Molecular Identification of Keratinase DgokerA from Deinococcus gobiensis for Feather Degradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, and Materials
2.2. Construction of Keratinase Expression Plasmid
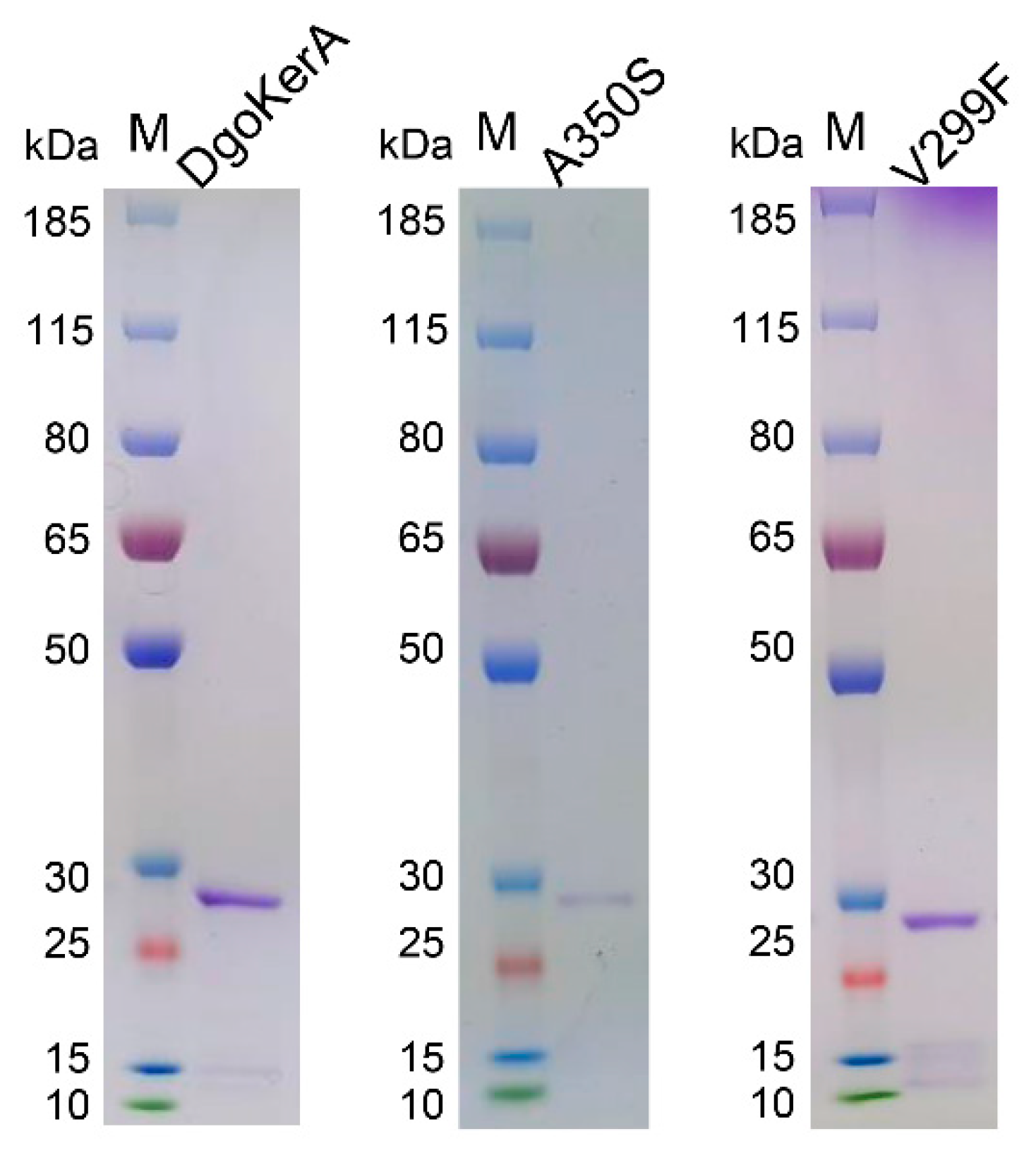
2.3. Expression and Purification of Keratinases
2.4. Keratinolytic Activity Assay
2.5. Effects of pH, Temperature, and Reagents on Enzyme Activity and Stability
2.6. Chicken Feather Degradation
3. Results
3.1. Identification of Keratinase DgoKerA
3.2. Expression and Activity of DgoKerA
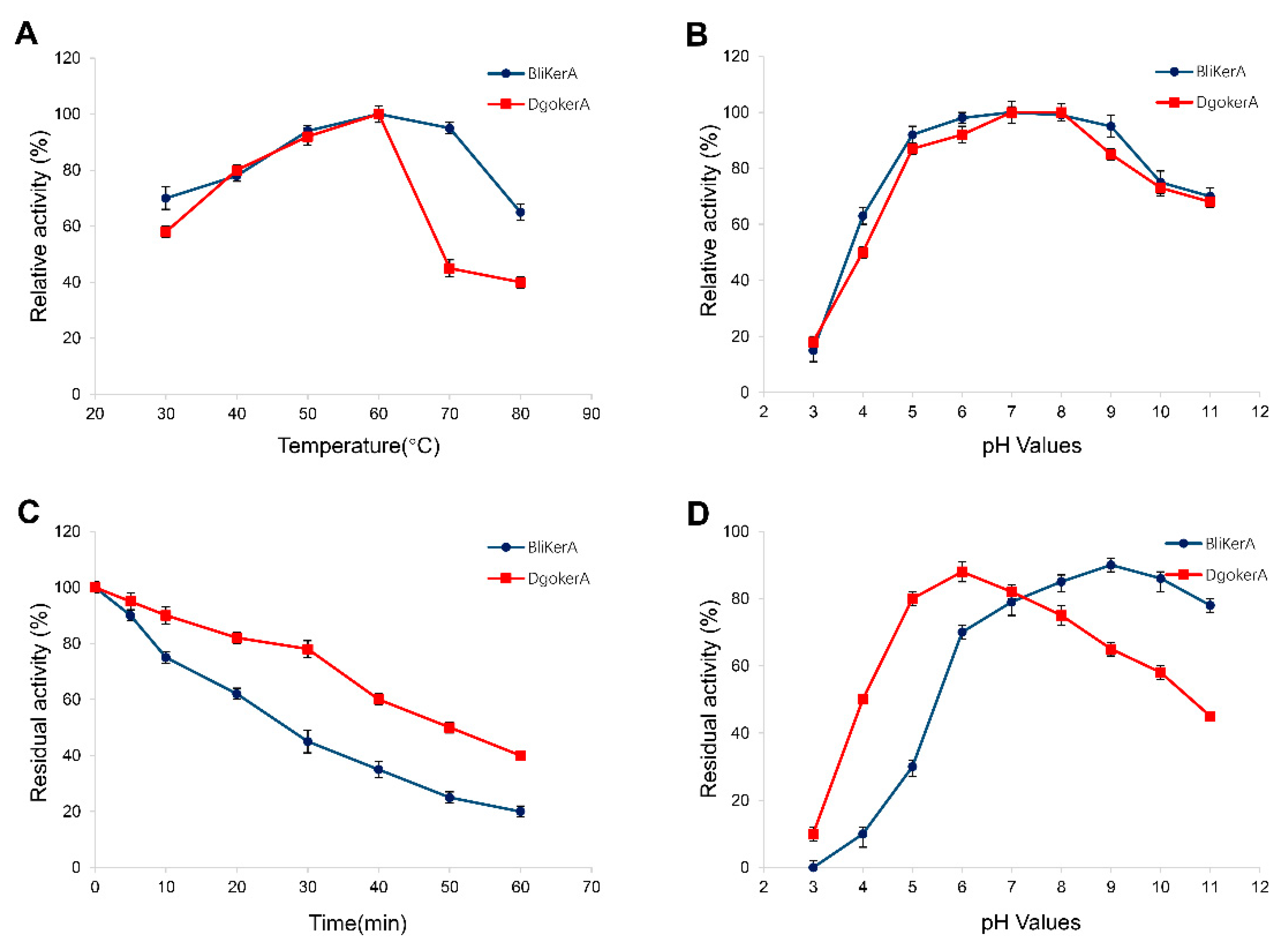
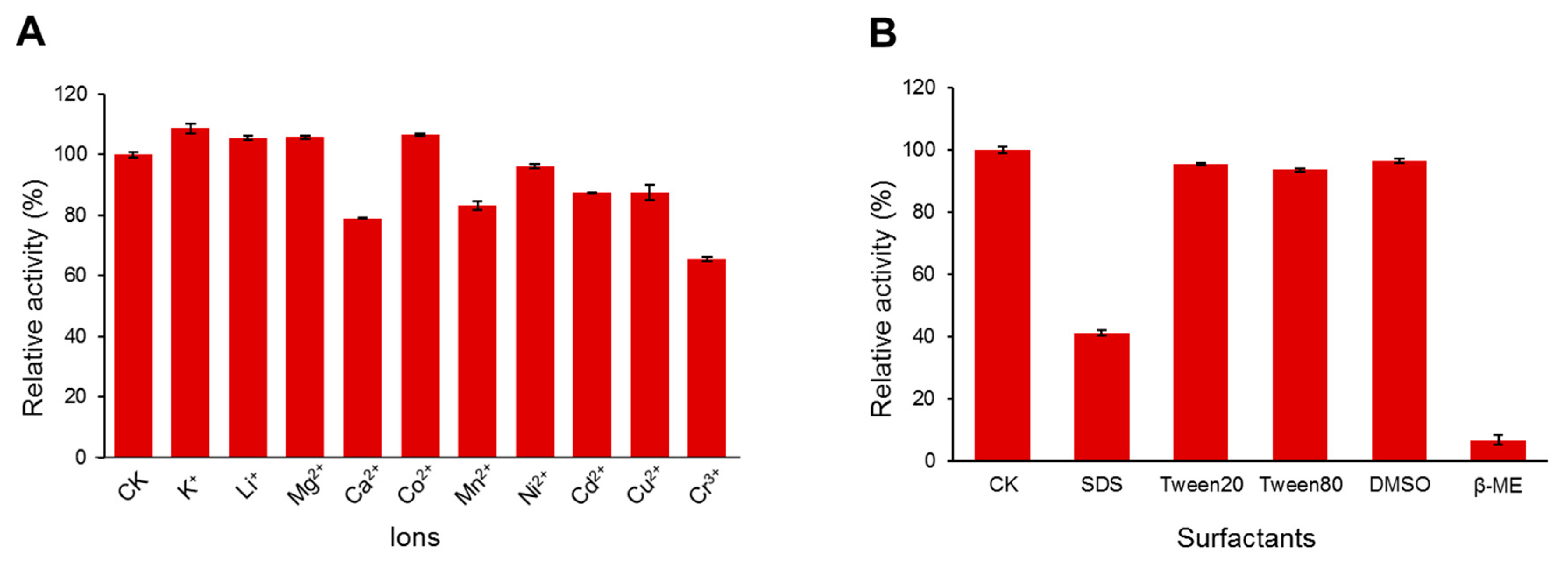
3.3. Effects of Temperature, pH, Metal Ions, and Chemical Agents on DgoKerA Activity
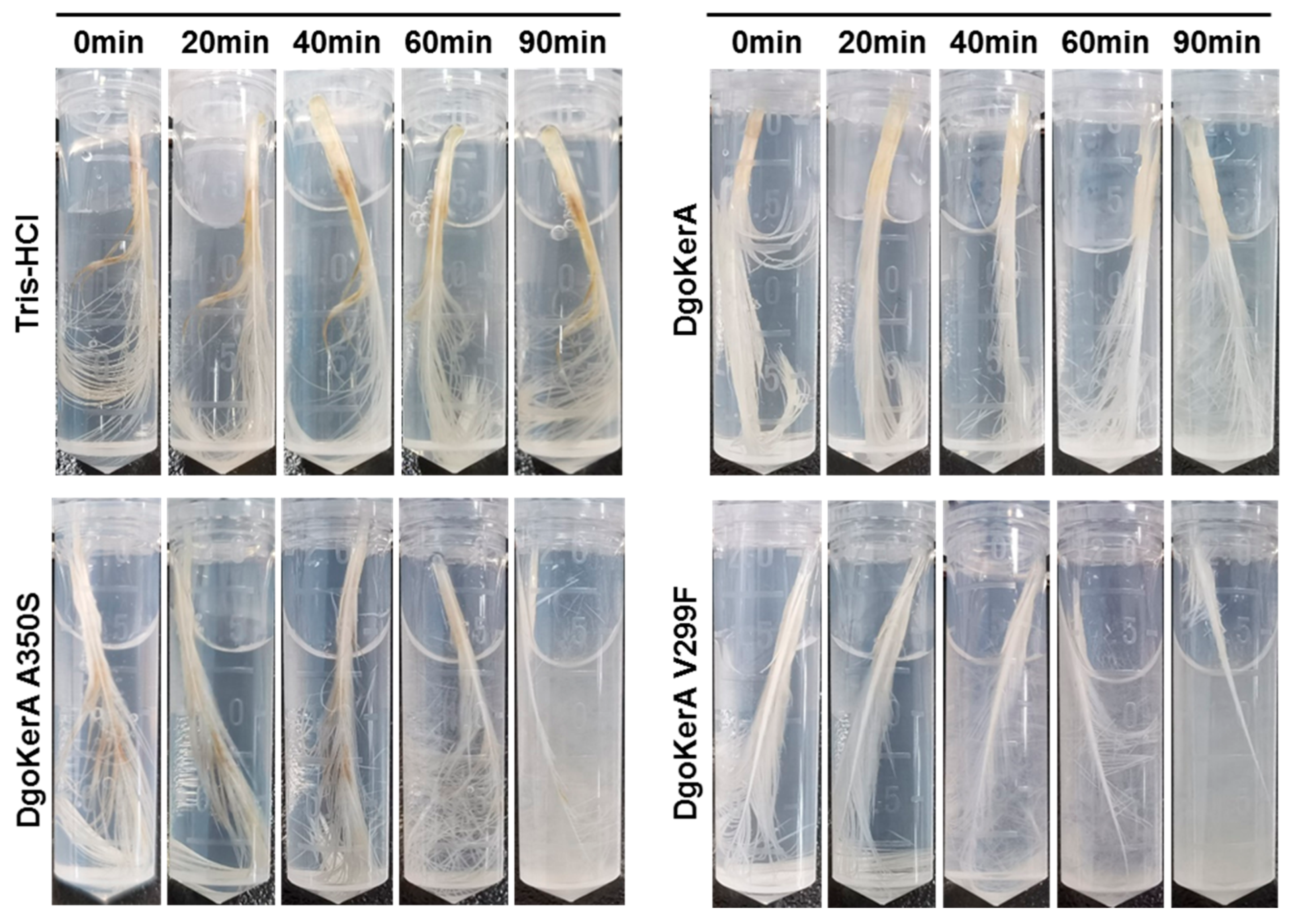
3.4. Chicken Feather Degradation of DgoKerA In Vitro
3.5. Enzymatic Activity and Feather Degradation Capacity of Mutants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lange, L.; Huang, Y.; Busk, P.K. Microbial decomposition of keratin in nature-a new hypothesis of industrial relevance. Appl. Microbiol. Biotechnol. 2016, 100, 2083–2096. [Google Scholar] [CrossRef] [Green Version]
- Li, Q. Progress in Microbial Degradation of Feather Waste. Front. Microbiol. 2019, 10, 2717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nnolim, N.E.; Udenigwe, C.C.; Okoh, A.I.; Nwodo, U.U. Microbial Keratinase: Next Generation Green Catalyst and Prospective Applications. Front. Microbiol. 2020, 11, 580164. [Google Scholar] [CrossRef]
- Gupta, R.; Ramnani, P. Microbial keratinases and their prospective applications: An overview. Appl. Microbiol. Biotechnol. 2006, 70, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Rajput, R.; Sharma, R.; Gupta, N. Biotechnological applications and prospective market of microbial keratinases. Appl. Microbiol. Biotechnol. 2013, 97, 9931–9940. [Google Scholar] [CrossRef]
