Immunoenhancement Effects of the Herbal Formula Hemomine on Cyclophosphamide-Induced Immunosuppression in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Study Design
2.3. Hematology
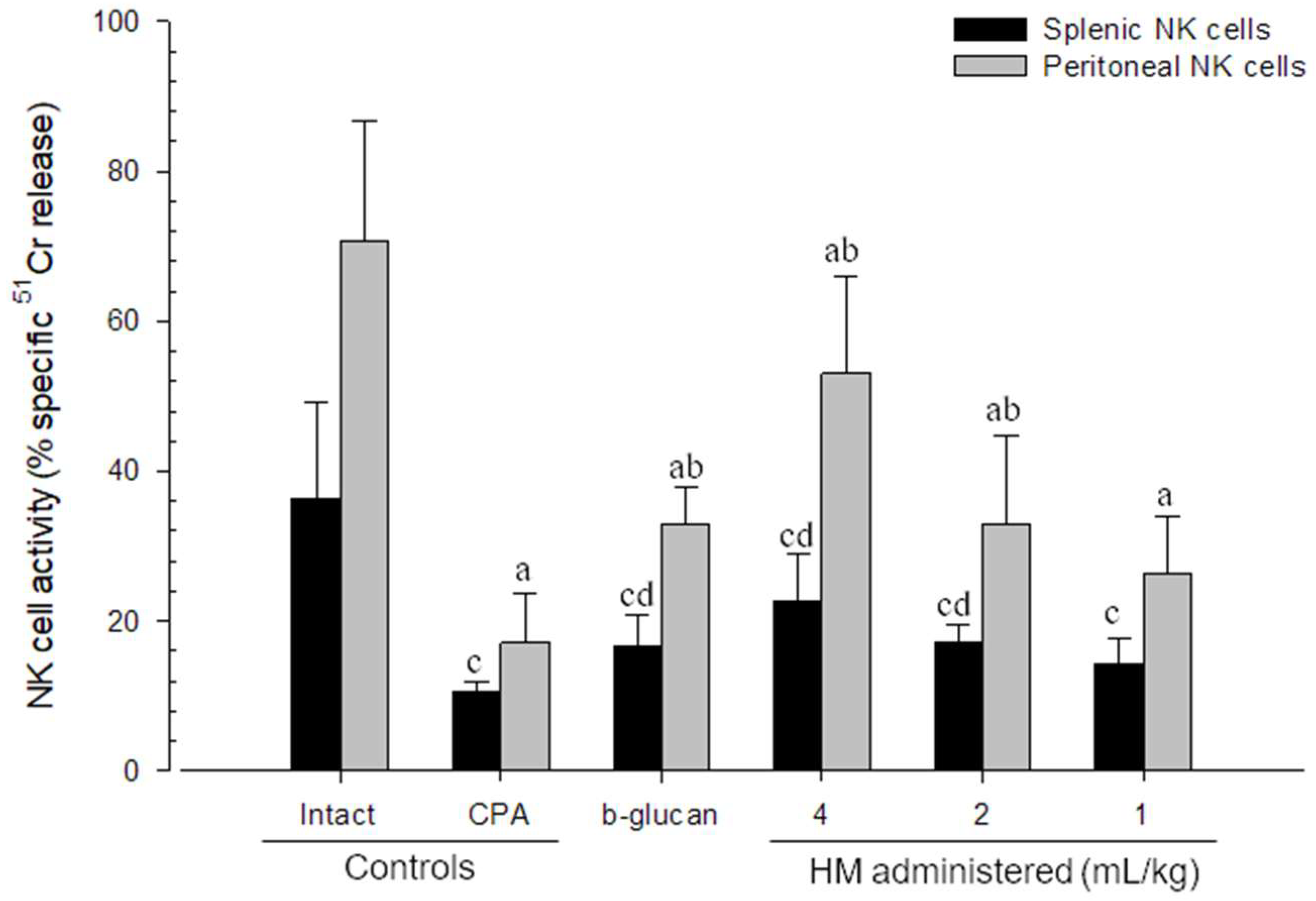
2.4. NK Cell Activities
2.5. Serum and Splenic Cytokine Measurements
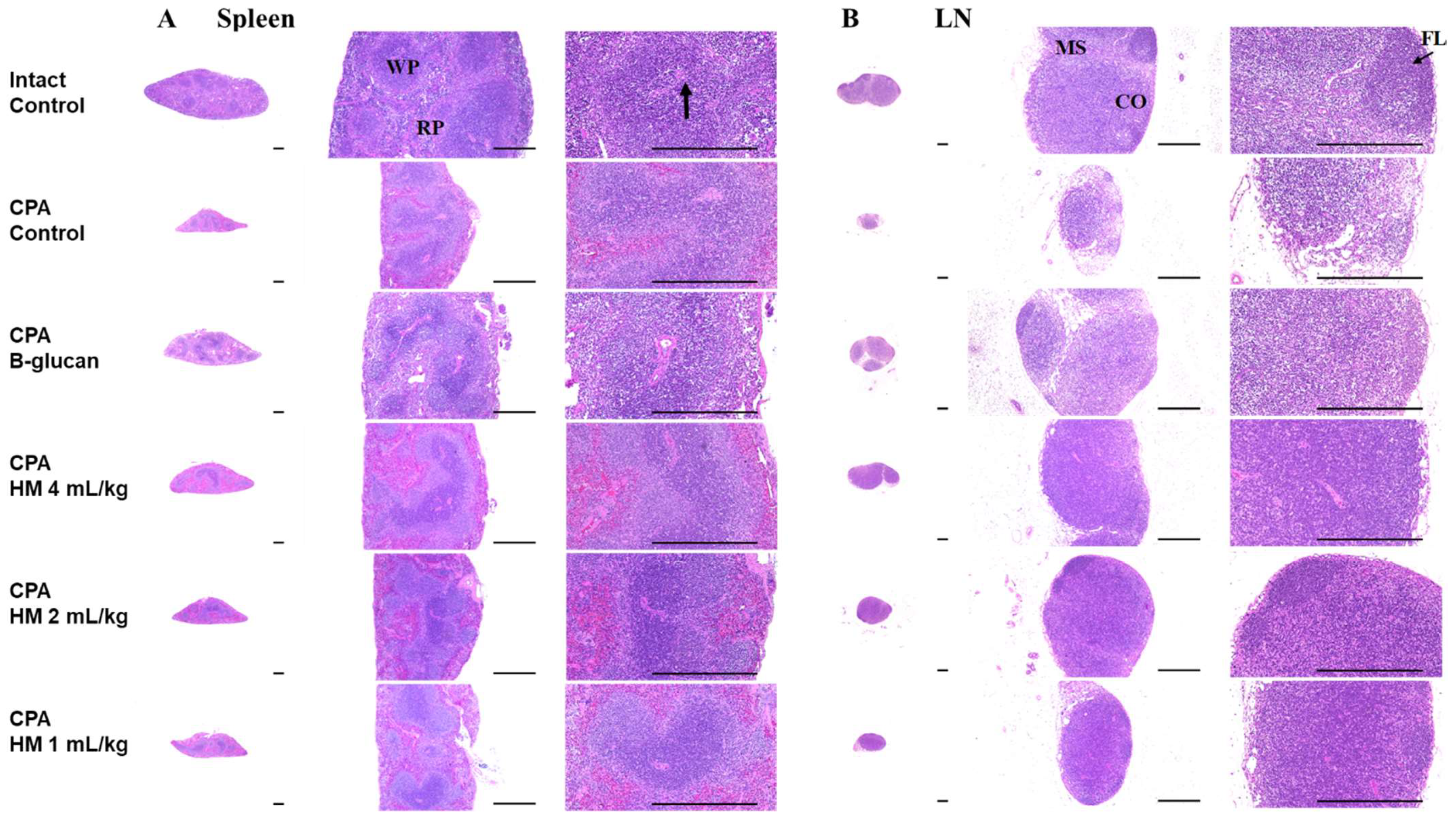
2.6. Histopathology
2.7. Statistical Analysis
3. Results
3.1. Changes in Body and Organ Weights
3.2. Hematological Changes
3.3. Changes in Serum and Splenic Cytokine Levels
3.4. Changes in NK Cell Activities
3.5. Effects on Splenic and Submandibular Lymph Node Histopathology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Carr, C.; Ng, J.; Wigmore, T. The side effects of chemotherapeutic agents. Curr. Anaesth. Crit. Care 2008, 19, 70–79. [Google Scholar] [CrossRef]
- Daniel, D.; Crawford, J. Myelotoxicity from Chemotherapy. In Seminars in Oncology; Elsevier: Amsterdam, The Netherlands, 2006; pp. 4–85. [Google Scholar]
- López, M.M.; Valenzuela, J.E.; Álvarez, F.C.; López-Álvarez, M.R.; Cecilia, G.S.; Paricio, P.P. Long-term problems related to immunosuppression. Transpl. Immunol. 2006, 17, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Chinen, J.; Kavanaugh, A. Immunomodulator therapy: Monoclonal antibodies, fusion proteins, cytokines, and immunoglobulins. J. Allergy Clin. Immunol. 2010, 125, S314–S323. [Google Scholar] [CrossRef] [PubMed]
- Jantan, I.; Ahmad, W.; Bukhari, S.N.A. Plant-derived immunomodulators: An insight on their preclinical evaluation and clinical trials. Front. Plant Sci. 2015, 6, 655. [Google Scholar] [CrossRef]
- Kim, J.; You, S. Samul-tang ameliorates oocyte damage due to cyclophosphamide-induced chronic ovarian dysfunction in mice. Sci. Rep. 2020, 10, 21925. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.K.; Kim, H.; Kim, J.W.; Kang, K.S.; Lee, H.-J. Ameliorating effects of herbal formula hemomine on experimental subacute hemorrhagic anemia in rats. J. Ethnopharmacol. 2017, 198, 205–213. [Google Scholar] [CrossRef]
