. Combined Effect of Dipping in Oxalic or in Citric Acid and Low O2 Modified Atmosphere, to Preserve the Quality of Fresh-Cut Lettuce during Storage. Foods 2020, 9, 988. [Google Scholar] [CrossRef] [PubMed]
- Chong, K.L.; Peng, N.; Yin, H.; Lipscomb, G.G.; Chung, T.S. Food sustainability by designing and modelling a membrane-controlled atmosphere storage system. J. Food Eng. 2013, 114, 361–374. [Google Scholar] [CrossRef]
- Teixeira, G.; Durigan, J.F. Storage of ‘Palmer’ mangoes in low-oxygen atmospheres. Fruits 2011, 66, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Zhang, Y.; Ma, Y.; Zhang, L.; Wang, D. Inhibitory mechanism of low-oxygen-storage treatment in postharvest internal bluing of radish (Raphanus sativus) roots. Food Chem. 2021, 364, 130423. [Google Scholar] [CrossRef]
- Zhang, X.H. Effects of Low Oxygen on Postharvest Energy Metabolism and Aroma Biosynthesis in Strawberry Fruits. Master’s Thesis, Bohai University, Jinzhou, China, 2020. [Google Scholar]
- Rocculi, P.; Cocci, E.; Sirri, F.; Cevoli, C.; Romani, S.; Rosa, M.D. Modified atmosphere packaging of hen table eggs, effects on functional properties of albumen. Poult. Sci. 2011, 90, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.B.; Murdock, L.L.; Dieudonne, B.; Wei, W. Storage of Maize in Purdue Improved Crop Storage (PICS) Bags. PLoS ONE 2017, 12, e0168624. [Google Scholar] [CrossRef]
- Xu, L.; Jia, F.; Luo, C.Y.; Zhao, F.H.; Gao, F.; Liu, Y.; Dai, D.T.; Li, X.M. Using low-field nuclear magnetic resonance technology to study the changes of egg white moisture in CO2 storage. Sci. Tech. Food Ind. 2017, 38, 6. [Google Scholar]
- Shao, Z.H.; Li, X.M. Comparative analysis of the effects of modified atmosphere packaging, cleaning coating and ultraviolet sterilization treatment on egg quality. Food Sci. Tech. 2019, 44, 73–79. [Google Scholar]
- Yuan, X.L.; Gao, J.X.; Du, Y.; Yao, X.J.; Chen, F.; Liu, Y.; Wang, H.W.; Li, X.M. Effects of Different Balanced Gases on the Fresh-keeping Quality of Eggs in Controlled Atmosphere Storage. Sci. Tech. Food Ind. 2014, 35, 303–307. [Google Scholar]
- Pasquali, F.; Manfreda, G.; Olivi, P.; Rocculi, P.; Sirri, F.; Meluzzi, A. Modified-atmosphere packaging of hen table eggs: Effects on pathogen and spoilage bacteria. Poult. Sci. 2012, 91, 3253–3259. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Wang, Q.H.; Zhu, Z.H.; Ma, Y.X. Correlation between Egg Internal Quality and Respiration Intensity. Food Sci. 2018, 39, 36–40. [Google Scholar]
- Wang, J.J.; Wang, Q.H.; Ma, M.H.; Wang, B. Correlation Between Egg External Quality and Respiration Intensity. Food Sci. 2018, 39, 42–46. [Google Scholar]
- Wang, Q.H.; Zhang, T.; Ma, M.H. Oxygen breathing regularity of eggs in storage period monitoring by non-invasive micro-test technique. Trans. Chin. Soc. Agric. Eng. 2014, 30, 255–261. [Google Scholar]
- Ma, Y.X.; Wang, Q.H.; Wang, X.B.; Tian, Y.N. Effect of Concentration of Carbon Dioxide on Respiration Intensity and Quality of Eggs. Chin. J. Anal. Chem. 2020, 48, 1728–1736. [Google Scholar]
- Haugh, R. The Haugh unit for measuring egg quality. Us Egg Poult. Mag. 1937, 43, 552–573. [Google Scholar]
- Curtis, A.C.; Anderson, E.A.; Musgrove, M.T. The effects of commercial cool water washing of shell eggs on Haugh unit, vitelline membrane strength, aerobic microorganisms, and fungi. Poult. Sci. 2010, 89, 160–168. [Google Scholar]
- Lalmi, D.; Benseddik, A.; Bensaha, H.; Bouzaher, M.T.; Arrif, T.; Guermoui, M.; Rabehi, A. Evaluation and estimation of the inside greenhouse temperature, numerical study with thermal and optical aspect. Int. J. Ambient. Energy 2021, 42, 1269–1280. [Google Scholar] [CrossRef]
- Yang, Q.Y. The Study of Thermal and Humid Environment Characteristics of Controlled Atmosphere (CA) Storage. Master’s Thesis, Xi’an University of Architecture and Technology, Xi’an, China, 2016. [Google Scholar]
- Zhou, B. Variation and Distribution of Species Concentration in CA Storage during Reduction Process of Oxygen. Master’s Thesis, Xi’an University of Architecture and Technology, Xi’an, China, 2015. [Google Scholar]
- Duncan, D.B. Multiple Range and Multiple F Tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Ezhilarasan, N.; Vijayalakshmi, C. Optimization of Fuzzy programming with TOPSIS Algorithm. Procedia Comput. Sci. 2020, 172, 473–479. [Google Scholar] [CrossRef]