- Lin, X.; Kelemen, D.W.; Miller, E.S.; Shih, J.C. Nucleotide sequence and expression of kerA, the gene encoding a keratinolytic protease of Bacillus licheniformis PWD-1. Appl. Environ. Microbiol. 1995, 61, 1469–1474. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.L.; Chen, M.Y.; Tu, I.F.; Lin, Y.C.; EswarKumar, N.; Chen, M.Y.; Ho, M.C.; Wu, S.H. The discovery of novel heat-stable keratinases from Meiothermus taiwanensis WR-220 and other extremophiles. Sci. Rep. 2017, 7, 4658. [Google Scholar] [CrossRef] [Green Version]
- Foophow, T.; Tanaka, S.; Koga, Y.; Takano, K.; Kanaya, S. Subtilisin-like serine protease from hyperthermophilic archaeon Thermococcus kodakaraensis with N- and C-terminal propeptides. Protein Eng. Des. Sel. 2010, 23, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Shinde, U.; Thomas, G. Insights from bacterial subtilases into the mechanisms of intramolecular chaperone-mediated activation of furin. Methods Mol. Biol. 2011, 768, 59–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Z.; Zhang, J.; Liu, B.H.; Du, G.C.; Chen, J. Insight into the substrate specificity of keratinase KerSMD from Stenotrophomonas maltophilia by site-directed mutagenesis studies in the S1 pocket. RSC Adv. 2015, 5, 74953–74960. [Google Scholar] [CrossRef]
- Wong, W.; Wijeyewickrema, L.C.; Kennan, R.M.; Reeve, S.B.; Steer, D.L.; Reboul, C.; Smith, A.I.; Pike, R.N.; Rood, J.I.; Whisstock, J.C.; et al. S1 pocket of a bacterially derived subtilisin-like protease underpins effective tissue destruction. J. Biol. Chem. 2011, 286, 42180–42187. [Google Scholar] [CrossRef] [Green Version]
- Foophow, T.; Tanaka, S.; Angkawidjaja, C.; Koga, Y.; Takano, K.; Kanaya, S. Crystal structure of a subtilisin homologue, Tk-SP, from Thermococcus kodakaraensis: Requirement of a C-terminal beta-jelly roll domain for hyperstability. J. Mol. Biol. 2010, 400, 865–877. [Google Scholar] [CrossRef] [PubMed]
