Non-Centrifugal Sugar (NCS) and Health: A Review on Functional Components and Health Benefits
Abstract
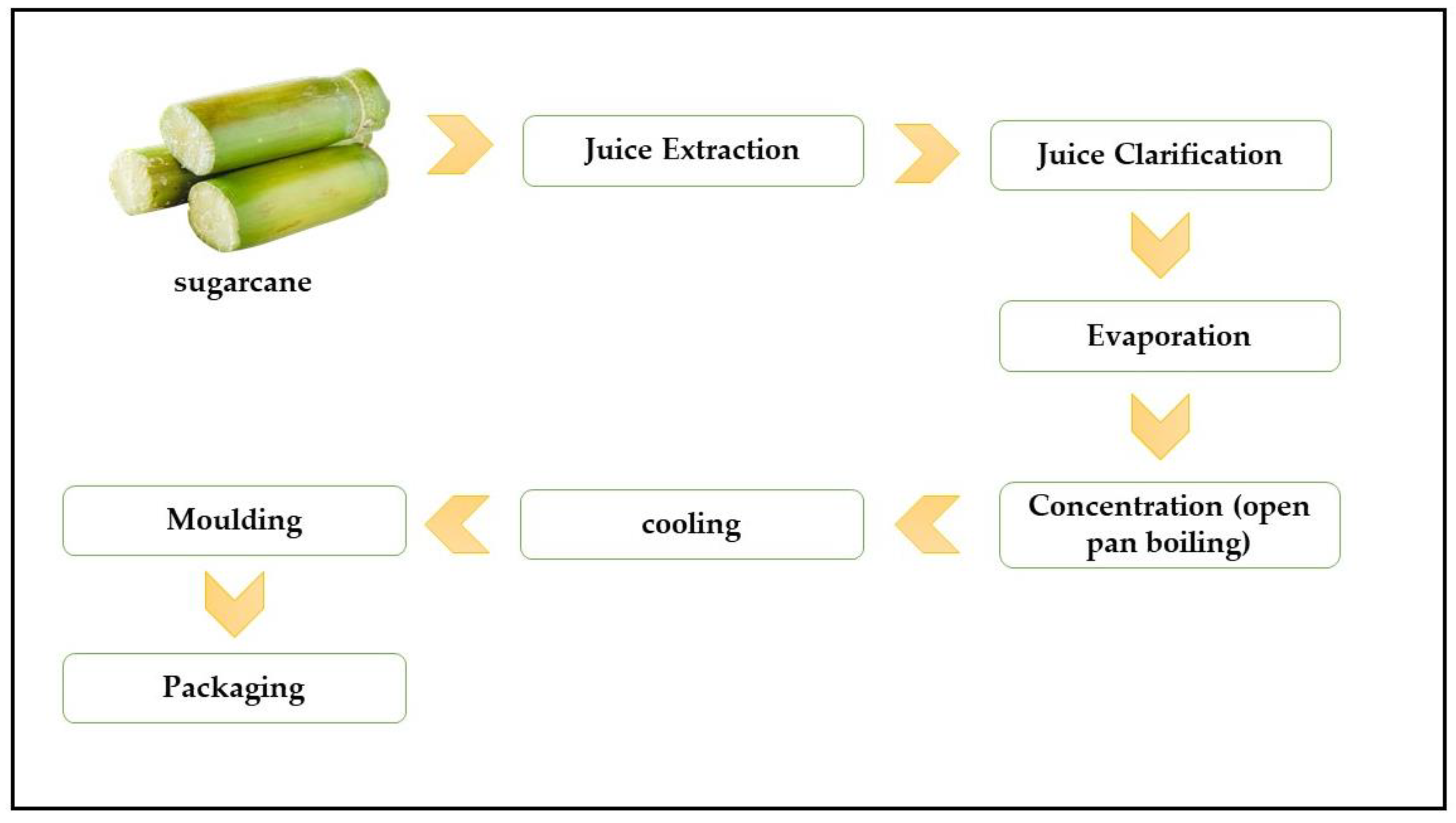
1. Introduction
2. Physical Properties
3. Chemical Composition
4. Phytochemicals Compounds
5. Type of Brown Sugar
6. Biological Properties of NCS
6.1. Antioxidant Properties
6.2. Anti-Inflammatory Properties
6.3. Non-Centrifugal Sugar and Metabolic Syndrome
6.3.1. Anti-Obesity Effect
6.3.2. Antidiabetic Effect
6.3.3. Hypolipidemic Effect of NCS
6.4. NCS and Neurodegeneration
7. Safety of NCS
8. Conclusions and Future Marks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Weerawatanakorn, M.; Asikin, Y.; Kamchonemenukool, S.; Tamaki, H.; Takara, K.; Wada, K. Physicochemical, antioxidant, volatile component, and mass spectrometry-based electronic nose analyses differentiated unrefined non-centrifugal cane, palm, and coconut sugars. J. Food Meas. Charact. 2021, 15, 1563–1577. [Google Scholar] [CrossRef]
- FAO. Definition and Classification of Commodities. Sugar Crops and Sweeteners and Derived Products. Available online: http://www.fao.org/es/faodef/fdef03e.HTM (accessed on 9 October 2021).
