Microwave Synthesis, Characterization and Perspectives of Wood Pencil-Derived Carbon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microwave Synthesis
2.2. Activation Process
2.3. Characterization Techniques
2.4. Batch Experiments
2.5. Adsorption Kinetic Models
2.6. Gibbs Free Energy Calculation
3. Results and Discussion
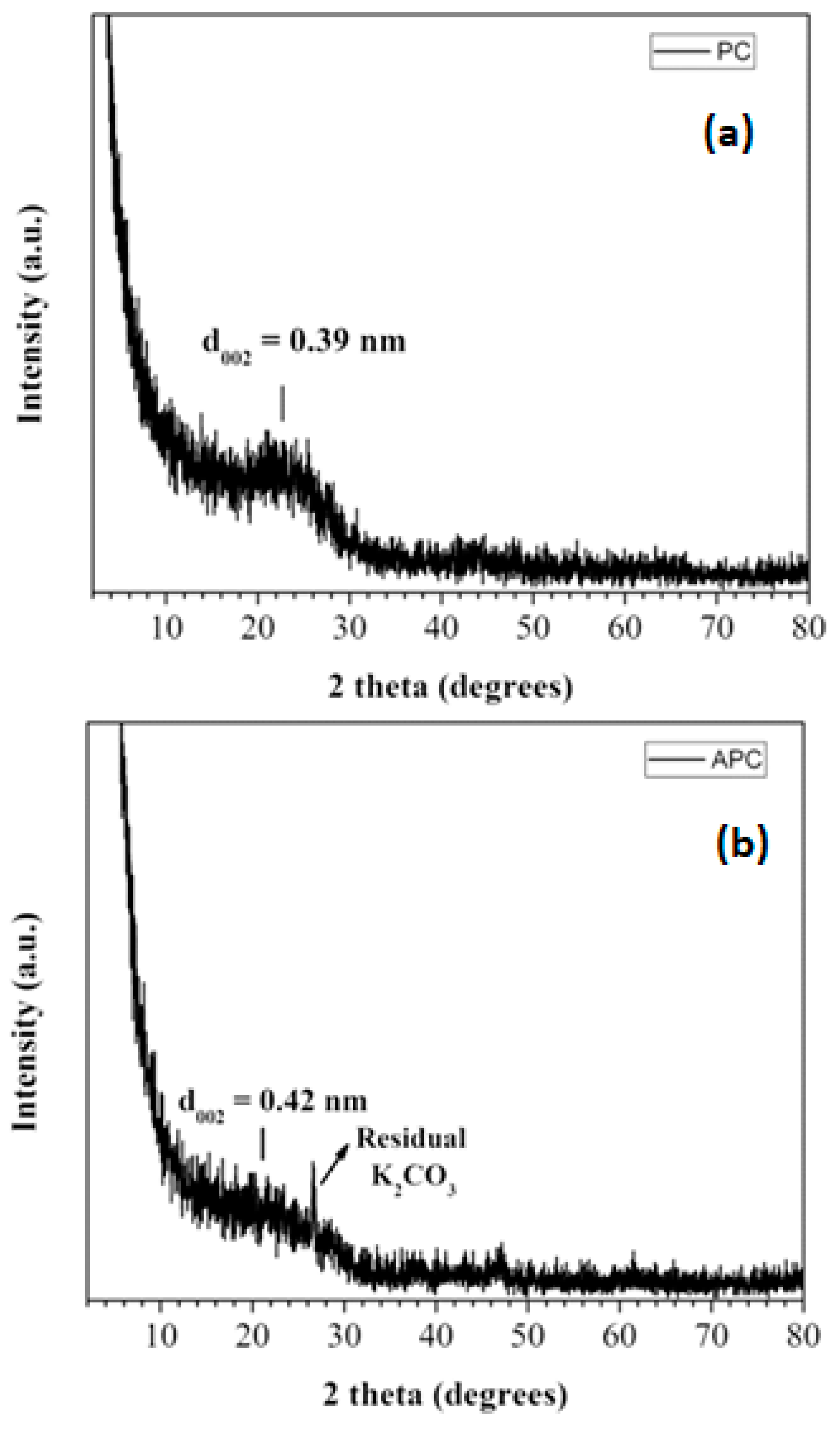
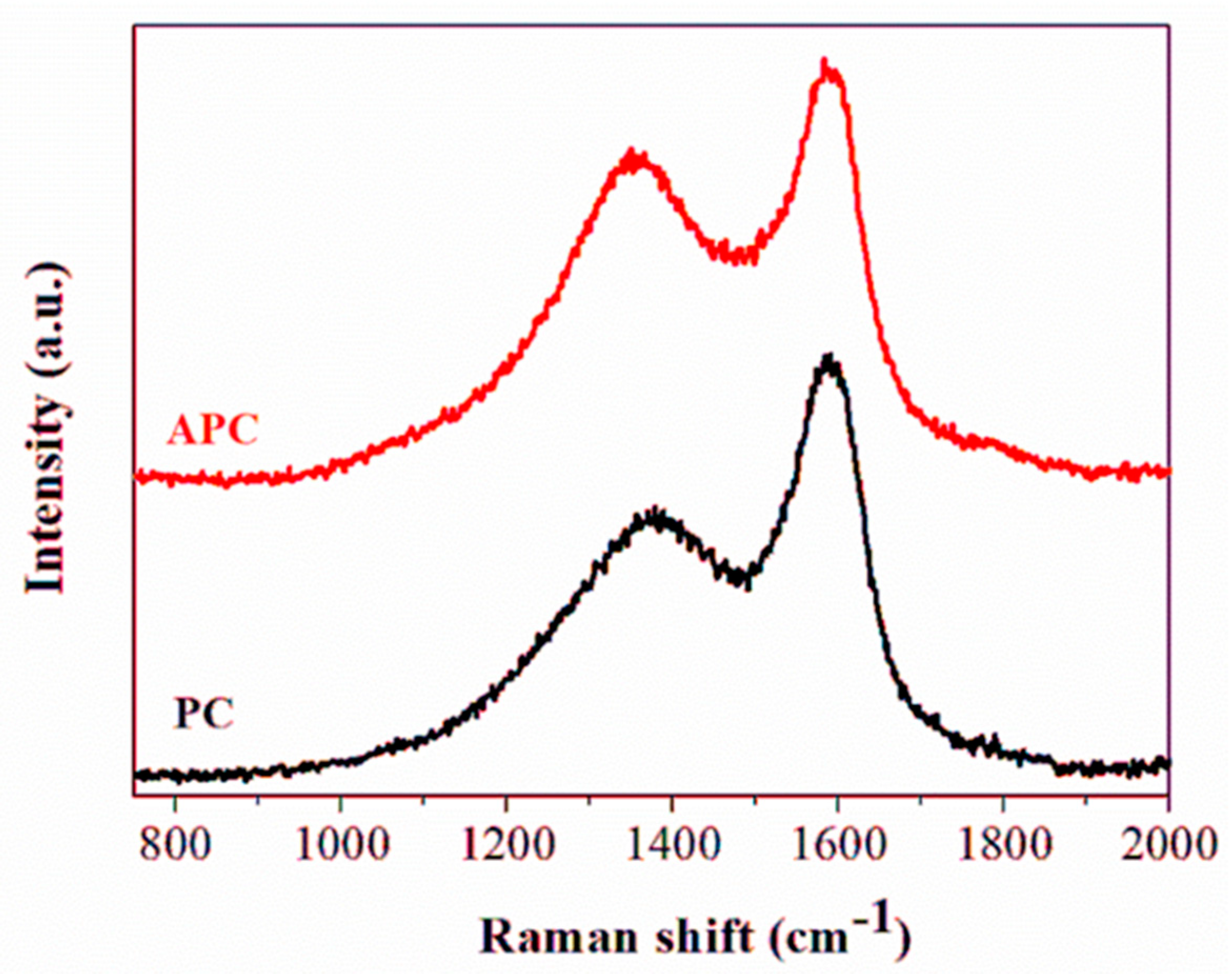
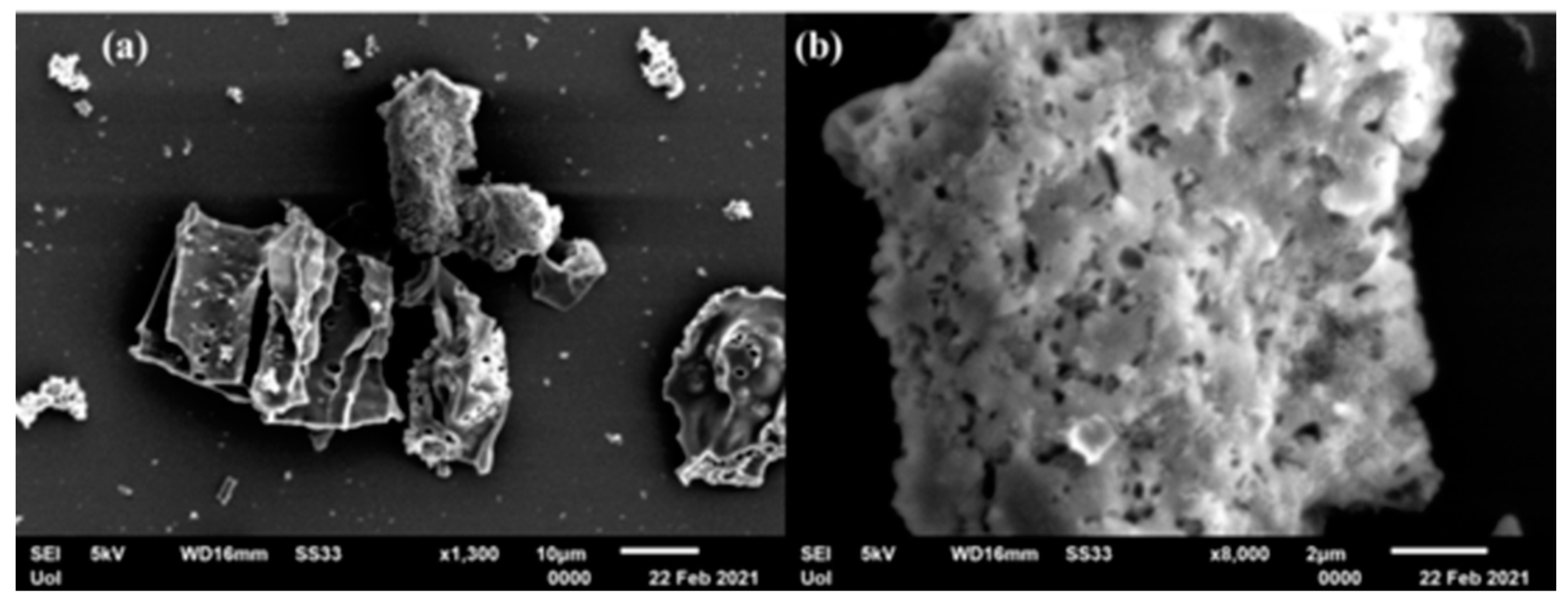
3.1. Structural Characterization and Morphology
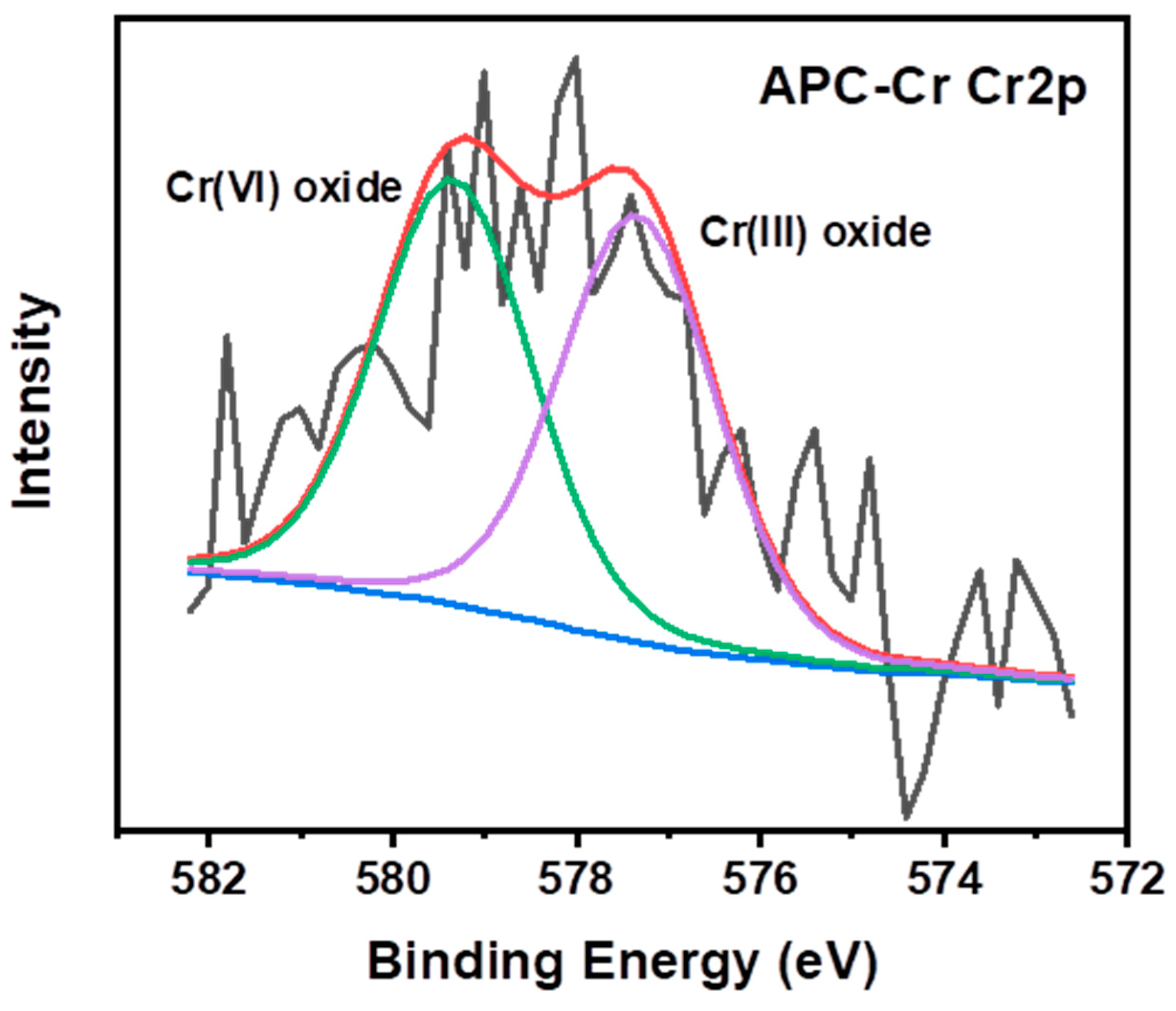
3.2. Practical Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Menéndez, J.A.; Arenillas, A.; Fidalgo, B.; Fernández, Y.; Zubizarreta, L.; Calvo, E.G.; Bermúdez, J.M. Microwave heating processes involving carbon materials. Fuel Process. Technol. 2010, 91, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekaran, S.; Basak, T.; Srinivasan, R. Microwave heating characteristics of graphite based powder mixtures. Int. Commun. Heat Mass Transf. 2013, 48, 22–27. [Google Scholar] [CrossRef]