- van den Burg, B. Extremophiles as a source for novel enzymes. Curr. Opin. Microbiol. 2003, 6, 213–218. [Google Scholar] [CrossRef]
- Dalmaso, G.Z.L.; Lage, C.A.S.; Mazotto, A.M.; Dias, E.P.D.; Caldas, L.A.; Ferreira, D.; Vermelho, A.B. Extracellular peptidases from Deinococcus radiodurans. Extremophiles 2015, 19, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.G.; Lu, W.; Wang, J.; Chen, M.; Zhang, W.; Lin, M.; Zhou, Z.F.; Liu, Z. Characterization of EstDR4, a Novel Cold-Adapted Insecticides-Metabolizing Esterase from Deinococcus radiodurans. Appl. Sci. 2021, 11, 1864. [Google Scholar] [CrossRef]
- Kiran, T.; Asad, W.; Siddiqui, S.; Ajaz, M.; Rasool, S.A. Industrially important hydrolytic enzyme diversity explored in stove ash bacterial isolates. Pak. J. Pharm. Sci. 2015, 28, 2035–2040. [Google Scholar]
- Khoshnevis, N.; Rezaei, S.; Forootanfar, H.; Faramarzi, M.A. Efficient Keratinolysis of Poultry Feather Waste by the Halotolerant Keratinase from Salicola Marasensis. Iran. J. Pharm. Res. 2019, 18, 1862–1870. [Google Scholar] [CrossRef]
- Tang, Y.; Guo, L.Z.; Zhao, M.M.; Gui, Y.; Han, J.H.; Lu, W.; Dai, Q.L.; Jiang, S.J.; Lin, M.; Zhou, Z.F.; et al. A Novel Thermostable Keratinase from Deinococcus geothermalis with Potential Application in Feather Degradation. Appl. Sci. 2021, 11, 3136. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Huang, Y.; Qin, W.J.; Quan, Z.X. The complete genome of extracellular protease-producing Deinococcus sp. D7000 isolated from the hadal region of Mariana Trench Challenger Deep. Mar. Genom. 2021, 57, 100832. [Google Scholar] [CrossRef]
- Zeng, Y.H.; Shen, F.T.; Tan, C.C.; Huang, C.C.; Young, C.C. The flexibility of UV-inducible mutation in Deinococcus ficus as evidenced by the existence of the imuB-dnaE2 gene cassette and generation of superior feather degrading bacteria. Microbiol. Res. 2011, 167, 40–47. [Google Scholar] [CrossRef]