- WCO (World Customs Organization). HS Nomenclature 2012 Edition, Section 0417-2012E, Chapter 17, Sugar and Sugar Confectionery. Available online: https://www.wcoomd.org/en/faq/~/link.aspx?_id=3F9BB5F791484D45810FE0A5B9782E4C&_z=z (accessed on 9 October 2021).
- Liu, J.; Wan, P.; Xie, C.; Chen, D.W. Key aroma-active compounds in brown sugar and their influence on sweetness. Food Chem. 2021, 345, 128826. [Google Scholar] [CrossRef]
- Asikin, Y.; Kamiya, A.; Mizu, M.; Takara, K.; Tamaki, H.; Wada, K. Changes in the physicochemical characteristics, including flavour components and Maillard reaction products, of non-centrifugal cane brown sugar during storage. Food Chem. 2014, 149, 170–177. [Google Scholar] [CrossRef]
- Velásquez, F.; Espitia, J.; Mendieta, O.; Escobar, S.; Rodríguez, J. Non-centrifugal cane sugar processing: A review on recent advances and the influence of process variables on qualities attributes of final products. J. Food Eng. 2019, 255, 32–40. [Google Scholar] [CrossRef]
- Mohan, N.; Singh, P. (Eds.) Sugar and Sugar Derivatives: Changing Consumer Preferences; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Asikin, Y.; Takahara, W.; Takahashi, M.; Hirose, N.; Ito, S.; Wada, K. Compositional and Electronic Discrimination Analyses of Taste and Aroma Profiles of Non-Centrifugal Cane Brown Sugars. Food Anal. Methods 2017, 10, 1844–1856. [Google Scholar] [CrossRef]
- Asikin, Y.; Takahashi, M.; Hirose, N.; Hou, D.X.; Takara, K.; Wada, K. Wax, policosanol, and long-chain aldehydes of different sugarcane (Saccharum officinarum L.) cultivars. Eur. J. Lipid Sci. Technol. 2012, 114, 583–591. [Google Scholar] [CrossRef]
- Cai, H.; Steward, W.P.; Gescher, A.J. Determination of the putative cancer chemopreventive flavone tricin in plasma and tissues of mice by HPLC with UV-visible detection. Biomed. Chromatogr. 2005, 19, 518–522. [Google Scholar] [CrossRef]
- Colombo, R.; Yariwake, J.H.; Queiroz, E.F.; Ndjoko, K.; Hostettmann, K. On-line identification of sugarcane (Saccharum officinarum L.) methoxyflavones by liquid chromatography-UV detection using post-column derivatization and liquid chromatography-mass spectrometry. J. Chromatogr. A 2005, 1082, 51–59. [Google Scholar] [CrossRef]
- Duarte-almeida, J.M.; Salatino, A.; Inés, M.; Lajolo, F.M. Phenolic composition and antioxidant activity of culms and sugarcane (Saccharum officinarum L.) products. Food Chem. 2021, 125, 660–664. [Google Scholar] [CrossRef]
- Jaffé, W.R. Health Effects of Non-Centrifugal Sugar (NCS): A Review. Sugar Tech 2012, 14, 87–94. [Google Scholar] [CrossRef]
- Hirpara, P.; Thakare, N.; Patel, D.; Kele, V.D. Jaggery: A natural sweetener. J. Pharmacogn. Phytochem. 2020, 9, 3145–3148. Available online: https://www.phytojournal.com (accessed on 21 December 2021).