- Kim, S.-J.; Ko, S.-M.; Choi, E.-J.; Ham, S.-H.; Kwon, Y.-D.; Lee, Y.-B.; Cho, H.-Y. Simultaneous determination of decursin, decursinol angelate, nodakenin, and decursinol of angelica gigas nakai in human plasma by uhplc-ms/ms: Application to pharmacokinetic study. Molecules 2018, 23, 1019. [Google Scholar] [CrossRef] [Green Version]
- Park, K.J.; Lee, B.C.; Lee, J.S.; Cho, M.H. Angelica gigas nakai extract ameliorates the effects of cyclophosphamide on immunological and hematopoietic dysfunction in mice. J. Med. Plant Res. 2014, 8, 657–663. [Google Scholar]
- Rim, H.-K.; Cho, W.; Sung, S.H.; Lee, K.-T. Nodakenin suppresses lipopolysaccharide-induced inflammatory responses in macrophage cells by inhibiting tumor necrosis factor receptor-associated factor 6 and nuclear factor-κb pathways and protects mice from lethal endotoxin shock. J. Pharmacol. Exp. Ther. 2012, 342, 654–664. [Google Scholar] [CrossRef]
- Kim, J.-H.; Jeong, J.-H.; Jeon, S.-T.; Kim, H.; Ock, J.; Suk, K.; Kim, S.-I.; Song, K.-S.; Lee, W.-H. Decursin inhibits induction of inflammatory mediators by blocking nuclear factor-κb activation in macrophages. Mol. Pharmacol. 2006, 69, 1783–1790. [Google Scholar] [CrossRef]
- Shehzad, A.; Parveen, S.; Qureshi, M.; Subhan, F.; Lee, Y.S. Decursin and decursinol angelate: Molecular mechanism and therapeutic potential in inflammatory diseases. Inflamm. Res. 2018, 67, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-J.; Lee, M.-Y.; Kim, J.-H.; Suk, K.; Lee, W.-H. Decursinol angelate blocks transmigration and inflammatory activation of cancer cells through inhibition of pi3k, erk and nf-κb activation. Cancer Lett. 2010, 296, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Auyeung, K.K.; Han, Q.-B.; Ko, J.K. Astragalus membranaceus: A review of its protection against inflammation and gastrointestinal cancers. Am. J. Chin. Med. 2016, 44, 1–22. [Google Scholar] [CrossRef] [PubMed]
- He, D.-Y.; Dai, S.-M. Anti-inflammatory and immunomodulatory effects of Paeonia lactiflora pall., a traditional chinese herbal medicine. Front. Pharmacol. 2011, 2, 10. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.-S.; Gao, T.; Cui, Y.-L.; Gao, L.-N.; Jiang, H.-L. Comparative studies of paeoniflorin and albiflorin from Paeonia lactiflora on anti-inflammatory activities. Pharm. Biol. 2014, 52, 1189–1195. [Google Scholar] [CrossRef]