- Yuan, M.; Chen, M.; Zhang, W.; Lu, W.; Wang, J.; Yang, M.; Zhao, P.; Tang, R.; Li, X.; Hao, Y.; et al. Genome sequence and transcriptome analysis of the radioresistant bacterium Deinococcus gobiensis: Insights into the extreme environmental adaptations. PLoS ONE 2012, 7, e34458. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Wang, H.; Lv, Y.; Bai, Y.; Luo, H.; Shi, P.; Huang, H.; Yao, B. Construction of a Rapid Feather-Degrading Bacterium by Overexpression of a Highly Efficient Alkaline Keratinase in Its Parent Strain Bacillus amyloliquefaciens K11. J. Agric. Food Chem. 2016, 64, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Luo, H.; Huang, H.; Hakulinen, N.; Wang, Y.; Wang, Y.; Su, X.; Bai, Y.; Zhang, J.; Yao, B.; et al. Improving the catalytic performance of Proteinase K from Parengyodontium album for use in feather degradation. Int. J. Biol. Macromol. 2020, 154, 1586–1595. [Google Scholar] [CrossRef]
- Niehaus, F.; Bertoldo, C.; Kahler, M.; Antranikian, G. Extremophiles as a source of novel enzymes for industrial application. Appl. Microbiol. Biot. 1999, 51, 711–729. [Google Scholar] [CrossRef] [PubMed]
- Eder, J.; Fersht, A.R. Pro-sequence-assisted protein folding. Mol. Microbiol. 1995, 16, 609–614. [Google Scholar] [CrossRef]
- Shinde, U.; Li, Y.; Chatterjee, S.; Inouye, M. Folding pathway mediated by an intramolecular chaperone. Proc. Natl. Acad. Sci. USA 1993, 90, 6924–6928. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Zhang, J.; Liu, B.; Jiang, L.; Du, G.; Chen, J. Cloning, heterologous expression and characterization of two keratinases from Stenotrophomonas maltophilia BBE11-1. Process Biochem. 2014, 49, 647–654. [Google Scholar] [CrossRef]