- Okabe, T.; Toda, T.; Inafuku, M.; Wada, K.; Iwasaki, H.; Oku, H. Antiatherosclerotic function of Kokuto, Okinawan non-centrifugal cane sugar. J. Agric. Food Chem. 2009, 57, 69–75. [Google Scholar] [CrossRef]
- Harish Nayaka, M.A.; Sathisha, U.V.; Manohar, M.P.; Chandrashekar, K.B.; Dharmesh, S.M. Cytoprotective and antioxidant activity studies of jaggery sugar. Food Chem. 2009, 115, 113–118. [Google Scholar] [CrossRef]
- Seguí, L.; Calabuig-Jiménez, L.; Betoret, N.; Fito, P. Physicochemical and antioxidant properties of non-refined sugarcane alternatives to white sugar. Int. J. Food Sci. 2015, 50, 2579–2588. [Google Scholar] [CrossRef]
- Cifuentes, J.; Salazar, V.A.; Cuellar, M.; Castellanos, M.C.; Rodríguez, J.; Cruz, J.C.; Muñoz-Camargo, C. Antioxidant and neuroprotective properties of non-centrifugal cane sugar and other sugarcane derivatives in an in vitro induced parkinson’s model. Antioxidants 2021, 10, 1040. [Google Scholar] [CrossRef] [PubMed]
- Jaffé, W.R. Nutritional and functional components of non-centrifugal cane sugar: A compilation of the data from the analytical literature. J. Food Compost. Anal. 2015, 43, 194–202. [Google Scholar] [CrossRef]
- Lee, J.S.; Ramalingam, S.; Guk Jo, I.; Som Kwon, Y.; Bahuguna, A.; Sook Oh, Y.; Kwon, O.-J.; Kim, M. Comparative study of the physicochemical, nutritional, and antioxidant properties of some commercial refined and non-centrifugal sugars. Food Res. Int. 2019, 122, 563. [Google Scholar] [CrossRef] [PubMed]
- Meerod, K.; Weerawatanakorn, M.; Pansak, W. Effect of Liming Process on Physicochemical Properties and Phytochemical Components of Non-Centrifugal Sugar from Different Sugarcane Cultivars. Agric. Res. 2020, 9, 35–45. [Google Scholar] [CrossRef]
- Alarcón, A.L.; Palacios, L.M.; Osorio, C.; César Narváez, P.; Heredia, F.J.; Orjuela, A.; Hernanz, D. Chemical characteristics and colorimetric properties of non-centrifugal cane sugar (“panela”) obtained via different processing technologies. Food Chem. 2021, 340, 128183. [Google Scholar] [CrossRef]
- Azlan, A.; Khoo, H.E.; Sajak, A.A.B.; Aizan Abdul Kadir, N.A.; Yusof, B.N.M.; Mahmood, Z.; Sultana, S. Antioxidant activity, nutritional and physicochemical characteristics, and toxicity of minimally refined brown sugar and other sugars. Food Sci. Nutr. 2020, 8, 5048–5062. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to iron and contribution to normal formation of haemoglobin and red blood cells pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2014, 12, 3515. [Google Scholar] [CrossRef][Green Version]
- Fontenelle, L.C.; Feitosa, M.M.; Morais, J.B.S.; Severo, J.S.; de Freitas, T.E.C.; Beserra, J.B.; Henriques, G.S.; Marreiro, D.D.N. The role of selenium in insulin resistance. Braz. J. Pharm. Sci. 2018, 54, 1–11. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Vo, T.S. An Updated Review on Pharmaceutical Properties of Gamma-Aminobutyric Acid. Molecules 2019, 24, 2678. [Google Scholar] [CrossRef]
- Funane, K.; Tokashiki, T.; Gibu, S.; Kawabata, Y.; Oguma, T.; Ito, H.; Nakachi, M.; Miyagi, S.; Kobayashi, M. Finding of Cyclodextrans and Attempts of their Industrialization for Cariostatic Oligosaccharides. J. Appl. Glycosci. 2007, 54, 103–107. [Google Scholar] [CrossRef]
