Dependence of Electric Pulse Mediated Growth Factor Release on the Platelet Rich Plasma Separation Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. PRP Systems and Protocols
2.2. Donors, Blood Collection and Preparation of PRP
2.3. PRP Study Design
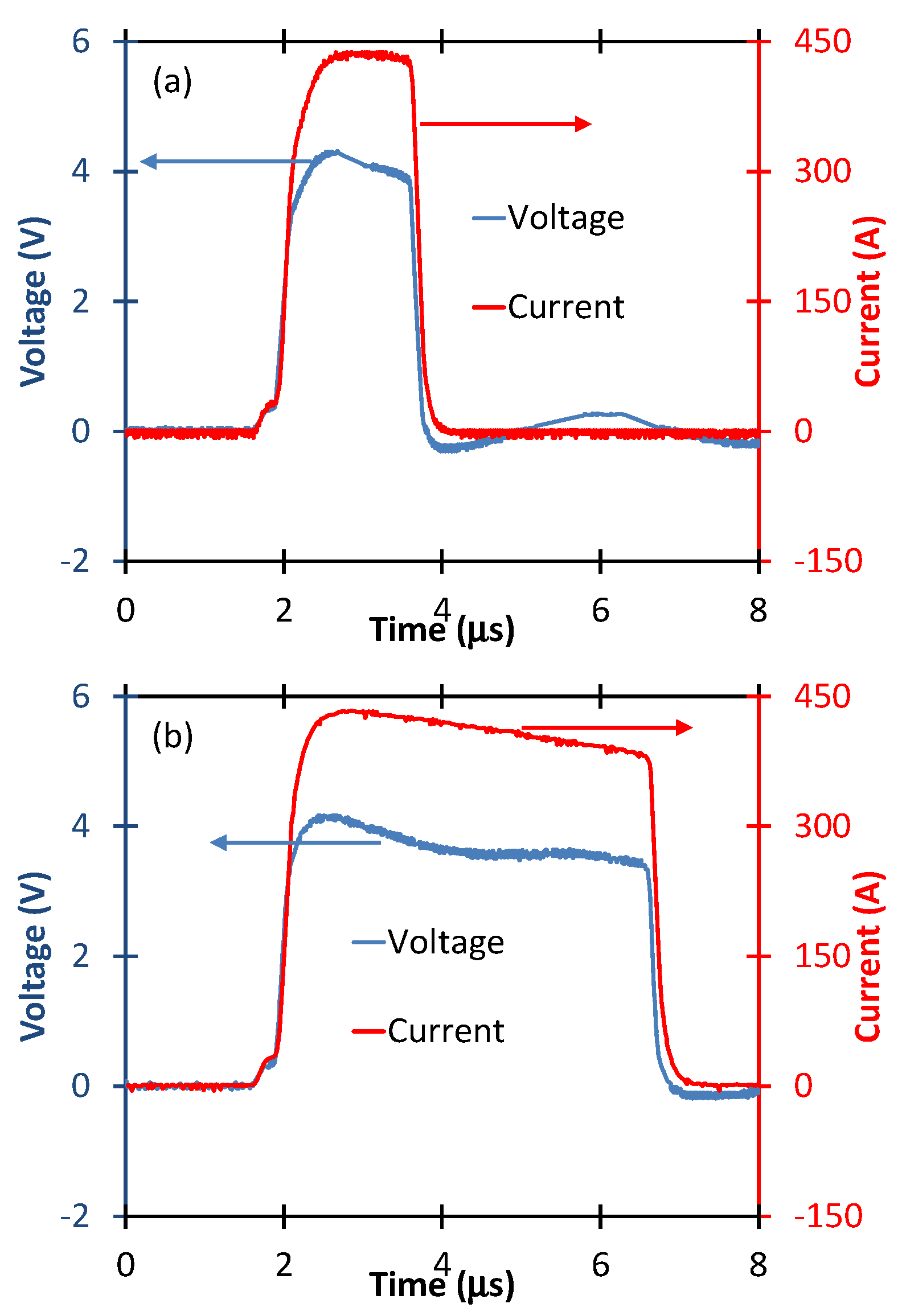
2.4. PEF Stimulation of PRP
2.5. Growth Factor Release
2.6. Statistical Analysis
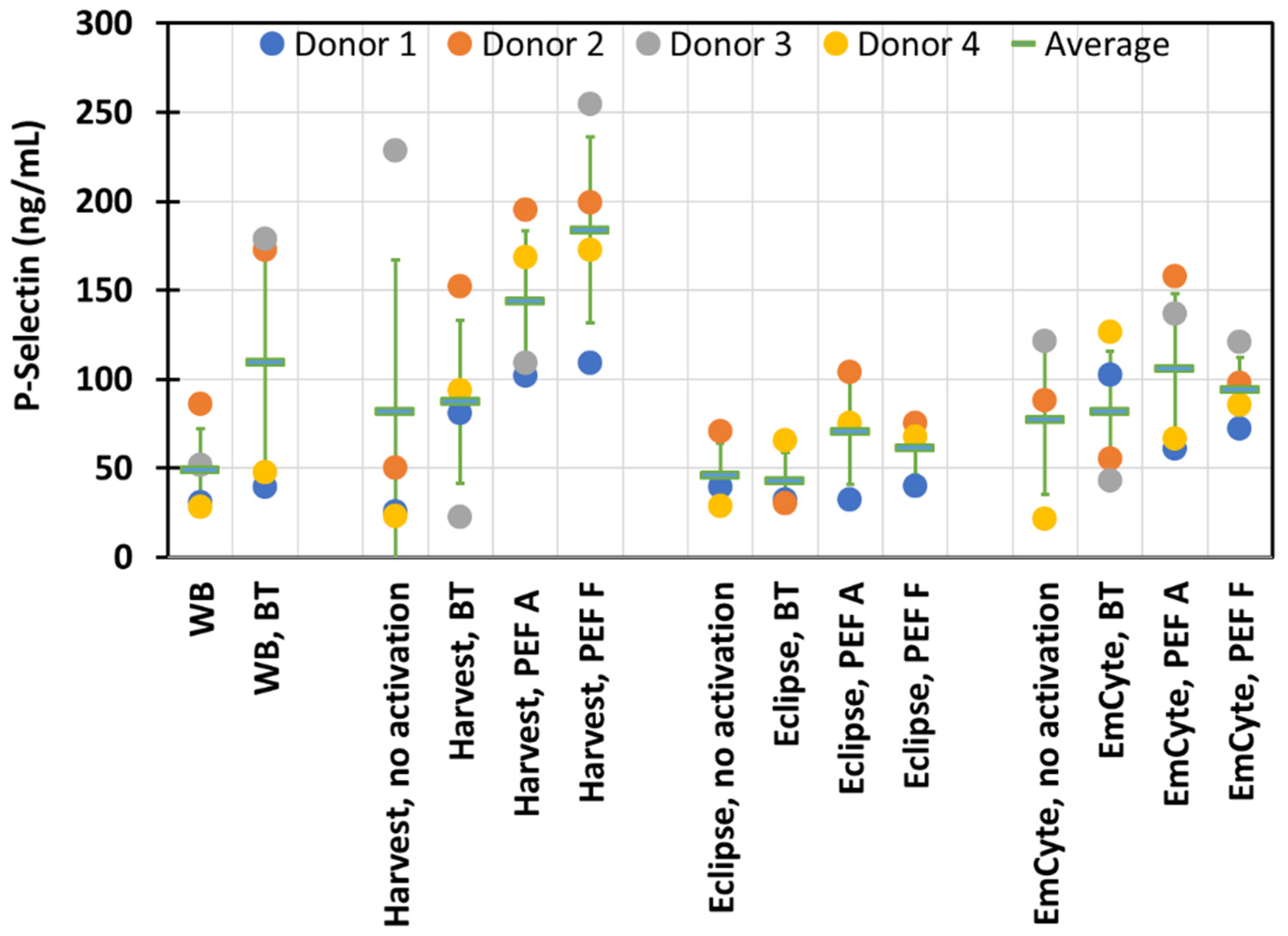
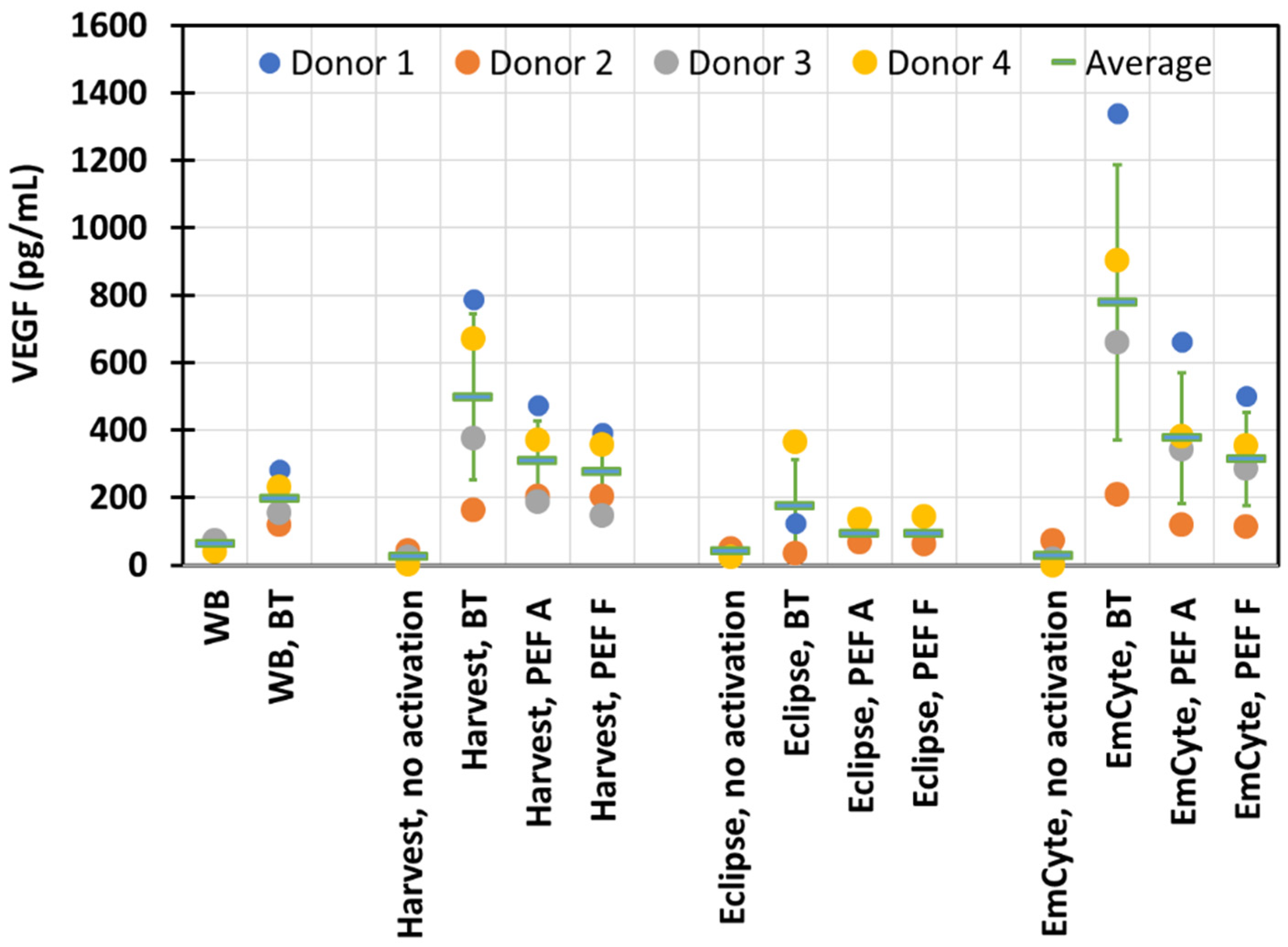
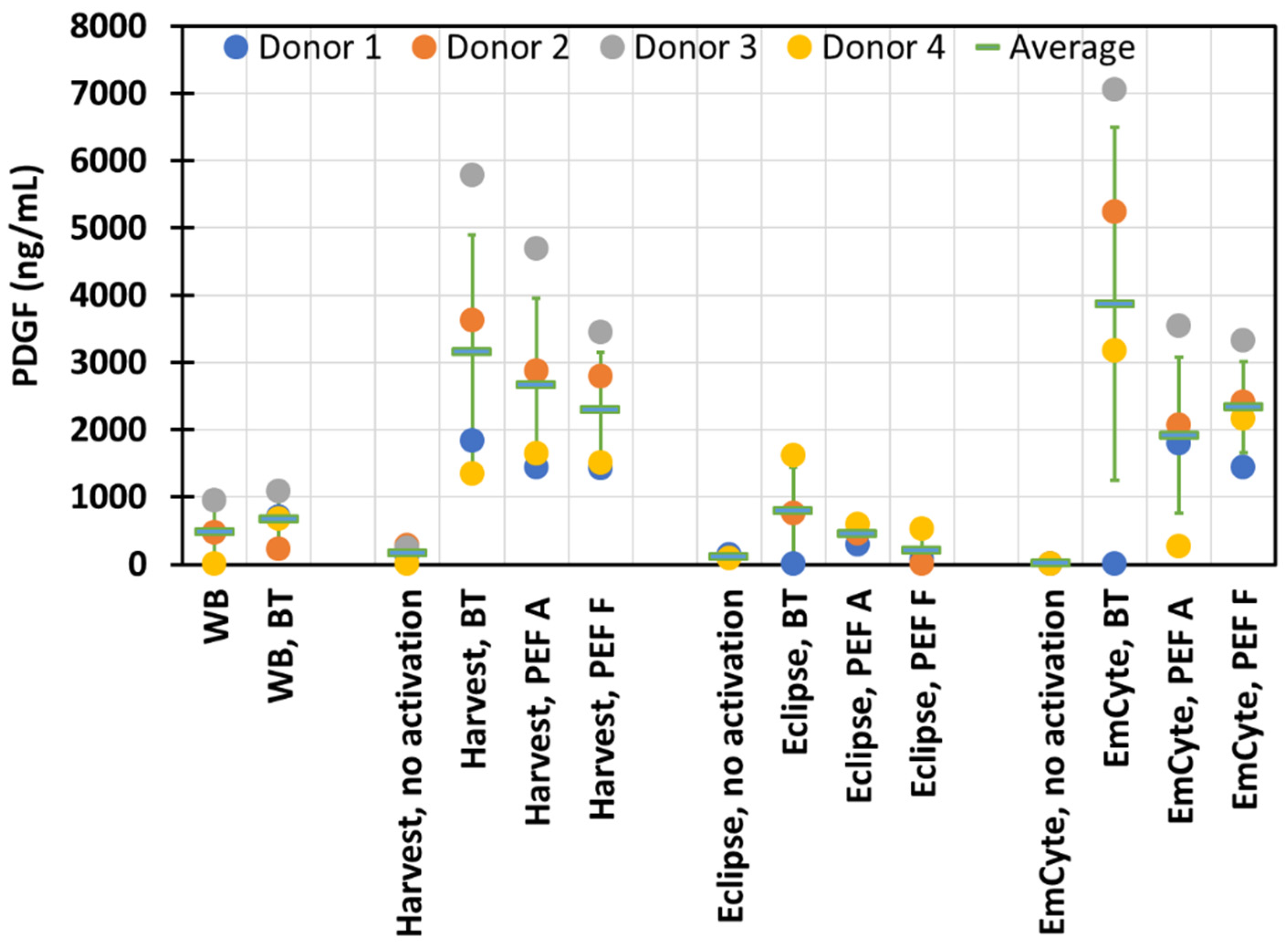
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Klement, G.L.; Shai, S.; Varon, D. The role of platelets in angiogenesis. In Platelets, 3rd ed.; Michelson, A.D., Ed.; Elsevier; Academic Press: San Diego, CA, USA, 2013; pp. 487–502. [Google Scholar]
- Lacci, K.M.; Dardik, A. Platelet-rich plasma: Support for its use in wound healing. Yale J. Biol. Med. 2010, 83, 1–9. [Google Scholar] [PubMed]
- Driver, V.R.; Hanft, J.; Fylling, C.P.; Beriou, J.M. Autologel Diabetic Foot Ulcer Study Group. A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manag. 2006, 52, 68–74. [Google Scholar]
- Gunaydin, S.; McCusker, K.; Sari, T.; Onur, M.; Gurpinar, A.; Sevim, H.; Atasoy, P.; Yorgancioglu, C.; Zorlutuna, Y. Clinical impact and biomaterial evaluation of autologous platelet gel in cardiac surgery. Perfusion 2008, 23, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Bielecki, T.M.; Gazdzik, T.S.; Arendt, J.; Szczepanski, T.; Krol, W.; Wielkoszynski, T. Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances: An in vitro study. J. Bone Joint Surg. 2007, 89-B, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef] [PubMed]
- Mlynarek, R.A.; Kuhn, A.W.; Bedi, A. Platelet-Rich Plasma (PRP) in Orthopedic Sports Medicine. Am. J. Orthop. 2016, 45, 290–326. [Google Scholar]
