Resistance and Resilience of Nine Plant Species to Drought in Inner Mongolia Temperate Grasslands of Northern China
Abstract
:1. Introduction
2. Materials and methods
2.1. Study Site
2.2. Experimental Design
2.3. Soil Temperature and Moisture
2.4. Variables of Plant Community
2.5. Plant Functional Trait
2.6. Data Analysis
3. Results
3.1. Soil Temperature and Moisture Induced by Drought Treatment
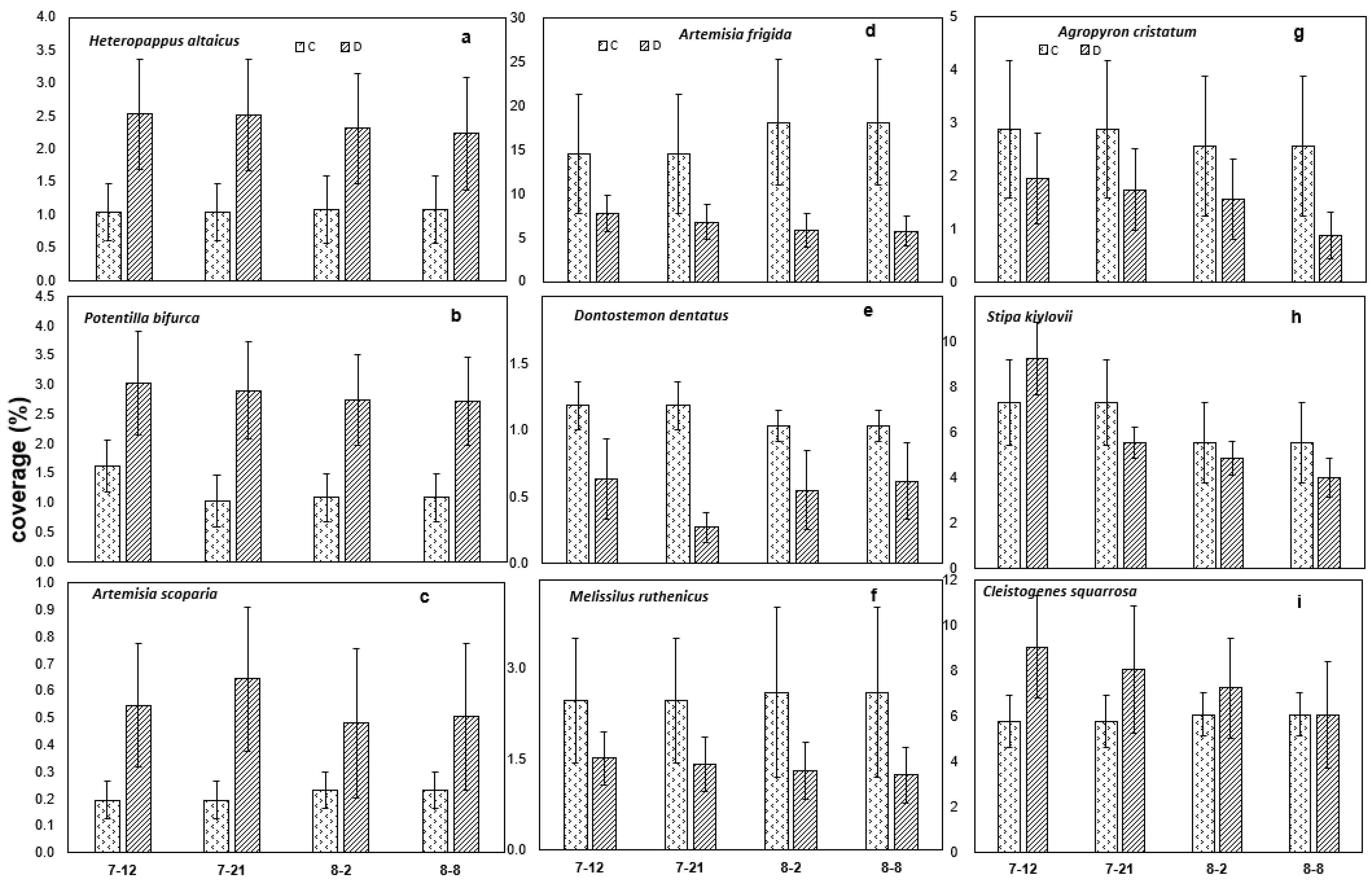
3.2. Effects of Drought on Coverage and Abundance of 9 Dominated Plant Species
3.3. Recovery of Coverage and Abundance Precipitation on Nine Plant Species after Drought Stress
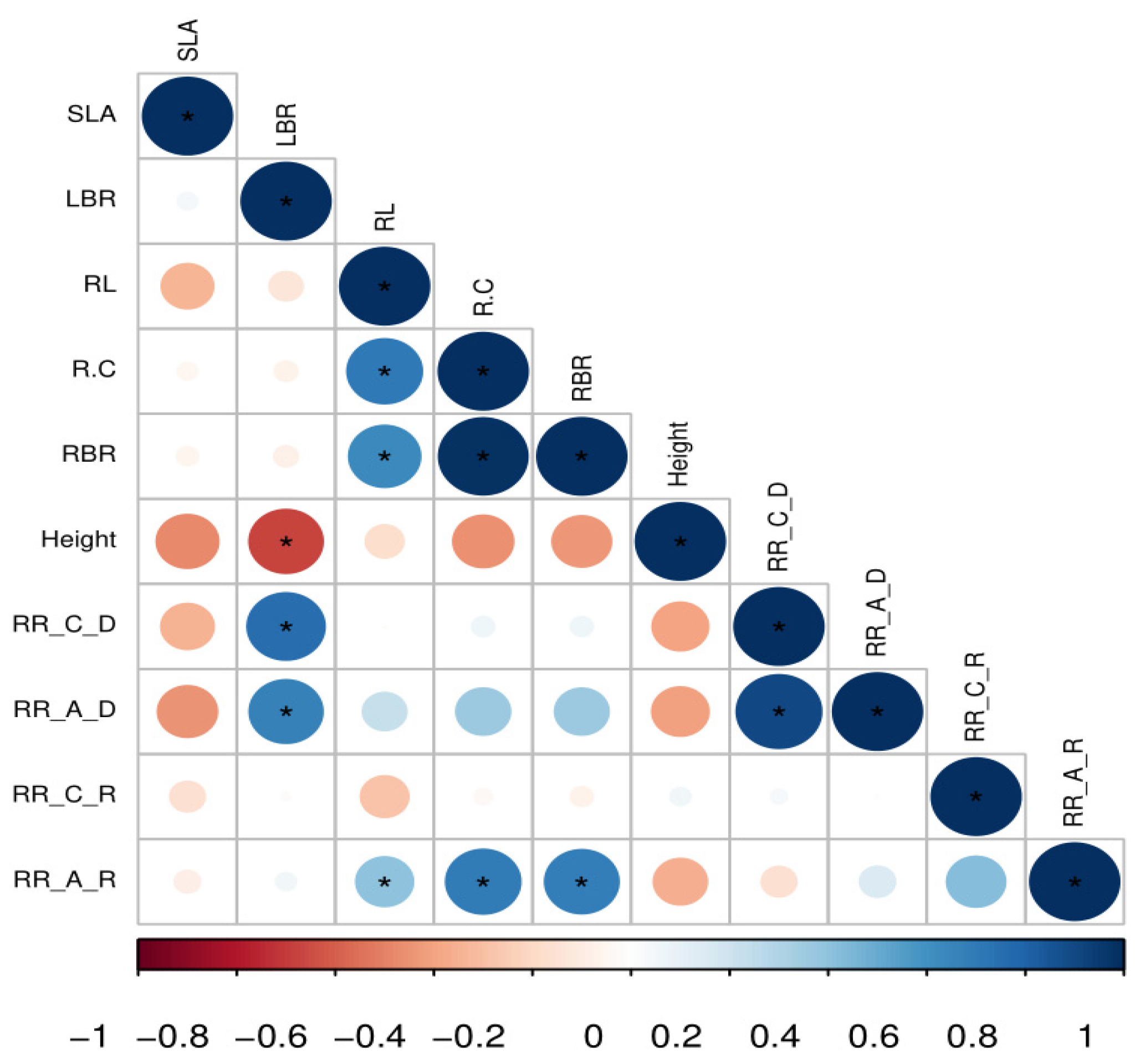
3.4. Relationships between Plant Abundance and Cover with Functional Trait
4. Discussion
4.1. Different Drought Resistance of the Plant Species
4.2. Recovery of Coverage and Abundance of Nine Species after Drought Stress
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christensen, J.H.; Kanikicharla, K.; Aldrian, E. Climate phenomena and their relevance for future regional climate change supplementary material. In Climate Change 2013: The Physical Science Basis; Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013; pp. 10–55. [Google Scholar]