- Zhou, C.; Qin, H.; Chen, X.; Zhang, Y.; Xue, Y.; Ma, Y. A novel alkaline protease from alkaliphilic Idiomarina sp. C9-1 with potential application for eco-friendly enzymatic dehairing in the leather industry. Sci. Rep. 2018, 8, 16467. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, J.; Du, G.; Chen, J. Rational protein engineering approaches to further improve the keratinolytic activity and thermostability of engineered keratinase KerSMD. Biochem. Eng. J. 2017, 127, 147–153. [Google Scholar] [CrossRef]
- Brandelli, A.; Daroit, D.J.; Riffel, A. Biochemical features of microbial keratinases and their production and applications. Appl. Microbiol. Biotechnol. 2010, 85, 1735–1750. [Google Scholar] [CrossRef]
- Hassan, M.A.; Taha, T.H.; Hamad, G.M.; Hashem, M.; Alamri, S.; Mostafa, Y.S. Biochemical characterisation and application of keratinase from Bacillus thuringiensis MT1 to enable valorisation of hair wastes through biosynthesis of vitamin B-complex. Int. J. Biol. Macromol. 2020, 153, 561–572. [Google Scholar] [CrossRef]
- Pereira, J.Q.; Ambrosini, A.; Passaglia, L.M.P.; Brandelli, A. A new cold-adapted serine peptidase from Antarctic Lysobacter sp. A03: Insights about enzyme activity at low temperatures. Int. J. Biol. Macromol. 2017, 103, 854–862. [Google Scholar] [CrossRef]
- Bouacem, K.; Bouanane-Darenfed, A.; Zarai Jaouadi, N.; Joseph, M.; Hacene, H.; Ollivier, B.; Fardeau, M.L.; Bejar, S.; Jaouadi, B. Novel serine keratinase from Caldicoprobacter algeriensis exhibiting outstanding hide dehairing abilities. Int. J. Biol. Macromol. 2016, 86, 321–328. [Google Scholar] [CrossRef]
- Dukunde, A.; Schneider, D.; Lu, M.; Brady, S.; Daniel, R. A novel, versatile family IV carboxylesterase exhibits high stability and activity in a broad pH spectrum. Biotechnol. Lett. 2017, 39, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Navone, L.; Speight, R. Understanding the dynamics of keratin weakening and hydrolysis by proteases. PLoS ONE 2018, 13, e0202608. [Google Scholar] [CrossRef]