- Hausauer, A.K.; Jones, D.H. Evaluating the Efficacy of Different Platelet-Rich Plasma Regimens for Management of Androgenetic Alopecia: A Single-Center, Blinded, Randomized Clinical Trial. Dermatol. Surg. 2018, 44, 1191–1200. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S.; Bielli, A.; Scioli, M.G.; Orlandi, A.; Cervelli, V. The Effect of Platelet-Rich Plasma in Hair Regrowth: A Randomized Placebo-Controlled Trial. Stem Cells Transl. Med. 2015, 4, 1317–1323. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S. Systematic Review of Platelet-Rich Plasma Use in Androgenetic Alopecia Compared with Minoxidil®, Finasteride®, and Adult Stem Cell-Based Therapy. Int. J. Mol. Sci. 2020, 21, 2702. [Google Scholar] [CrossRef] [Green Version]
- Alves, R.; Grimalt, R. A Review of Platelet-Rich Plasma: History, Biology, Mechanism of Action, and Classification. Skin Appendage Disord. 2018, 4, 18–24. [Google Scholar] [CrossRef]
- Emer, J. Platelet-Rich Plasma (PRP): Current Applications in Dermatology. Skin Ther. Lett. 2019, 24, 1–6. [Google Scholar] [PubMed]
- Diesen, D.L.; Lawson, J.H. Bovine thrombin: History, use, and risk in the surgical patient. Vascular 2008, 16 (Suppl. 1), S29–S36. [Google Scholar] [CrossRef] [PubMed]
- Ofosu, F.A.; Crean, S.; Reynolds, M.W. A safety review of topical bovine thrombin-induced generation of antibodies to bovine proteins. Clin. Ther. 2009, 31, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Chapman, W.C.; Singla, N.; Genyk, Y.; McNeil, J.W.; Renkens, K.L., Jr.; Reynolds, T.C.; Murphy, A.; Weaver, F.A. A phase 3, randomized, double-blind comparative study of the efficacy and safety of topical recombinant human thrombin and bovine thrombin in surgical hemostasis. J. Amer. Coll. Surg. 2007, 205, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Cada, D.J.; Levien, T.; Baker, D.E. Thrombin, Topical (Recombinant). Hosp. Pharm. 2008, 43, 577–585. [Google Scholar] [CrossRef]