- Oshima, H.; Kimura, I.; Izumori, K. Psicose contents in various food products and its origin. Food Sci. Technol. 2006, 12, 137–143. [Google Scholar] [CrossRef]
- Weerawatanakorn, M.; Asikin, Y.; Takahashi, M.; Tamaki, H.; Wada, K.; Ho, C.T.; Chuekittisak, R. Physico-chemical properties, wax composition, aroma profiles, and antioxidant activity of granulated non-centrifugal sugars from sugarcane cultivars of Thailand. J. Food Sci. Technol. 2016, 53, 4084–4092. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Barrera, C.; Betoret, N.; Seguí, L. Phenolic Profile of Cane Sugar Derivatives Exhibiting Antioxidant and Antibacterial Properties. Sugar Tech 2020, 22, 798–811. [Google Scholar] [CrossRef]
- Eggleston, G.; Aita, G. Exploration of sugarcane products as a major source of antioxidant phenolic extracts in commercial foods and beverages. Int. Sugar J. 2020, 126, 690–696. [Google Scholar]
- Shang, Y.; Xie, C.; Meng, L.; Cui, F.; Lu, H.; Li, W.; Li, K. Polyphenol Profiles and Antioxidant Activities of Non-centrifugal Sugars Derived from Different Varieties of Membrane-Clarified Sugarcane Juice. Sugar Tech 2021, 23, 1–12. [Google Scholar] [CrossRef]
- Zhu, Z.; Xie, C.; Li, W.; Hang, F.; Li, K.; Shi, C.; Doherty, W.O.S. Nutritional and antioxidant properties of non-centrifugal cane sugar derived from membrane clarified juice. LWT 2020, 131, 109717. [Google Scholar] [CrossRef]
- Kinjo, Y.; Takahashi, M.; Hirose, N.; Mizu, M.; Hou, D.X.; Wada, K. Anti-stress and antioxidant effects of non- centrifuged cane sugar, Kokuto, in restraint-stressed mice. J. Oleo Sci. 2019, 68, 183–191. [Google Scholar] [CrossRef]
- Eggleston, G.; Aita, G.; Triplett, A. Circular Sustainability of Sugarcane: Natural, Nutritious, and Functional Unrefined Sweeteners That Meet New Consumer Demands. Sugar Tech 2021, 23, 964–973. [Google Scholar] [CrossRef]
- Eggleston, G. Positive aspects of cane sugar and sugar cane derived products in food and nutrition. J. Agric. Food Chem. 2018, 66, 4007–4012. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.M.; Carlsen, M.H.; Blomhoff, R. Total antioxidant content of alternatives to refined sugar. J. Am. Diet. Assoc. 2009, 109, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Azlan, A.; Ebadi, S.; Yusof, B.N.M.; Othman, N.M.H.; Kannar, D.; Sultana, S.; Mahmood, Z. Determination of Satiety, Glycaemic Profiles, Total Antioxidant Capacity and Postprandial Glycemic Responses of Minimally Refined Sugar and Others in Malaysian Healthy Adults. Nutrition, 2021; in press. [Google Scholar] [CrossRef]
- Salgo, A.; Nagy, J.; Mikó, É.; Boros, I. Application of near infrared spectroscopy in the sugar industry. J. Near Infrared Spectrosc. 1998, 6, A101–A106. [Google Scholar] [CrossRef]
- Harbeck, C.; Faurie, R.; Scheper, T. Application of near-infrared spectroscopy in the sugar industry for the detection of betaine. Anal. Chim. Acta 2004, 501, 249–253. [Google Scholar] [CrossRef]
- Gajdoš Kljusurić, J.; Mihalev, K.; Bečić, I.; Polović, I.; Georgieva, M.; Djaković, S.; Kurtanjek, Ž. Near-infrared spectroscopic analysis of total phenolic content and antioxidant activity of berry fruits. Food Technol. Biotechnol. 2016, 54, 236–242. [Google Scholar] [CrossRef]
- Closed Loop Sugar Processing. Available online: https://shop.exchange.se.com/en-US/apps/60696/closed-loop-sugar-processing (accessed on 21 December 2021).