- Peng, Z.; Mao, X.; Zhang, J.; Du, G.; Chen, J. Biotransformation of keratin waste to amino acids and active peptides based on cell-free catalysis. Biotechnol. Biofuels 2020, 13, 61. [Google Scholar] [CrossRef]
- Pourjavaheri, F.; Pour, S.O.; Jones, O.A.H.; Smooker, P.M.; Brkljaca, R.; Sherkat, F.; Blanch, E.W.; Gupta, A.; Shanks, R.A. Extraction of keratin from waste chicken feathers using sodium sulfide and L-cysteine. Process Biochem. 2019, 82, 205–214. [Google Scholar] [CrossRef]
- Gong, J.S.; Ye, J.P.; Tao, L.Y.; Su, C.; Qin, J.; Zhang, Y.Y.; Li, H.; Li, H.; Xu, Z.H.; Shi, J.S. Efficient keratinase expression via promoter engineering strategies for degradation of feather wastes. Enzym. Microb. Technol. 2020, 137, 109550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ultsch, M.; Skelton, N.J.; Burdick, D.J.; Beresini, M.H.; Li, W.; Kong-Beltran, M.; Peterson, A.; Quinn, J.; Chiu, C.; et al. Discovery of a cryptic peptide-binding site on PCSK9 and design of antagonists. Nat. Struct. Mol. Biol. 2017, 24, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Tsai, C.J.; Nussinov, R. Factors enhancing protein thermostability. Protein Eng. 2000, 13, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Sittipol, D.; Rodpan, S.; Ajingi, Y.S.; Lohnoo, T.; Lerksuthirat, T.; Kumsang, Y.; Yingyong, W.; Khunrae, P.; Rattanarojpong, T.; Pattanapanyasat, K.; et al. Identification, overexpression, purification, and biochemical characterization of a novel hyperthermostable keratinase from Geoglobus acetivorans. 3 Biotech 2021, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Jakob, F.; Martinez, R.; Mandawe, J.; Hellmuth, H.; Siegert, P.; Maurer, K.H.; Schwaneberg, U. Surface charge engineering of a Bacillus gibsonii subtilisin protease. Appl. Microbiol. Biotechnol. 2013, 97, 6793–6802. [Google Scholar] [CrossRef] [PubMed]