- Textor, J.A.; Tablin, F. Activation of equine platelet-rich plasma: Comparison of methods and characterization of equine autologous thrombin. Vet. Surg. 2012, 41, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Nuccitelli, R. Application of pulsed electric fields to cancer therapy. Bioelectricity 2019, 1, 30–34. [Google Scholar] [CrossRef]
- Garner, A.L. Pulsed electric field inactivation of microorganisms: From fundamental biophysics to synergistic treatments. Appl. Microbiol. Biotechnol. 2019, 103, 7917–7929. [Google Scholar] [CrossRef]
- Zhang, J.; Blackmore, P.F.; Hargrave, B.Y.; Xiao, S.; Beebe, S.J.; Schoenbach, K.H. Nanosecond pulse electric field (nanopulse): A novel non-ligand agonist for platelet activation. Arch. Biochem. Biophys. 2008, 471, 240–248. [Google Scholar] [CrossRef]
- LaPlante, N.; Neculaes, V.B.; Lee, B.D.; Torres, A.S.; Conway, K.; Klopman, S.; Caiafa, A. Ex-vivo Platelet Activation using Electric Pulse Stimulation. In Proceedings of the 6th International Conference on Biomedical Electronics and Devices (BIODEVICES 2013), Barcelona, Spain, 11–14 February 2013; pp. 202–208. [Google Scholar] [CrossRef] [Green Version]
- Caiafa, A.; Neculaes, V.B.; Garner, A.L.; Jiang, Y.; Klopman, S.; Torres, A.; LaPlante, N. Compact solid state pulsed power architecture for biomedical workflows: Modular topology, programmable pulse output and experimental validation on Ex vivo platelet activation. In Proceedings of the 2014 IEEE International Power Modulator and High Voltage Conference (IPMHVC), Santa Fe, MO, USA, 1–5 June 2014; pp. 35–40. [Google Scholar] [CrossRef]
- Torres, A.S.; Caiafa, A.; Garner, A.L.; Klopman, S.; LaPlante, N.; Morton, C.; Conway, K.; Michelson, A.D.; Frelinger, A.L.; Neculaes, V.B. Platelet activation using electric pulse stimulation: Growth factor profile and clinical implications. J. Trauma Acute Care Surg. 2014, 77 (Suppl. 2), S94–S100. [Google Scholar] [CrossRef]