- Jeong, J.B.; Hong, S.C.; Jeong, H.J.; Koo, J.S. Anti-inflammatory effects of ethyl acetate fraction from cnidium officinale makino on lps-stimulated raw 264.7 and thp-1 cells. Korean J. Plant Res. 2012, 25, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Han, M.H.; Lee, M.H.; Hong, S.H.; Choi, Y.H.; Moon, J.S.; Song, M.K.; Kim, M.J.; Shin, S.J.; Hwang, H.J. Comparison of anti-inflammatory activities among ethanol extracts of sophora flavescens, glycyrrhiza uralensis and dictamnus dasycarpus, and their mixtures in raw 246.7 murine macrophages. J. Life Sci. 2014, 24, 329–335. [Google Scholar] [CrossRef]
- Park, E.; Kum, S.; Wang, C.; Park, S.Y.; Kim, B.S.; Schuller-Levis, G. Anti-inflammatory activity of herbal medicines: Inhibition of nitric oxide production and tumor necrosis factor-α secretion in an activated macrophage-like cell line. Am. J. Chin. Med. 2005, 33, 415–424. [Google Scholar] [CrossRef]
- Crook, T.R.; Souhami, R.L.; Mclean, A.E. Cytotoxicity, DNA cross-linking, and single strand breaks induced by activated cyclophosphamide and acrolein in human leukemia cells. Cancer Res. 1986, 46, 5029–5034. [Google Scholar]
- Emadi, A.; Jones, R.J.; Brodsky, R.A. Cyclophosphamide and cancer: Golden anniversary. Nat. Rev. Clin. Oncol. 2009, 6, 638. [Google Scholar] [CrossRef]
- Angulo, I.; de las Heras, F.G.; Garcıa-Bustos, J.F.; Gargallo, D.; Munoz-Fernández, M.A.; Fresno, M. Nitric oxide-producing cd11b+ ly-6g (gr-1)+ cd31 (er-mp12)+ cells in the spleen of cyclophosphamide–treated mice: Implications for t-cell responses in immunosuppressed mice. Blood 2000, 95, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Xun, C.; Thompson, J.; Jennings, C.; Brown, S.; Widmer, M. Effect of total body irradiation, busulfan-cyclophosphamide, or cyclophosphamide conditioning on inflammatory cytokine release and development of acute and chronic graft-versus-host disease in h-2-incompatible transplanted scid mice. Blood 1994, 83, 2360–2367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Wang, M.; Chen, J.; Tang, Y.; Dou, J.; Yu, J.; Xi, T.; Zhou, C. A polysaccharide from strongylocentrotus nudus eggs protects against myelosuppression and immunosuppression in cyclophosphamide-treated mice. Int. Immunopharmacol. 