| Name of Primers | Sequences (5′ to 3′) |
|---|---|
| DgokerA-F | aaggagatataccatgggcATGTGCGGAACCTCGACTACCC |
| DgokerA-R | gtggtggtggtggtgctcgagGAAGTTCAGGGTGTACAGCAGCT |
| A350S-F1 | aaggagatataccatgggcATGTGCGGAACCTCGACTACCC |
| A350S-R1 | gtgccgctgatggtgttggtGCTGGTCGTGCTGCCGAT |
| A350S-F2 | gatcggcagcacgaccagcACCAACACCATCAGCGGCA |
| A350S-R2 | gtggtggtggtggtgctcgagGAAGTTCAGGGTGTACAGCAGCT |
| V299F-F1 | aaggagatataccatgggcATGTGCGGAACCTCGACTACCC |
| V299F-R1 | ctggcacgcgccgggctGAAGTTGCAGGCGTCCTGG |
| V299F-F2 | aggacgcctgcaacttcAGCCCGGCGCGTGCCAGC |
| V299F-R2 | gtggtggtggtggtgctcgagGAAGTTCAGGGTGTACAGCAGCT |
| The Enzymatic Activity (U/mg) | ||||
|---|---|---|---|---|
| BliKerA | DgoKerA | DgoKerA A350S | DgoKerA V299F | |
| 1% Soluble Keratin | 45,730.65 ± 244.30 | 51,147.65 ± 442.80 | 66,872.84 ± 137.41 | 49,367.23 ± 346.51 |
| Feather Powder | 1771.63 ± 25.29 | 3277.88 ± 172.62 | 3312.14 ± 201.10 | 1914.17 ± 156.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Tang, Y.; Zhang, X.; Wang, J.; Zhou, Z. Molecular Identification of Keratinase DgokerA from Deinococcus gobiensis for Feather Degradation. Appl. Sci. 2022, 12, 464. https://doi.org/10.3390/app12010464
Meng Y, Tang Y, Zhang X, Wang J, Zhou Z. Molecular Identification of Keratinase DgokerA from Deinococcus gobiensis for Feather Degradation. Applied Sciences. 2022; 12(1):464. https://doi.org/10.3390/app12010464
Chicago/Turabian StyleMeng, Yong, Yin Tang, Xiuhong Zhang, Jin Wang, and Zhengfu Zhou. 2022. "Molecular Identification of Keratinase DgokerA from Deinococcus gobiensis for Feather Degradation" Applied Sciences 12, no. 1: 464. https://doi.org/10.3390/app12010464