- Neculaes, V.B.; Torres, A.; Caiafa, A.; Morton, C.; Larriera, A.; Klopman, S.; Conway, K.; Garner, A.L. Ex vivo platelet activation with extended duration pulse electric fields for autologous platelet gel applications. EWMA J. 2015, 15, 15–19. [Google Scholar]
- Neculaes, V.B.; Torres, A.S.; Caiafa, A.; Lee, B.D.L.; Garner, A.L. Platelet Activation and Growth Factor Release Using Electric Pulses. U.S. Patent 9,452,199, 27 September 2016. [Google Scholar]
- Frelinger, A.L., III; Torres, A.S.; Caiafa, A.; Morton, C.A.; Berny-Lang, M.A.; Gerrits, A.J.; Carmichael, S.L.; Neculaes, V.B.; Michelson, A.D. Platelet-rich plasma stimulated by pulse electric fields: Platelet activation, procoagulant markers, growth factor release and cell proliferation. Platelets 2016, 27, 128–135. [Google Scholar] [CrossRef]
- Garner, A.L.; Caiafa, A.; Jiang, Y.; Klopman, S.; Morton, C.; Torres, A.S.; Loveless, A.M.; Neculaes, V.B. Design, characterization and experimental validation of a compact, flexible pulsed power architecture for ex vivo platelet activation. PLoS ONE 2017, 12, e0181214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frelinger, A.L., III; Gerrits, A.J.; Neculaes, V.B.; Gremmel, T.; Torres, A.S.; Caiafa, A.; Carmichael, S.L.; Michelson, A.D. Tunable activation of therapeutic platelet-rich plasma by pulse electric field: Differential effects on clot formation, growth factor release, and platelet morphology. PLoS ONE 2018, 13, e0203557. [Google Scholar] [CrossRef] [PubMed]
- Garner, A.L.; Frelinger, A.L.; Gerrits, A.J.; Gremmel, T.; Forde, E.E.; Carmichael, S.L.; Michelson, A.D.; Neculaes, V.B. Using extracellular calcium concentration and electric pulse conditions to tune platelet-rich plasma growth factor release and clotting. Med. Hypotheses 2019, 125, 100–105. [Google Scholar] [CrossRef]