2011, 11, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Shruthi, S.; Vijayalaxmi, K.; Shenoy, K.B. Immunomodulatory effects of gallic acid against cyclophosphamide-and cisplatin-induced immunosuppression in swiss albino mice. Indian J. Pharm. Sci. 2018, 80, 150–160. [Google Scholar] [CrossRef] [Green Version]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Yoo, S.-R.; Jeong, S.-J.; Shin, H.-K. Single oral dose toxicity evaluation of samul-tang, a traditional herbal formula, in crl: Cd (sd) rats. J. Korean Med. Sci. 2014, 35, 28–33. [Google Scholar] [CrossRef]
- Yoon, H.-S.; Kim, J.-W.; Cho, H.-R.; Moon, S.-B.; Shin, H.-D.; Yang, K.-J.; Lee, H.-S.; Kwon, Y.-S.; Ku, S.-K. Immunomodulatory effects of aureobasidium pullulans sm-2001 exopolymers on cyclophosphamide-treated mice. J. Microbiol. Biotechnol. 2010, 20, 438–445. [Google Scholar] [CrossRef]
- Kim, J.W.; Choi, J.-S.; Seol, D.J.; Choung, J.J.; Ku, S.K. Immunomodulatory effects of kuseonwangdogo-based mixed herbal formula extracts on a cyclophosphamide-induced immunosuppression mouse model. Evid. Based Complement. Altern. Med. 2018, 2018, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Hotchkiss, R.; Osborne, D.; Lappas, G.; Karl, I. Calcium antagonists decrease plasma and tissue concentrations of tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-1 alpha in a mouse model of endotoxin. Shock 1995, 3, 337–342. [Google Scholar]
- Hubbell, H.R.; Kvalnes-Krick, K.; Carter, W.A.; Strayer, D.R. Antiproliferative and immunomodulatory actions of β-interferon and double-stranded rna, individually and in combination, on human bladder tumor xenografts in nude mice. Cancer Res. 1985, 45, 2481–2486. [Google Scholar]
- Clark, B.D.; Bedrosian, I.; Schindler, R.; Cominelli, F.; Cannon, J.G.; Shaw, A.R.; Dinarello, C.A. Detection of interleukin 1 alpha and 1 beta in rabbit tissues during endotoxemia using sensitive radioimmunoassays. J. Appl. Physiol. 1991, 71, 2412–2418. [Google Scholar] [CrossRef] [PubMed]