- Kim, T.; Lee, J.; Lee, K.-H. Microwave heating carbon-based solid materials. Carbon Lett. 2014, 15, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Abioye, A.; Ani, F. Advancement in the production of activated carbon from biomass using microwave heating. J. Teknol. 2017, 79. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.; Shen, W. Carbon nanosheets: Synthesis and application. ChemSusChem 2015, 8, 2004–2027. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhuang, X.; Lei, C.; Lei, L.; Hou, Y.; Mai, Y.; Feng, X. Porous carbon nanosheets: Synthetic strategies and electrochemical energy related applications. Nano Today 2019, 24, 103–119. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Yang, H.M.; Zhang, D.H.; Chen, Y.; Ran, M.J.; Gu, J.C. Study on the application of KOH to produce activated carbon to realize the utilization of distiller’s grains. IOP Conf. Ser. Earth Environ. Sci. 2017, 69, 012051. [Google Scholar] [CrossRef] [Green Version]
- Androutsopoulos, G.P.; Salmas, C.E. A new model for capillary condensation−evaporation hysteresis based on a random corrugated pore structure concept: prediction of intrinsic pore size distributions. 1. Model formulation. Ind. Eng. Chem. Res. 2000, 39, 3747–3763. [Google Scholar] [CrossRef]
- Androutsopoulos, G.P.; Salmas, C.E. A new model for capillary condensation−evaporation hysteresis based on a random corrugated pore structure concept: prediction of intrinsic pore size distribution. 2. Model application. Ind. Eng. Chem. Res. 2000, 39, 3764–3777. [Google Scholar] [CrossRef]
- Salmas, C.E.; Androutsopoulos, G.P. Rigid sphere molecular model enables an assessment of the pore curvature effect upon realistic evaluations of surface areas of mesoporous and microporous materials. Langmuir 2005, 21, 11146–11160. [Google Scholar] [CrossRef]
- Salmas, C.E.; Ladavos, A.K.; Skaribas, S.P.; Pomonis, P.J.; Androutsopoulos, G.P. Evaluation of microporosity, pore tortuosity, and connectivity of montmorillonite solids pillared with LaNiOx binary oxide. A combined application of the CPSM model, the αs-plot method and a pore percolation–connectivity model. Langmuir 2003, 19, 8777–8786. [Google Scholar] [CrossRef]
- Salmas, C.E.; Androutsopoulos, G.P. A novel pore structure tortuosity concept based on nitrogen sorption hysteresis data. Ind. Eng. Chem. Res. 2001, 40, 721–730. [Google Scholar] [CrossRef]
- DOWNLOADS, CPSM_Nitrogen. Available online: http://users.uoi.gr/ksalmas/ (accessed on 28 June 2021).