- Neculaes, B.; Garner, A.L.; Klopman, S.; Morton, C.A.; Torres, A.S. A multi-donor ex vivo platelet activation and growth factor release study using electric pulses with durations up to 100 microseconds. IEEE Access 2021, 9, 31340–31349. [Google Scholar] [CrossRef]
- Garner, A.L.; Torres, A.S.; Klopman, S.; Neculaes, B. Electrical stimulation of whole blood for growth factor release and potential clinical implications. Med. Hypotheses 2020, 143, 110105. [Google Scholar] [CrossRef]
- Neculaes, B.; Frelinger, A.L., III; Gerrits, A.J.; Gremmel, T.; Forde, E.E.; Klopman, S.; Carmichael, S.L.; Michelson, A.D. Activation of platelet-rich plasma by pulse electric fields: Voltage, pulse width and calcium concentration can be used to control and tune the release of growth factors, serotonin and hemoglobin. PLoS ONE 2021, 16, e0249209. [Google Scholar] [CrossRef]
- Frelinger, A.L., III; Gerrits, A.J.; Garner, A.L.; Torres, A.S.; Caiafa, A.; Morton, C.A.; Berny-Lang, M.; Carmichael, S.L.; Neculaes, V.B.; Michelson, A.D. Modification of pulsed electric field conditions results in distinct activation profiles of platelet-rich plasma. PLoS ONE 2016, 11, e0160933. [Google Scholar] [CrossRef] [Green Version]
- Hargrave, B.Y. Autologous platelet rich plasma (platelet gel): An appropriate intervention for salvaging cardiac myocytes under oxidative stress after myocardial infarction. Anat. Physiol. 2014, 4, 134. [Google Scholar] [CrossRef] [Green Version]
- Hargrave, B.; Li, F. Nanosecond pulse electric field activated-platelet rich plasma enhances the return of blood flow to large and ischemic wounds in a rabbit model. Physiol. Rep. 2015, 3, e12461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hargrave, B.; Varghese, F.; Barabutis, N.; Catravas, J.; Zemlin, C. Nanosecond pulsed platelet-rich plasma (ns PRP) improves mechanical and electrical cardiac function following myocardial reperfusion injury. Physiol. Rep. 2016, 4, e12710. [Google Scholar] [CrossRef] [PubMed]