- Tabidi, M.H. Impact of storage period and quality on composition of table egg. J. Environ. Biol. 2011, 5, 856–861. [Google Scholar]
- Nematinia, E.; Mehdizadeh, S.A. Assessment of Egg Freshness by Prediction of Haugh Unit and Albumen pH Using an Artificial Neural Network. J. Food Meas. Charact. 2018, 12, 1449–1459. [Google Scholar] [CrossRef]
- Fu, D.D.; Wang, Q.H. Predictive Models for the Detection of Egg Freshness, Acidity and Viscosity Using Hyper-Spectral Imaging. Food Sci. 2016, 37, 173–179. [Google Scholar]
- Putnik, P.; Kovačević, D.B.; Herceg, K.; Levaj, B. Influence of Respiration on Predictive Microbial Growth of Aerobic Mesophilic Bacteria and Enterobacteriaceae in Fresh-Cut Apples Packaged Under Modified Atmosphere. J. Food Saf. 2017, 37, e12284. [Google Scholar] [CrossRef]
- Abdelkrim, A.; Lynda, M.; Youcef, H. Dealing with value constraints in decision making using MCDM methods. J. Comput. Sci. 2020, 44, 101154. [Google Scholar]
- Han, S.H.; Han, D.J.; Lee, S.J. Modeling respiration rates of Ipomoea batatas (sweet potato) under hermetic storage system. Food Sci. Biotechnol. 2020, 29, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Kader, A.A. Biochemical and physiological basis for effects of controlled and modified atmospheres on fruits and vegetables. Food Technol. 1986, 40, 99–104. [Google Scholar]
- Omana, D.A.; Liang, Y.; Kav, N.V.; Wu, J. Proteomic analysis of egg white proteins during storage. Proteomics 2011, 11, 144–153. [Google Scholar] [CrossRef]
- Qiu, N.; Ma, M.; Zhao, L.; Liu, W.; Li, Y.Q. Comparative Proteomic Analysis of Egg White Proteins under Various Storage Temperatures. J. Agric. Food Chem. 2012, 60, 7746–7753. [Google Scholar] [CrossRef]
- Saenmuang, S.; Al-Haq, M.I.; Samarakoon, H.C.; Makino, Y.; Kawagoe, Y.; Oshita, S. Evaluation of Models for Spinach Respiratory Metabolism Under Low Oxygen Atmospheres. Food Bioprocess Technol. 2012, 5, 1950–1962. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Samapundo, S.; Pothakos, V.; Sürengil, G.; Devlieghere, F. Effect of high oxygen and high carbon dioxide atmosphere packaging on the microbial spoilage and shelf-life of fresh-cut honeydew melon. Int. J. Food Microbiol. 2013, 166, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Keuschnig, C.; Gorfer, M.; Li, G.; Mania, D.; Frostegrd, S.; Bakken, L.; Larose, C. NO and N2O transformations of diverse fungi in hypoxia, evidence for anaerobic respiration only in Fusarium strains. Environ. Microbiol. 2020, 22, 2182–2195. [Google Scholar] [CrossRef]
- Kawasaki, S.; Suzuki, I.; Niimura, Y. Oxygen adaptation mechanism of anaerobic bacteria Microbial ecology under 0–21% oxygen concentrations. Jpn. J. Lact. Acid Bact. 2013, 24, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Nassos, P.S.; Jr, K.; Stafford, A.E. Lactic Acid Concentration and Microbial Spoilage in Anaerobically and Aerobically Stored Ground Beef. J. Food Sci. 2010, 50, 710–712. [Google Scholar] [CrossRef]
- Talasila, P.C.; Chau, K.V.; Brecht, J.K. Design of rigid modified atmosphere packages for fresh fruits and vegetables. J. Food Sci. 1995, 60, 758–769. [Google Scholar] [CrossRef]
- Pretel, M.T.; Souty, M.; Romojaro, F. Use of passive and active modified atmosphere packaging to prolong the postharvest life of three varieties of apricot (Prunus armeniaca, L.). Eur. Food Res. Tech. 2000, 211, 191–198. [Google Scholar] [CrossRef]
| Group | A (0% O2) | B (5% O2) | C (10% O2) | D (15% O2) | E (20% O2) |
|---|---|---|---|---|---|
| Score | 0.022 | 0.025 | 0.021 | 0.018 | 0.011 |
| Sorting | 2 | 1 | 3 | 4 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Wang, X.; Tian, Y.; Wang, Q. Effect of a Hypoxia-Controlled Atmosphere Box on Egg Respiration Intensity and Quality. Appl. Sci. 2022, 12, 380. https://doi.org/10.3390/app12010380
Ma Y, Wang X, Tian Y, Wang Q. Effect of a Hypoxia-Controlled Atmosphere Box on Egg Respiration Intensity and Quality. Applied Sciences. 2022; 12(1):380. https://doi.org/10.3390/app12010380
Chicago/Turabian StyleMa, Yixiao, Xianbo Wang, Yaning Tian, and Qiaohua Wang. 2022. "Effect of a Hypoxia-Controlled Atmosphere Box on Egg Respiration Intensity and Quality" Applied Sciences 12, no. 1: 380. https://doi.org/10.3390/app12010380
APA StyleMa, Y., Wang, X., Tian, Y., & Wang, Q. (2022). Effect of a Hypoxia-Controlled Atmosphere Box on Egg Respiration Intensity and Quality. Applied Sciences, 12(1), 380. https://doi.org/10.3390/app12010380