- Levene, A. Pathological factors influencing excision of tumours in the head and neck. Part I. Clin. Otolaryngol. Allied Sci. 1981, 6, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Kanno, T.Y.N.; Sensiate, L.A.; Paula, N.A.d.; Salles, M.J.S. Toxic effects of different doses of cyclophosphamide on the reproductive parameters of male mice. Braz. J. Pharm. Sci. 2009, 45, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Harada, T.; Miura, N.; Adachi, Y.; Nakajima, M.; Yadomae, T.; Ohno, N. Effect of scg, 1, 3-β-d-glucan from sparassis crispa on the hematopoietic response in cyclophosphamide induced leukopenic mice. Biol. Pharm. Bull. 2002, 25, 931–939. [Google Scholar] [CrossRef] [Green Version]
- Bascones-Martinez, A.; Mattila, R.; Gomez-Font, R.; Meurman, J.H. Immunomodulatory drugs: Oral and systemic adverse effects. Med. Oral Patol. Oral Cir. Bucal. 2014, 19, e24–e31. [Google Scholar] [CrossRef]
- Peng, S.; Lyford-Pike, S.; Akpeng, B.; Wu, A.; Hung, C.-F.; Hannaman, D.; Saunders, J.R.; Wu, T.-C.; Pai, S.I. Low-dose cyclophosphamide administered as daily or single dose enhances the antitumor effects of a therapeutic hpv vaccine. Cancer Immunol. Immunother. 2013, 62, 171–182. [Google Scholar] [CrossRef]
- Eid, R.A.; Razavi, G.S.E.; Mkrtichyan, M.; Janik, J.; Khleif, S.N. Old-school chemotherapy in immunotherapeutic combination in cancer, a low-cost drug repurposed. Cancer Immunol. Res. 2016, 4, 377–382. [Google Scholar] [CrossRef] [Green Version]
- Tzianabos, A.O. Polysaccharide immunomodulators as therapeutic agents: Structural aspects and biologic function. Clin. Microbiol. Rev. 2000, 13, 523–533. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, H.-C.; He, S.-B.; Zhang, X.-F.; Ling, Y.-H.; Li, X.-Y.; Zhang, H.; Hou, D.-D. The immunoenhancement effects of sea buckthorn pulp oil in cyclophosphamide-induced immunosuppressed mice. Food Funct. 2021, 12, 7954–7963. [Google Scholar] [CrossRef]
- Botnick, L.E.; Hannon, E.C.; Vigneulle, R.; Hellman, S. Differential effects of cytotoxic agents on hematopoietic progenitors. Cancer Res. 1981, 41, 2338–2342. [Google Scholar]