- Rego, D.; Redondo, L.; Casaleiro, S.; Sousa, A.P.; Abreu, S.; Serra, M.; Santo, V.E. Application of pulsed electric fields for the valorization of platelets with no therapeutic value for transfusion medicine. Technology 2019, 7, 40–45. [Google Scholar] [CrossRef]
- Bradley, J.P.; Lawyer, T.J.; Ruef, S.; Towers, J.D.; Arner, J.W. Platelet-rich plasma shortens return to play in National Football League players with acute hamstring injuries. Orthop. J. Sports Med. 2020, 8, 2325967120911731. [Google Scholar] [CrossRef]
- Blanco-Rivera, J.; Elizondo-Rodríguez, J.; Simental-Mendía, M.; Vilchez-Cavazos, F.; Peña-Martínez, V.M.; Acosta-Olivo, C. Treatment of lateral ankle sprain with platelet-rich plasma: A randomized clinical study. Foot Ankle Sur. 2020, 26, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Keene, D.J.; Alsousou, J.; Harrison, P.; Hulley, P.; Wagland, S.; Parsons, S.R.; Thompson, J.Y.; O’Connor, H.M.; Schlüssel, M.M.; Dutton, S.J.; et al. Platelet rich plasma injection for acute Achilles tendon rupture: PATH-2 randomised, placebo controlled, superiority trial. BMJ 2019, 367, 16132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, D. Platelet-rich plasma. In Ultrasound for Interventional Pain Management; Peng, P., Finlayson, R., Lee, S.H., Bhatia, A., Eds.; Springer: Cham, Switzerland, 2020; pp. 317–324. [Google Scholar]
- Straum, O.K. The optimal platelet concentration in platelet-rich plasma for proliferation of human cells in vitro—diversity, biases, and possible basic experimental principles for further research in the field: A review. PeerJ 2020, 8, e10303. [Google Scholar] [CrossRef]
- Castle, J.; Smith, R.; Davis, B.; Klopman, S.; Torres, A.; Robinson, V.S.; Neculaes, V.B.; Garner, A.L. Pilot study assessing the impact of platelet activation electric stimulation protocols on hematopoietic and mesenchymal stem cell proliferation. In Proceedings of the 2016 IEEE International Power Modulator and High Voltage Conference (IPMHVC), San Francisco, CA, USA, 6–9 July 2016; pp. 88–90. [Google Scholar] [CrossRef]