- Roberts, C.K.; Sindhu, K.K. Oxidative stress and metabolic syndrome. Life Sci. 2009, 84, 705–712. [Google Scholar] [CrossRef]
- Iqbal, M.; Afzal Qamar, M.; Bokhari, T.H.; Abbas, M.; Hussain, F.; Masood, N.; Keshavarzi, A.; Qureshi, N.; Nazir, A. Total phenolic, chromium contents and antioxidant activity of raw and processed sugars. Inf. Processing Agric. 2017, 4, 83–89. [Google Scholar] [CrossRef]
- Ranilla, L.G.; Kwon, Y.I.; Genovese, M.I.; Lajolo, F.M.; Shetty, K. Antidiabetes and antihypertension potential of commonly consumed carbohydrate sweeteners using in vitro models. J. Med. Food 2008, 11, 337–348. [Google Scholar] [CrossRef]
- Singh, N.; Kumar, D.; Lal, K.; Raisuddin, S.; Sahu, A.P. Adverse health effects due to arsenic exposure: Modification by dietary supplementation of jaggery in mice. Toxicol. Appl. Pharmacol. 2010, 242, 247–255. [Google Scholar] [CrossRef]
- Grabek-Lejko, D.; Tomczyk-Ulanowska, K. Phenolic content, antioxidant and antibacterial activity of selected natural sweeteners available on the Polish market. J. Environ. Sci. Health B 2013, 48, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tapia, M.; Martínez-Medina, J.; Tovar, A.R.; Torres, N. Natural and artificial sweeteners and high fat diet modify differential taste receptors, insulin, and TLR4-mediated inflammatory pathways in adipose tissues of rats. Nutrients 2019, 11, 880. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.M.; Calder, P.C. Obesity, inflammation, toll-like receptor 4 and fatty acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tapia, M.; Miller, A.W.; Granados-Portillo, O.; Tovar, A.R.; Torres, N. The development of metabolic endotoxemia is dependent on the type of sweetener and the presence of saturated fat in the diet. Gut Microbes 2020, 12, 1801301. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A consensus statement from the international diabetes federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Shamsi-Goushki, A.; Mortazavi, Z.; Mirshekar, M.A.; Behrasi, F.; Moradi-Kor, N.; Taghvaeefar, R. Effects of high white and brown sugar consumption on serum level of brain-derived neurotrophic factor, insulin resistance, and body weight in albino rats. J. Obes. Metab. Syndr. 2021, 29, 320–324. [Google Scholar] [CrossRef]
- Burgeiro, A.; Cerqueira, M.G.; Varela-Rodríguez, B.M.; Nunes, S.; Neto, P.; Pereira, F.C.; Reis, F.; Carvalho, E. Glucose and Lipid Dysmetabolism in a Rat Model of Prediabetes Induced by a High-Sucrose Diet. Nutrients 2017, 9, 638. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.; Madhu, S.; Makkar, B.; Ghosh, S.; Saboo, B.; Kalra, S. RSSDI-ESI clinical practice recommendations for the management of type 2 diabetes mellitus 2020. Indian J. Endocrinol. Metab. 2020, 24, 1–122. [Google Scholar] [CrossRef]
- Ramli, N.Z.; Chin, K.Y.; Zarkasi, K.A.; Ahmad, F. A review on the protective effects of honey against metabolic syndrome. Nutrients 2018, 10, 1009. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ito, Y.; Taga, A. Comparison of the enhancement of plasma glucose levels in type 2 diabetes Otsuka Long-Evans Tokushima Fatty rats by oral administration of sucrose or maple syrup. J. Oleo Sci. 2013, 62, 737–743. [Google Scholar] [CrossRef]