- Zhou, Y.; Chen, X.; Yi, R.; Li, G.; Sun, P.; Qian, Y.; Zhao, X. Immunomodulatory effect of tremella polysaccharides against cyclophosphamide-induced immunosuppression in mice. Molecules 2018, 23, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivashkiv, L.B. Ifnγ: Signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zhang, Z.; Ye, S.; Hong, X.; Jin, H.; Huang, F.; Yang, Z.; Tang, Y.; Chen, Y.; Ding, G. Immunoenhancement effects of pentadecapeptide derived from cyclina sinensis on immune-deficient mice induced by cyclophosphamide. J. Funct. Foods 2019, 60, 103408. [Google Scholar] [CrossRef]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The role of interleukin-1 in general pathology. Inflamm. Regen. 2019, 39, 12. [Google Scholar] [CrossRef] [Green Version]
- Couper, K.N.; Blount, D.G.; Riley, E.M. Il-10: The master regulator of immunity to infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef]
- Pontrelli, P.; Rascio, F.; Castellano, G.; Grandaliano, G.; Gesualdo, L.; Stallone, G. The role of natural killer cells in the immune response in kidney transplantation. Front. Immunol. 2020, 11, 1454. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X.; Jin, W.; Guo, Y. Immunomodulatory effects of a low-molecular weight polysaccharide from enteromorpha prolifera on raw 264.7 macrophages and cyclophosphamide-induced immunosuppression mouse models. Mar. Drugs 2020, 18, 340. [Google Scholar] [CrossRef]
- Lim, J.-Y.; Lee, J.-H.; Yun, D.-H.; Lee, Y.-M.; Kim, D.-K. Inhibitory effects of nodakenin on inflammation and cell death in lipopolysaccharide-induced liver injury mice. Phytomedicine 2020, 81, 153411. [Google Scholar] [CrossRef]
- Zhou, X.; Seto, S.W.; Chang, D.; Kiat, H.; Razmovski-Naumovski, V.; Chan, K.; Bensoussan, A. Synergistic effects of chinese herbal medicine: A comprehensive review of methodology and current research. Front. Pharmacol. 2016, 7, 201. [Google Scholar] [CrossRef] [Green Version]

| Group | Weight Gain 1 (g) | Relative Organ Weight 2 (% of Body Weights) | |
|---|---|---|---|
| Spleen | LN | ||
| Intact Control | 3.35 ± 0.62 | 0.297 ± 0.050 | 0.057 ± 0.016 |
| CPA-Control | −2.19 ± 0.89 a | 0.124 ± 0.030 a | 0.014 ± 0.007 a |