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Horvath, G.; Kawazoe, K. Method for the calculation of effective pore size distribution in molecular sieve carbon. J. Chem. Eng. Jpn. 1983, 16, 470–475. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.G.; Yang, R.T. Theoretical basis for the potential theory adsorption isotherms. The Dubinin-Radushkevich and Dubinin-Astakhov equations. Langmuir 1994, 10, 4244–4249. [Google Scholar] [CrossRef]
- Clesceri, L.S.; Greenberg, A.E.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998; pp. 3–71. [Google Scholar]
- Asimakopoulos, G.; Baikousi, M.; Kostas, V.; Papantoniou, M.; Bourlinos, A.B.; Zbořil, R.; Karakassides, M.A.; Salmas, C.E. Nanoporous activated carbon derived via pyrolysis process of spent coffee: Structural characterization. Investigation of its use for hexavalent chromium removal. Appl. Sci. 2020, 10, 8812. [Google Scholar] [CrossRef]
- Asimakopoulos, G.; Baikousi, M.; Salmas, C.; Bourlinos, A.B.; Zboril, R.; Karakassides, M.A. Advanced Cr(VI) sorption properties of activated carbon produced via pyrolysis of the “Posidonia oceanica” seagrass. J. Hazard. Mater. 2021, 405, 124274. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.-S. Structural study of the activated carbon fiber using laser raman spectroscopy. Carbon Lett. 2008, 9, 127–130. [Google Scholar] [CrossRef]
- Deraman, M.; Sazali, N.E.S.; Hanappi, M.F.Y.M.; Tajuddin, N.S.M.; Hamdan, E.; Suleman, M.; Othman, M.A.R.; Omar, R.; Hashim, M.A.; Basri, N.H.; et al. Graphene/semicrystalline-carbon derived from amylose films for supercapacitor application. J. Phys. Conf. Ser. 2016, 739, 012085. [Google Scholar] [CrossRef] [Green Version]
- Molodets, A.M.; Golyshev, A.A. Structural transformations of amorphous carbon (glassy carbon) at high shock pressures. J. Exp. Theor. Phys. 2018, 126, 772–778. [Google Scholar] [CrossRef]