- Castle, J.; Garner, A.L.; Smith, R.; Davis, B.; Klopman, S.; Dinn, S.R.; Torres, A.S.; Neculaes, V.B. Hematopoietic stem cell and fibroblast proliferation during electrically stimulated platelet activation. IEEE Access 2019, 6, 56395–56401. [Google Scholar] [CrossRef]
- Amin, H.M.; Ahmad, S.; Walenga, J.M.; Hoppensteadt, D.A.; Leitz, H.; Fareed, J. Soluble P-selectin in human plasma: Effect of anticoagulant matrix and its levels in patients with cardiovascular disorders. Clin. Appl. Thromb. Hemost. 2000, 6, 71–76. [Google Scholar] [CrossRef]
- Ferroni, P.; Martini, F.; Riondino, S.; La Farina, F.; Magnapera, A.; Ciatti, F.; Guadagni, F. Soluble P-selectin as a marker of in vivo platelet activation. Clin. Chim. Acta. 2009, 399, 88–91. [Google Scholar] [CrossRef]
- Katayama, M.; Handa, M.; Araki, Y.; Ambo, H.; Kawai, Y.; Watanabe, K.; Ikeda, Y. Soluble P-selectin is present in normal circulation and its plasma level is elevated in patients with thrombotic thrombocytopenic purpura and haemolytic uraemic syndrome. Br. J. Haematol. 1993, 84, 702–710. [Google Scholar] [CrossRef] [PubMed]

| Average | Concentration Ratio (Compared to Whole Blood) | |
|---|---|---|
| Whole Blood | ||
| WBC (×109 L−1) | 7.0 ± 1.26 | |
| RBC (×1012 L−1) | 4.06 ± 0.78 | |
| HCT (%) | 35.4 ± 6.70 | |
| PLT (×109 L−1) | 358 ± 94 | |
| Harvest | ||
| WBC (×109 L−1) | 10.8 ± 9.30 | 1.5 ± 1.28 |
| RBC (×1012 L−1) | 0.86 ± 0.41 | 0.21 ± 0.09 |
| HCT (%) | 6.9 ± 2.42 | 0.19 ± 0.09 |
| PLT (×109 L−1) | 1242 ± 361 | 3.8 ± 0.54 |
| Eclipse | ||
| WBC (×109 L−1) | 1.8 ± 1.11 | 0.3 ± 0.13 |
| RBC (×1012 L−1) | 0.05 ± 0.06 | 0.01 ± 0.02 |
| HCT (%) | 0.3 ± 0.42 | 0.02 ± 0.01 |