| CPA-β-glucan | 0.48 ± 0.83 ab | 0.184 ± 0.038 ab | 0.024 ± 0.007 ab |
| CPA-HM 4 mL/kg | 1.13 ± 0.86 ab | 0.201 ± 0.032 ab | 0.037 ± 0.009 ab |
| CPA-HM 2 mL/kg | 0.63 ± 1.22 ab | 0.186 ± 0.032 ab | 0.027 ± 0.008 ab |
| CPA-HM 1 mL/kg | −0.40 ± 0.66 ab | 0.167 ± 0.020 ab | 0.022 ± 0.006 a |
| Groups Items | Controls | Reference | HM | |||
|---|---|---|---|---|---|---|
| Intact | CPA | β-Glucan | 4 mL/kg | 2 mL/kg | 1 mL/kg | |
| WBC (K/μL) | 9.02 ± 3.33 | 0.31 ± 0.11 c | 0.79 ± 0.12 cd | 1.24 ± 0.26 cd | 0.82 ± 0.19 cd | 0.56 ± 0.13 cd |
| LYM (%) | 77.49 ± 7.31 | 76.10 ± 5.21 | 77.15 ± 8.49 | 76.38 ± 5.68 | 76.79 ± 7.27 | 75.74 ± 6.48 |
| NEU (%) | 15.92 ± 5.56 | 17.69 ± 4.94 | 16.47 ± 8.42 | 17.07 ± 5.19 | 17.00 ± 5.30 | 18.01 ± 6.48 |
| MON (%) | 3.94 ± 1.27 | 3.86 ± 1.27 | 3.85 ± 1.84 | 3.94 ± 1.57 | 3.85 ± 2.03 | 3.89± 1.86 |
| EOS (%) | 0.93 ± 0.90 | 0.96 ± 0.84 | 0.97 ± 0.45 | 0.99 ± 0.66 | 0.97 ± 0.59 | 0.99 ± 0.47 |
| BAS (%) | 0.81 ± 0.70 | 0.82 ± 0.50 | 0.81 ± 0.60 | 0.78 ± 0.46 | 0.81 ± 0.58 | 0.83 ± 0.36 |
| RBC (M/μL) | 9.68 ± 0.79 | 4.92 ± 0.73 a | 7.14 ± 0.64 ab | 8.25 ± 1.19 ab | 7.18 ± 0.51 ab | 6.53 ± 0.59 ab |
| HGB (g/dL) | 18.10 ± 1.19 | 12.87 ± 1.91 a | 17.00 ± 0.89 ab | 17.76 ± 1.16 b | 17.02 ± 0.61 ab | 15.90 ± 0.95 ab |
| HCT (%) | 44.08 ± 2.15 | 38.64 ± 2.60 a | 42.27 ± 0.99 ab | 44.26 ± 2.65 b | 41.99 ± 1.33 ab | 41.02 ± 0.88 ab |
| MCV (fl) | 52.04 ± 2.99 | 51.62 ± 4.06 | 51.23 ± 3.23 | 51.33 ± 2.44 | 52.08 ± 1.97 | 51.77 ± 2.37 |
| MCH (pg) | 18.20 ± 0.91 | 17.81 ± 1.40 | 17.75 ± 1.07 | 17.77 ± 1.03 | 17.92 ± 1.34 | 17.80 ± 1.34 |
| MCHC (g/dL) | 19.61 ± 1.73 | 19.56 ± 1.50 | 19.48 ± 1.51 | 19.64 ± 1.36 | 19.73 ± 1.23 | 19.41 ± 1.16 |
| PLT (M/μL) | 1665.4 ± 217.6 | 881.8 ± 155.3 a | 1204.8 ± 97.6 ab | 1407.7 ± 111.6 ab | 1209.6 ± 174.7 ab | 1079.6 ± 120.6 ab |
| Serum | Spleen | |||
|---|---|---|---|---|
| Groups | IFN-γ (pg/mL) | TNF-α (pg/mg Protein) | IL-1β (pg/mg Protein) | IL-10 (pg/mg Protein) |
| Intact Control | 276.08 ± 38.51 | 109.91 ± 28.30 | 66.45 ± 10.04 | 99.46 ± 22.61 |
| CPA Control | 75.15 ± 21.29 a | 32.15 ± 10.06 c | 19.92 ± 10.54 a | 32.42 ± 10.20 a |