- Ren, S.; Deng, L.; Zhang, B.; Lei, Y.; Ren, H.; Lv, J.; Zhao, R.; Chen, X. Effect of air oxidation on texture, surface properties and dye adsorption of wood-derived porous carbon materials. Materials 2019, 12, 1675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-H.; Kelly, P.B.; Clifford, A.J. Biological/biomedical accelerator mass spectrometry targets. 2. Physical, morphological, and structural characteristics. Anal. Chem. 2008, 80, 7661–7669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsirka, K.; Katsiki, A.; Chalmpes, N.; Gournis, D.; Paipetis, A.S. Mapping of graphene oxide and single layer graphene flakes—defects annealing and healing. Front. Mater. 2018, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Chalmpes, N.; Asimakopoulos, G.; Spyrou, K.; Vasilopoulos, K.C.; Bourlinos, A.B.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Functional carbon materials derived through hypergolic reactions at ambient conditions. Nanomaterials 2020, 10, 566. [Google Scholar] [CrossRef] [Green Version]
- Baikousi, M.; Chalmpes, N.; Spyrou, K.; Bourlinos, A.B.; Avgeropoulos, A.; Gournis, D.; Karakassides, M.A. Direct production of carbon nanosheets by self-ignition of pyrophoric lithium dialkylamides in air. Mater. Lett. 2019, 254, 58–61. [Google Scholar] [CrossRef]
- Chalmpes, N.; Spyrou, K.; Bourlinos, A.B.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Synthesis of highly crystalline graphite from spontaneous ignition of in situ derived acetylene and chlorine at ambient conditions. Molecules 2020, 25, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dombrowski, R.J.; Lastoskie, C.M.; Hyduke, D.R. The Horvath–Kawazoe method revisited. Colloids Surf. A Physicochem. Eng. Asp. 2001, 187, 23–39. [Google Scholar] [CrossRef]
- Chalmpes, N.; Tantis, I.; Bakandritsos, A.; Bourlinos, A.B.; Karakassides, M.A.; Gournis, D. Rapid carbon formation from spontaneous reaction of ferrocene and liquid bromine at ambient conditions. Nanomaterials 2020, 10, 1564. [Google Scholar] [CrossRef]
- Chalmpes, N.; Moschovas, D.; Bourlinos, A.B.; Spyrou, K.; Vasilopoulos, K.C.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Hypergolic ignition of 1,3-cyclodienes by fuming nitric acid toward the fast and spontaneous formation of carbon nanosheets at ambient conditions. Micro 2021, 1, 15–27. [Google Scholar] [CrossRef]