| PLT (×109 L−1) | 662 ± 480 | 2.0 ± 1.18 |
| EmCyte | ||
| WBC (×109 L−1) | 17.5 ± 6.50 | 2.5 ± 0.63 |
| RBC (×1012 L−1) | 0.20 ± 0.09 | 0.05 ± 0.02 |
| HCT (%) | 1.5 ± 0.58 | 0.04 ± 0.02 |
| PLT (×109 L−1) | 2014 ± 608 | 5.8 ± 0.80 |
| PLT | VEGF | EGF | PDGF | |||||
|---|---|---|---|---|---|---|---|---|
| Harvest PRP and PEF A | r | p | r | p | r | p | r | p |
| VEGF | −0.30 | 0.70 | −0.40 | 0.60 | −0.86 | 0.14 | ||
| EGF | 0.72 | 0.28 | −0.40 | 0.60 | 0.70 | 0.30 | ||
| PDGF | 0.29 | 0.72 | −0.86 | 0.14 | 0.70 | 0.30 | ||
| EmCyte PRP and PEF A | ||||||||
| VEGF | 0.04 | 0.96 | −0.05 | 0.96 | −0.15 | 0.85 | ||
| EGF | 0.73 | 0.27 | −0.05 | 0.96 | 0.72 | 0.28 | ||
| PDGF | 0.05 | 0.95 | −0.15 | 0.85 | 0.72 | 0.28 | ||
| Harvest PRP and BT | ||||||||
| VEGF | −0.01 | 0.99 | −0.99 | 0.02 | −0.69 | 0.1 | ||
| EGF | 0.18 | 0.83 | −0.99 | 0.02 | 0.66 | 0.34 | ||
| PDGF | 0.12 | 0.88 | −0.69 | 0.1 | 0.66 | 0.34 | ||
| EmCyte PRP and BT | ||||||||
| VEGF | 0.11 | 0.89 | −0.16 | 0.84 | −0.80 | 0.20 | ||
| EGF | 0.56 | 0.44 | −0.16 | 0.84 | 0.68 | 0.32 | ||
| PDGF | 0.43 | 0.58 | −0.80 | 0.20 | 0.68 | 0.32 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neculaes, B.; Garner, A.L.; Klopman, S.; Longman, E.A. Dependence of Electric Pulse Mediated Growth Factor Release on the Platelet Rich Plasma Separation Method. Appl. Sci. 2022, 12, 4965. https://doi.org/10.3390/app12104965
Neculaes B, Garner AL, Klopman S, Longman EA. Dependence of Electric Pulse Mediated Growth Factor Release on the Platelet Rich Plasma Separation Method. Applied Sciences. 2022; 12(10):4965. https://doi.org/10.3390/app12104965
Chicago/Turabian StyleNeculaes, Bogdan, Allen L. Garner, Steven Klopman, and Emme A. Longman. 2022. "Dependence of Electric Pulse Mediated Growth Factor Release on the Platelet Rich Plasma Separation Method" Applied Sciences 12, no. 10: 4965. https://doi.org/10.3390/app12104965