| CPA-β-glucan | 147.35 ± 32.12 ab | 63.30 ± 12.65 cd | 39.26 ± 10.35 ab | 62.14 ± 11.26 ab |
| CPA-HM 4 mL/kg | 183.88 ± 33.80 ab | 77.64 ± 17.22 cd | 48.67 ± 10.23 ab | 78.06 ± 16.76 ab |
| CPA-HM 2 mL/kg | 148.37 ± 14.65 ab | 63.43 ± 13.12 cd | 39.28 ± 14.32 ab | 62.97 ± 19.12 ab |
| CPA-HM 1 mL/kg | 104.65 ± 22.03 ab | 52.98 ± 11.37 cd | 33.79 ± 10.46 ab | 47.71 ± 11.01 ab |
| Groups Items | Controls | Reference | HM | |||
|---|---|---|---|---|---|---|
| Intact | CPA | β-Glucan | 4 mL/kg | 2 mL/kg | 1 mL/kg | |
| Spleen thickness | ||||||
| Total (μm) | 1762.17 ± 161.91 | 836.47 ± 178.99 a | 1397.67 ± 194.24 ab | 1451.95 ± 193.96 ab | 1375.67 ± 160.47 ab | 1134.98 ± 250.88 ab |
| Cortex (μm) | 414.55 ± 52.99 | 197.79 ± 29.12 a | 294.63 ± 36.89 ab | 356.94 ± 41.23 ab | 300.42 ± 41.84 ab | 254.18 ± 44.89 ab |
| WP number (/mm2) | 14.20 ± 2.15 | 4.20 ± 0.92 c | 9.80 ± 1.32 cd | 12.60 ± 1.43 d | 10.20 ± 1.40 cd | 7.50 ± 0.71 cd |
| LN thickness | ||||||
| Total (μm) | 1148.41 ± 110.11 | 448.88 ± 115.39 a | 917.77 ± 104.45 ab | 1028.52 ± 127.04 ab | 934.67 ± 105.72 ab | 733.50 ± 182.62 ab |
| Cortex (μm) | 833.29 ± 118.01 | 279.90 ± 101.44 a | 599.21 ± 119.30 ab | 774.40 ± 132.92 b | 612.95 ± 137.40 ab | 483.44 ± 128.17 ab |
| Follicle number (/mm2) | 13.50 ± 2.80 | 2.10 ± 1.20 c | 6.10 ± 1.20 cd | 9.30 ± 2.45 cd | 6.20 ± 1.69 cd | 3.80 ± 1.03 cd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Kim, J.W.; Kim, Y.-K.; Ku, S.K.; Lee, H.-J. Immunoenhancement Effects of the Herbal Formula Hemomine on Cyclophosphamide-Induced Immunosuppression in Mice. Appl. Sci. 2022, 12, 4935. https://doi.org/10.3390/app12104935
Kim H, Kim JW, Kim Y-K, Ku SK, Lee H-J. Immunoenhancement Effects of the Herbal Formula Hemomine on Cyclophosphamide-Induced Immunosuppression in Mice. Applied Sciences. 2022; 12(10):4935. https://doi.org/10.3390/app12104935
Chicago/Turabian StyleKim, Hyemee, Joo Wan Kim, Yeon-Kye Kim, Sae Kwang Ku, and Hae-Jeung Lee. 2022. "Immunoenhancement Effects of the Herbal Formula Hemomine on Cyclophosphamide-Induced Immunosuppression in Mice" Applied Sciences 12, no. 10: 4935. https://doi.org/10.3390/app12104935