- Mejia, E.; Pearlman, M. Natural Alternative Sweeteners and Diabetes Management. Curr. Diab. Rep. 2019, 19, 142. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Okuda, H.; Arichi, S. Effects on non-sugar fraction in black sugar on lipid and carbohydrate metabolism; Part I. Planta Med. 1984, 50, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Rozanska, O.; Uruska, A.; Zozulinska-Ziolkiewicz, D. Brain-derived neurotrophic factor and diabetes. Int. J. Mol. Sci. 2020, 21, 841. [Google Scholar] [CrossRef]
- Ait-Omar, A.; Monteiro-Sepulveda, M.; Poitou, C.; Le Gall, M.; Cotillard, A.; Gilet, J.; Garbin, K.; Houllier, A.; Château, D.; Lacombe, A.; et al. GLUT2 accumulation in enterocyte apical and intracellular membranes: A study in morbidly obese human subjects and ob/ob and high fat-fed mice. Diabetes 2011, 60, 2598–2607. [Google Scholar] [CrossRef]
- Leguisamo, N.M.; Lehnen, A.M.; Machado, U.F.; Okamoto, M.M.; Markoski, M.M.; Pinto, G.H.; Schaan, B.D. GLUT4 content decreases along with insulin resistance and high levels of inflammatory markers in rats with metabolic syndrome. Cardiovasc. Diabetol. 2012, 11, 100. [Google Scholar] [CrossRef]
- Anjum, A. Comparative Effects of Cane Sugar, Honey & Jaggery on Plasma Glucose Level & Body Weight of Alloxan Induced Diabetic Rats. Proc. Shaikh Zayed Med. Complex Lahore 2021, 35, 44–49. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Diwedi, V.; Bhardwaj, Y. In vitro α -amylase and α-glucosidase inhibitory potential of Trigonella foenum-graecum leaves extract. Ayu 2013, 34, 109. [Google Scholar] [CrossRef]
- Cefalu, W.T.; Hu, F.B. Role of Chromium in Human Health and in Diabetes. Diabetes Care 2004, 27, 2741–2751. [Google Scholar] [CrossRef]
- Hummel, M.; Standl, E.; Schnell, O. Chromium in metabolic and cardiovascular disease. Horm. Metab. Res. 2007, 39, 743–751. [Google Scholar] [CrossRef]
- Stapleton, S.R. Selenium: An insulin-mimetic. Cell. Mol. Life Sci. 2000, 57, 1874–1879. [Google Scholar] [CrossRef] [PubMed]
- Saibandith, B.; Spencer, J.P.E.; Rowland, I.R.; Commane, D.M. Olive Polyphenols and the Metabolic Syndrome. Molecules 2017, 22, 1082. [Google Scholar] [CrossRef] [PubMed]
- Inafuku, M.; Toda, T.; Okabe, T.; Wada, K.; Takara, K.; Iwasaki, H.; Oku, H. Effect of Kokuto, a non-centrifugal cane sugar, on the development of experimental atherosclerosis in Japanese quail and apolipoprotein E deficient mice. Food Sci. Technol. Res. 2007, 13, 61–66. [Google Scholar] [CrossRef][Green Version]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. Oxidative Stress in Autoimmune and Neurodegenerative Diseases. Role Oxidative Stress Neurodegener. Dis. 2015, 24, 325–340. [Google Scholar] [CrossRef]
- Sai, Y.; Wu, Q.; Le, W.; Ye, F.; Li, Y.; Dong, Z. Rotenone-induced PC12 cell toxicity is caused by oxidative stress resulting from altered dopamine metabolism. Toxicol. Vitr. 2008, 22, 1461–1468. [Google Scholar] [CrossRef]
- Cenini, G.; Lloret, A.; Cascella, R. Oxidative Stress in Neurodegenerative Diseases: From a Mitochondrial Point of View. Oxidative Med. Cell. Longev. 2019, 2019, 2105607. [Google Scholar] [CrossRef] [PubMed]