- Chalmpes, N.; Bourlinos, A.B.; Talande, S.; Bakandritsos, A.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Nanocarbon from rocket fuel waste: The case of furfuryl alcohol-fuming nitric acid hypergolic pair. Nanomaterials 2021, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Ng, K.M.; Weng, L.-T.; Chan, C.-M. Characterization of hydrogenated graphite powder by X-ray photoelectron spectroscopy and time-of-flight secondary ion mass spectrometry. RSC Adv. 2016, 6, 80649–80654. [Google Scholar] [CrossRef]
- Chalmpes, N.; Moschovas, D.; Tantis, I.; Bourlinos, A.B.; Bakandritsos, A.; Fotiadou, R.; Patila, M.; Stamatis, H.; Avgeropoulos, A.; Karakassides, M.A.; et al. Carbon nanostructures derived through hypergolic reaction of conductive polymers with fuming nitric acid at ambient conditions. Molecules 2021, 26, 1595. [Google Scholar] [CrossRef] [PubMed]
- Chalmpes, N.; Bourlinos, A.B.; Šedajová, V.; Kupka, V.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Hypergolic materials synthesis through reaction of fuming nitric acid with certain cyclopentadienyl compounds. C 2020, 6, 61. [Google Scholar] [CrossRef]
- Baikousi, M.; Dimos, K.; Bourlinos, A.B.; Zbořil, R.; Papadas, I.; Deligiannakis, Y.; Karakassides, M.A. Surface decoration of carbon nanosheets with amino-functionalized organosilica nanoparticles. Appl. Surf. Sci. 2012, 258, 3703–3709. [Google Scholar] [CrossRef]
- Kumar, A.; Jena, H.M. Removal of methylene blue and phenol onto prepared activated carbon from Fox nutshell by chemical activation in batch and fixed-bed column. J. Clean. Prod. 2016, 137, 1246–1259. [Google Scholar] [CrossRef]
- Üner, O.; Geçgel, Ü.; Bayrak, Y. Adsorption of methylene blue by an efficient activated carbon prepared from citrullus lanatus rind: Kinetic, isotherm, thermodynamic, and mechanism analysis. Water Air Soil Pollut. 2016, 227, 247. [Google Scholar] [CrossRef]
- Naiman, K.; Dračínský, M.; Hodek, P.; Martínková, M.; Schmeiser, H.H.; Frei, E.; Stiborová, M. Formation, persistence, and identification of DNA adducts formed by the carcinogenic environmental pollutant o-anisidine in rats. Toxicol. Sci. 2012, 127, 348–359. [Google Scholar] [CrossRef] [Green Version]
- Casadevall, M.; da Cruz Fresco, P.; Kortenkamp, A. Chromium(VI)-mediated DNA damage: Oxidative pathways resulting in the formation of DNA breaks and abasic sites. Chem.-Biol. Interact. 1999, 123, 117–132. [Google Scholar] [CrossRef]
- Mitra, S.; Sarkar, A.; Sen, S. Removal of chromium from industrial effluents using nanotechnology: A review. Nanotechnol. Environ. Eng. 2017, 2, 11. [Google Scholar] [CrossRef]
- Pakade, V.E.; Tavengwa, N.T.; Madikizela, L.M. Recent advances in hexavalent chromium removal from aqueous solutions by adsorptive methods. RSC Adv. 2019, 9, 26142–26164. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Candela, M.; Martín-Martínez, J.; Torregrosa-Maciá, R. Chromium(VI) removal with activated carbons. Water Res. 1995, 29, 2174–2180. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U. Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water. J. Hazard. Mater. 2006, 137, 762–811. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, M.; Khan, S.; Ali Baig, S.; Munir, S.; Naz, A.; Ahmad, S.S.; Khan, A. Removal of potentially toxic elements from aqueous solutions and industrial wastewater using activated carbon. Water Sci. Technol. 2017, 75, 2571–2579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutherland, C.; Venkobachar, C. A diffusion-chemisorption kinetic model for simulating biosorption using forest macro-fungus, fomes fasciatus. Int. Res. J. Plant Sci. 2010, 1, 107–117. [Google Scholar]
- Hubbe, M.; Azizian, S.; Douven, S. Implications of apparent pseudo-second-prder adsorption kinetics onto cellulosic materials: A review. Bioresources 2019, 14, 7582–7626. [Google Scholar] [CrossRef]
- Perlach, J.R. Activated Carbon Adsorption for Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 1981; p. 260. [Google Scholar]
- Pholosi, A.; Naidoo, E.B.; Ofomaja, A.E. Intraparticle diffusion of Cr(VI) through biomass and magnetite coated biomass: A comparative kinetic and diffusion study. S. Afr. J. Chem. Eng. 2020, 32, 39–55. [Google Scholar] [CrossRef]
- Bandara, P.C.; Peña-Bahamonde, J.; Rodrigues, D.F. Redox mechanisms of conversion of Cr(VI) to Cr(III) by graphene oxide-polymer composite. Sci. Rep. 2020, 10, 9237. [Google Scholar] [CrossRef]
- Zhu, K.; Gao, Y.; Tan, X.; Chen, C. Polyaniline-modified Mg/Al layered double hydroxide composites and their application in efficient removal of Cr(VI). ACS Sustain. Chem. Eng. 2016, 4, 4361–4369. [Google Scholar] [CrossRef]