- Lasso, J.V.; Pérez, Y.T.; Suárez, F.T.; Caballero, L.C. Determinación de acrilamida en el procesamiento de la panela por cromatografía líquida (Acrylamide Determintion in the Sugar Cane Juice Process by the Liquid Chromatography Technique). Ciencia en Desarrollo 2014, 5, 99–106. [Google Scholar] [CrossRef]
- Virk-Baker, M.K.; Nagy, T.R.; Barnes, S.; Groopman, J. Dietary Acrylamide and Human Cancer: A Systematic Review of Literature. Nutr. Cancer 2014, 66, 774–790. [Google Scholar] [CrossRef]
- NTP (National Toxicology Program). Technical Report on the Toxicology and Carcinogenesis Studies of Acrylamide in F344/N Rats and B6C3F1 Mice (Feed and Drinking Water Studies). Natl. Toxicol. Program 2012, 79, 1–234. [Google Scholar] [CrossRef]
- Lipworth, L.; Sonderman, J.S.; Tarone, R.E.; McLaughlin, J.K. Review of epidemiologic studies of dietary acrylamide intake and the risk of cancer. Eur. J. Cancer Prev. 2012, 21, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Dybing, E.; Farmer, P.B.; Andersen, M.; Fennell, T.R.; Lalljie, S.P.D.; Müller, D.J.G.; Olin, S.; Petersen, B.J.; Schlatter, J.; Scholz, G.; et al. Human exposure and internal dose assessments of acrylamide in food. Food Chem. Toxicol. 2005, 43, 365–410. [Google Scholar] [CrossRef] [PubMed]
- Waheed, S.; Rahman, S.; Gill, K.P. INAA and AAS of different products from sugar cane industry in Pakistan: Toxic trace elements for nutritional safety. J. Radioanal. Nucl. Chem. 2009, 279, 725–731. [Google Scholar] [CrossRef]
- Silva, F.S.; Cristale, J.; Ribeiro, M.L.; de Marchi, M.R.R. Polycyclic aromatic hydrocarbons (PAHs) in raw cane sugar (rapadura) in Brazil. J. Food Compost. Anal. 2011, 24, 346–350. [Google Scholar] [CrossRef]
- Wojtczak, M.; Antczak, A.; Lisik, K. Contamination of commercial cane sugars by some organic acids and some inorganic anions. Food Chem. 2013, 136, 193–198. [Google Scholar] [CrossRef]
- Drewnowski, A.; Tappy, L.; Forde, C.G.; McCrickerd, K.; Tee, E.S.; Chan, P.; Amarra, M.S. Sugars and sweeteners: Science, innovations, and consumer guidance for Asia. Asia Pac. J. Clin. Nutr. 2019, 28, 645–663. [Google Scholar]
- Guerra, M.J.; Mujica, M.V. Physical and chemical properties of granulated cane sugar “panelas”. Food Sci. Technol. 2010, 30, 250–257. [Google Scholar] [CrossRef]
- Generoso, W.C.; Borges, M.T.M.R.; Ceccato-Antonini, S.R.; Marino, A.F.; Silva, M.V.M.; Nassu, R.T.; Verruma-Bernardi, M.R. Physical-chemical and microbiological evaluation of commercial brown sugar. J. Adolfo Lutz Inst. 2009, 68, 259–268. [Google Scholar]

| Nutrients | Unit | Quantity |
|---|---|---|
| Moisture | g | 1.5–15.8 |
| Carbohydrate | g | 83.90–97.2 |
| Total sugar | g | 87.5–95.4 |
| Sucrose | g | 76.55–89.48 |
| Reducing sugar | g | 3.69–10.5 |
| Fiber | g | 0.00 |
| Protein | g | 0.37–1.7 |
| Fat | g | 0..00–0.10 |
| Ash | g | 0.3–3.6 |
| Amino Acids | mg | 205.22–805.28 |
| Total Minerals | mg | 1648.11–2971.90 |
| Minerals | Unit | Quantity |
|---|---|---|
| Calcium | mg | 13.70–240.00 |
| Chloride | mg | 5.30–250.00 |
| Cobalt | µg | 9.90 |
| Copper | mg | 0.17–8.50 |
| Chromium | µg | 11.90–16.00 |
| Iodine | µg | 0.01 |
| Iron | mg | 1.60–12.50 |