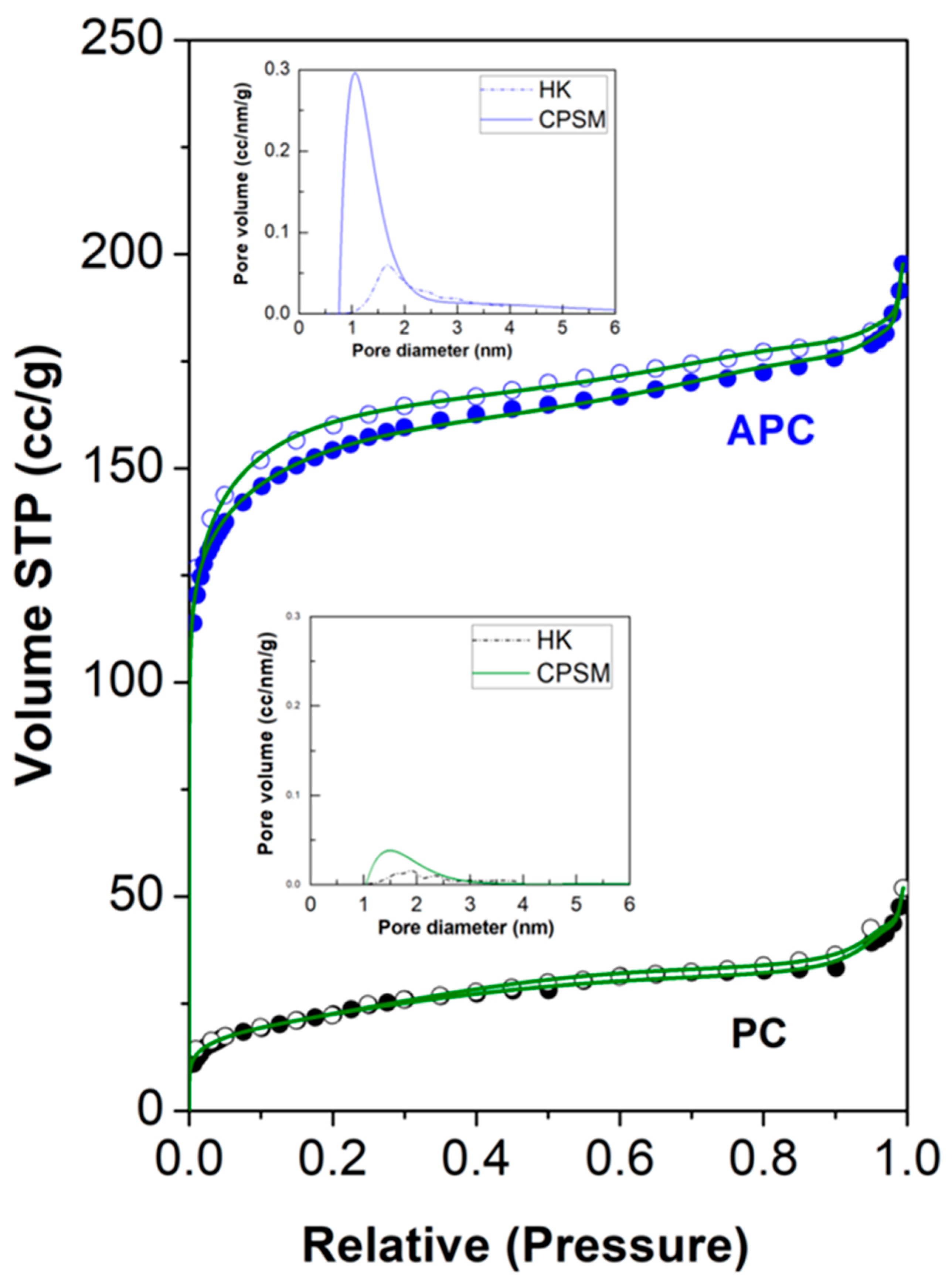
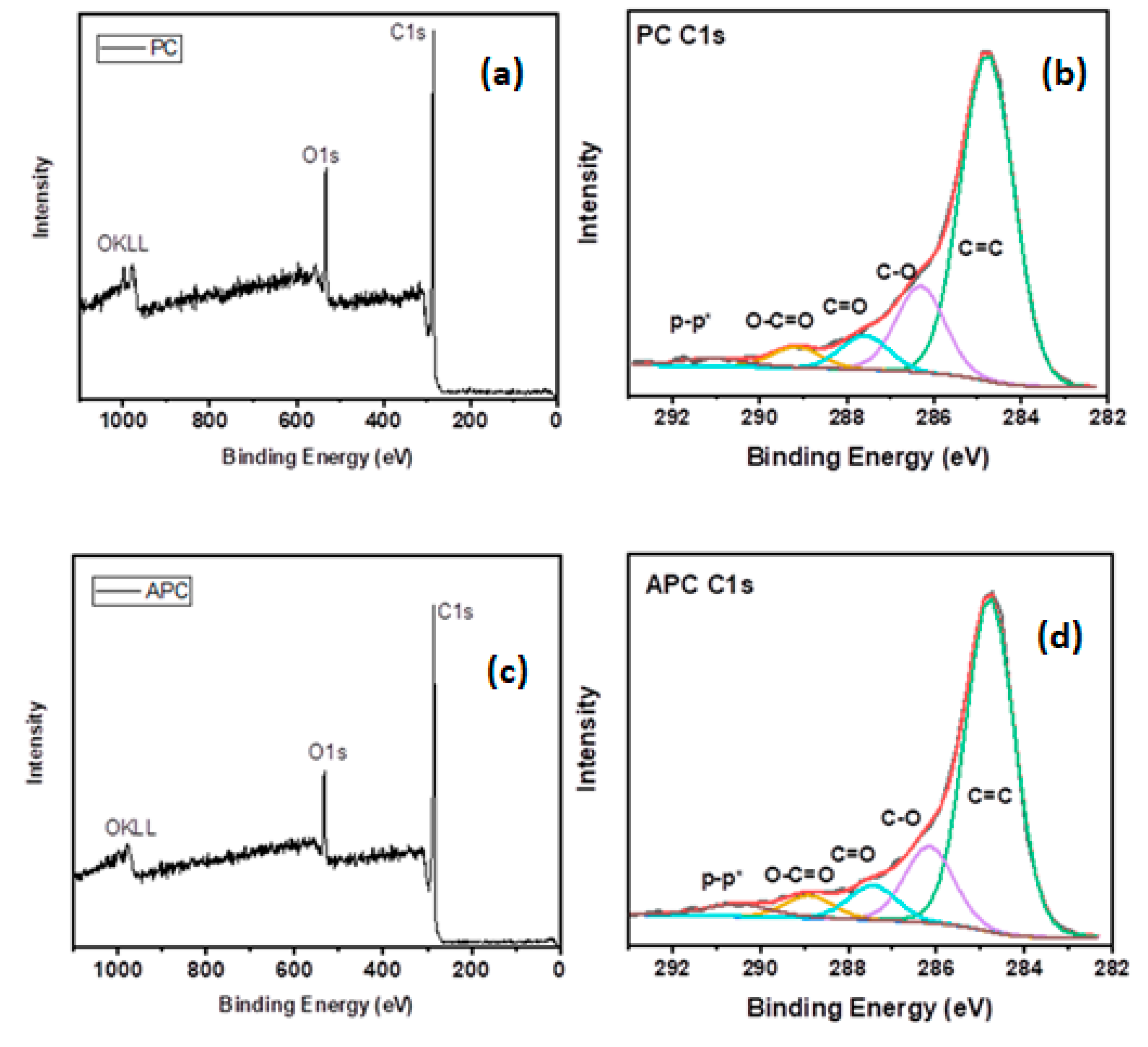
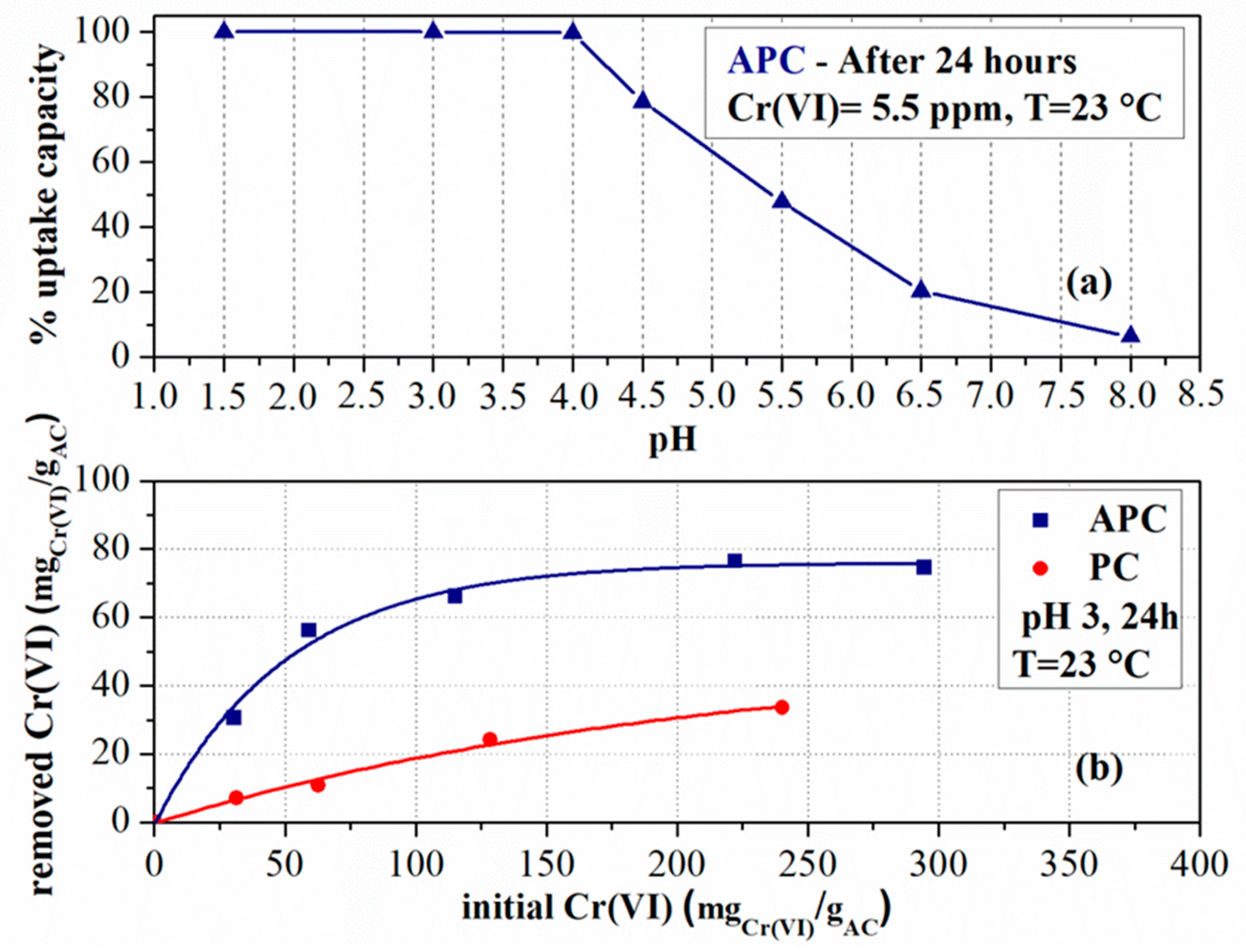
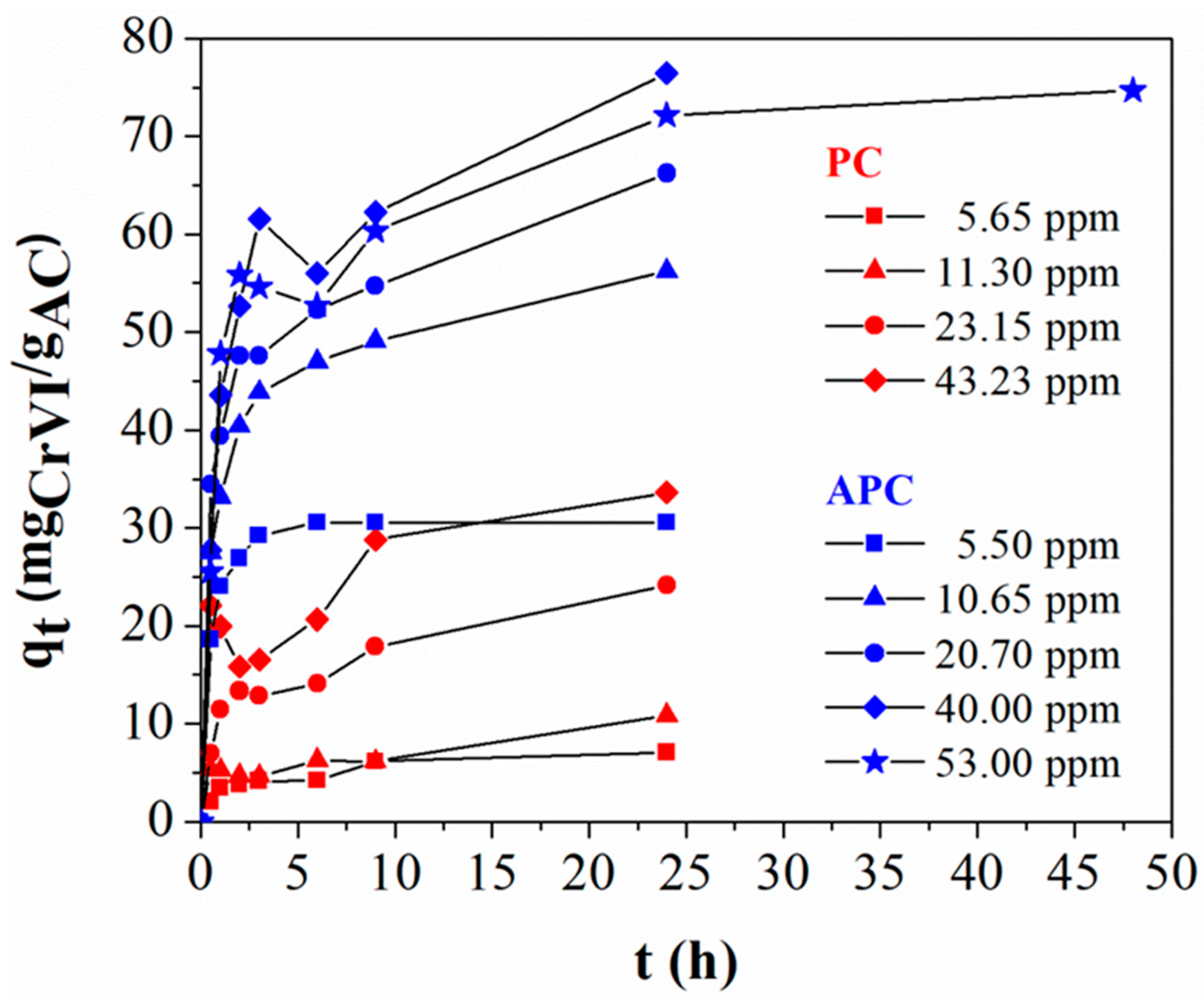
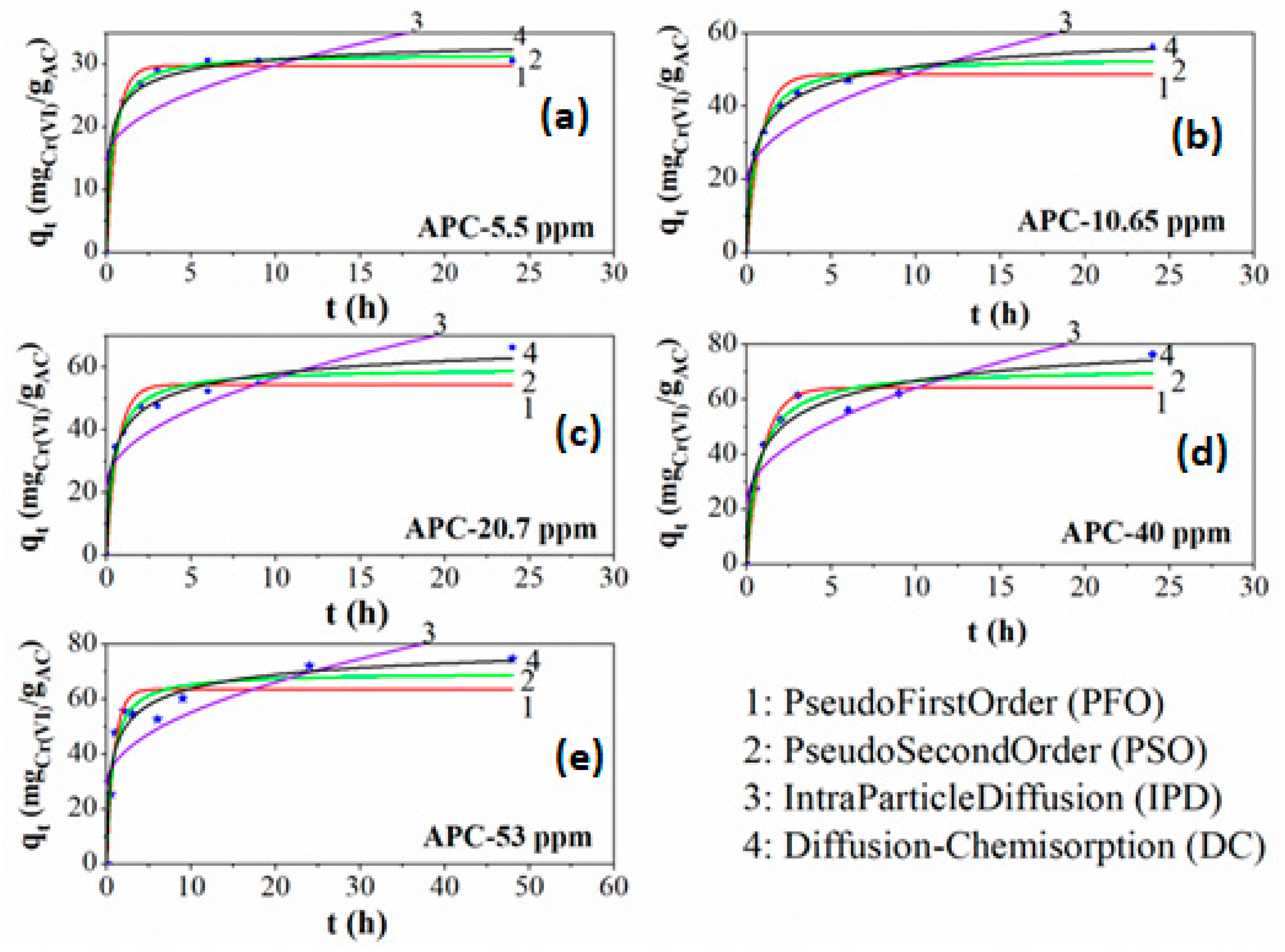
| Sample Code | Vpore (cm3/g) | VD–Rmicro (cm3/g) | VCPSMmicro (% cm3/g) | DCPSMNmean (nm) | DCPSMVmean (nm) | DHKVmean (nm) |
|---|---|---|---|---|---|---|
| PC | 0.080 | 0.034 | 0.030 | 1.38 | 1.50 | 1.90 |
| APC | 0.306 | 0.24 | 0.21 | 0.97 | 1.06 | 1.67 |
| Sample Code | SBET (m2/g) | SCPSM (m2/g) |
|---|---|---|
| PC | 82 | 105 |
| APC | 509 | 776 |
| APC-Cinit (ppm) | PFO R2 | PSO R2 | IPD R2 | DC R2 |
|---|---|---|---|---|
| 5.50 | 0.999 | 0.998 | 0.503 | 0.984 |
| 10.65 | 0.945 | 0.986 | 0.706 | 0.998 |
| 20.70 | 0.912 | 0.962 | 0.710 | 0.989 |
| 40.00 | 0.914 | 0.963 | 0.727 | 0.961 |
| 53.00 | 0.909 | 0.943 | 0.736 | 0.948 |
| APC-Cinit (ppm) | qe (mgCr(VI)/gAC) (PSO) | qe (mgCr(VI)/gAC) (DC) |
|---|---|---|
| 5.50 | 31.8 | 35.8 |
| 10.65 | 53.5 | 66.8 |
| 20.70 | 59.8 | 74.1 |
| 40.00 | 71.6 | 93.2 |
| 53.00 | 69.8 | 85.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalmpes, N.; Asimakopoulos, G.; Baikousi, M.; Salmas, C.E.; Moschovas, D.; Avgeropoulos, A.; Bourlinos, A.B.; Tantis, I.; Bakandritsos, A.; Gournis, D.; et al. Microwave Synthesis, Characterization and Perspectives of Wood Pencil-Derived Carbon. Appl. Sci. 2022, 12, 410. https://doi.org/10.3390/app12010410
Chalmpes N, Asimakopoulos G, Baikousi M, Salmas CE, Moschovas D, Avgeropoulos A, Bourlinos AB, Tantis I, Bakandritsos A, Gournis D, et al. Microwave Synthesis, Characterization and Perspectives of Wood Pencil-Derived Carbon. Applied Sciences. 2022; 12(1):410. https://doi.org/10.3390/app12010410
Chicago/Turabian StyleChalmpes, Nikolaos, Georgios Asimakopoulos, Maria Baikousi, Constantinos E. Salmas, Dimitrios Moschovas, Apostolos Avgeropoulos, Athanasios B. Bourlinos, Iosif Tantis, Aristides Bakandritsos, Dimitrios Gournis, and et al. 2022. "Microwave Synthesis, Characterization and Perspectives of Wood Pencil-Derived Carbon" Applied Sciences 12, no. 1: 410. https://doi.org/10.3390/app12010410