| Magnesium | mg | 31.00–120.00 |
| Manganese | mg | 0.35–1.66 |
| Phosphorus | mg | 2.00–125.00 |
| Potassium | mg | 14.05–1100.00 |
| Selenium | µg | 0.02 |
| Sodium | mg | 15.50–79.00 |
| Zinc | mg | 0.10–1.76 |
| Vitamins | Unit | Quantity |
|---|---|---|
| Vitamin A | mg | 1.90 |
| B1 (Thiamin) | mg | 0.03 |
| B2 (Riboflavin) | mg | 0.07 |
| B3 (Niacin) | mg | 2.14 |
| B5 (Pantothenic acid) | mg | 0.70 |
| B6 (Pyridoxine) | mg | 0.21 |
| B9 (Folic acid) | µg | 3.33 |
| Vitamin C | mg | 4.23 |
| Vitamin E | mg | 55.0 |
| Phytochemical Molecules | Quantity (mg/100 g) | References |
|---|---|---|
| Phenolic Acids | ||
| p-hydroxybenzoic acid | 0.022–0.44 | [18,34] |
| Benzoic acid | 0.47–0.84 | [23] |
| Caffeic acid | 0.065–7.6 | [23,31,34] |
| Vanillic acid | 0.36–1.86 | [18,22,23,34] |
| p-coumaric acid | 0.080- 1.98 | [22,23,31,34,35] |
| Gallic acids | 0.15–1.43 | [34] |
| Ferulic acid | 0.0035–5.6 | [18,22,23,31,34] |
| Rosmarinic acid | <0.01 | [18] |
| Syringic acid | 0.076–0.547 | [22,23,34] |
| Chlorogenic acid | 0.089–28 | [34,36,37] |
| Protocatechuic acid | 0.012–0.089 | [22,34] |
| Cinnamic acids | 0.49–38 | [31] |
| Flavonoids | ||
| Luteolin | 0.48–2.5 | [18,31] |
| Kaempferol | 0.081 | [18] |
| Naringenin | 0.006 | [18] |
| Apigenin | 0.008–19 | [18,31] |
| Tricin | 0.71–13.0 | [23,31] |
| Flavones | 2.4–33 | [31] |
| Anthocyanins | ||
| 3-rutinoside cyanidin | 0.13 | [18] |
| Cyanidin | 0.22 | [18] |
| Pelargonidin | 0.034 | [18] |
| Name | Country |
|---|---|
| Vollrohrzucker | Germany |
| Black sugar (Kurosato), and Kokuto | Japan |
| Evaporated cane juice and raw sugar | Europe, North America and USA |
| Chancaca | Peru and Bolivia |
| Azúcar Integral and Azúcar Panela | Latin America, Argentina |
| Gula Java and Gula Merah | Indonesia |
| Gula Melaka | Malaysia |
| Dulce and Tapa Dulce | Costa Rica and Nicaragua |
| Panocha and Panutsa | Philippines |
| Gur | India and Pakistan |
| Hakuru and Vellam | Sri Lanka |
| Rapadura | Brazil and Guatemala |
| Jaggery | India, Nigeria, Kenya and South Africa |
| Moscovado | Philippines |
| Panela | Colombia, Ecuador, Guatemala, Panama and Venezuela |
| Papelón | Venezuela |
| Piloncillo | Mexico |
| Namtan Tanode | Thailand |
| Unrefined Muscovado | UK |
| Sukari Njumru | Swahili-speaking countries |
| Raspadura | Panama |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zidan, D.; Azlan, A. Non-Centrifugal Sugar (NCS) and Health: A Review on Functional Components and Health Benefits. Appl. Sci. 2022, 12, 460. https://doi.org/10.3390/app12010460
Zidan D, Azlan A. Non-Centrifugal Sugar (NCS) and Health: A Review on Functional Components and Health Benefits. Applied Sciences. 2022; 12(1):460. https://doi.org/10.3390/app12010460
Chicago/Turabian StyleZidan, Dina, and Azrina Azlan. 2022. "Non-Centrifugal Sugar (NCS) and Health: A Review on Functional Components and Health Benefits" Applied Sciences 12, no. 1: 460. https://doi.org/10.3390/app12010460
APA StyleZidan, D., & Azlan, A. (2022). Non-Centrifugal Sugar (NCS) and Health: A Review on Functional Components and Health Benefits. Applied Sciences, 12(1), 460. https://doi.org/10.3390/app12010460