- Xu, C.G.; McDowell, N.G.; Fisher, R.A.; Wei, L.; Sevanto, S.; Christoffersen, B.O.; Weng, E.; Middleton, R.S. Increasing impacts of extreme droughts on vegetation productivity under climate change. Nat. Clim. Chang. 2019, 9, 948–953. [Google Scholar] [CrossRef] [Green Version]
- Routson, C.C.; McKay, N.P.; Kaufman, D.S.; Erb, M.P.; Goosse, H.; Shuman, B.N.; Rodysill, J.R.; Ault, T. Mid-latitude net precipitation decreased with Arctic warming during the Holocene. Nature 2019, 568, 83–88. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Schoof, J.T.; Pryor, S.C.; Supernant, J. Development of daily precipitation projections for the United States based on probabilistic downscaling. J. Geophys. Res. Atmos. 2010, 115, D13106. [Google Scholar] [CrossRef]
- Huang, J.P.; Ji, M.X.; Xie, Y.K.; Wang, S.S.; He, Y.L.; Ran, J.J. Global semi-arid climate change over last 60 years. EGU Gen. Assem. Conf. Abstr. 2016, 46, 1131–1150. [Google Scholar] [CrossRef] [Green Version]
- Dai, A.G. Drought under global warming: A review. Wiley Interdiscip. Rev. Clim. Chang. 2011, 2, 45–65. [Google Scholar] [CrossRef] [Green Version]
- Craine, J.M.; Ocheltree, T.W.; Nippert, J.B.; Towne, E.G.; Skibbe, A.M.; Kembel, S.W.; Fargione, J.E. Global diversity of drought tolerance and grassland climate-change resilience. Nat. Clim. Chang. 2012, 3, 63–67. [Google Scholar] [CrossRef]
- Kamara, M.M.; Rehan, M.; Mohamed, A.M.; El Mantawy, R.F.; Kheir, A.M.S.; Abd El-Moneim, D.; Safhi, F.A.; ALshamrani, S.M.; Hafez, E.M.; Behiry, S.I.; et al. Genetic Potential and Inheritance Patterns of Physiological, Agronomic and Quality Traits in Bread Wheat under Normal and Water Deficit Conditions. Plants 2022, 11, 952. [Google Scholar] [CrossRef]
- Van Loon, A.F.; Van Lanen, H.A.J. Making the distinction between water scarcity and drought using an observation-modeling framework. Water Resour. Res. 2013, 49, 1483–1502. [Google Scholar] [CrossRef]
- Ahmed, M.; Kheir, A.M.S.; Mehmood, M.Z.; Ahmad, S.; Hasanuzzaman, M. Changes in Germination and Seedling Traits of Sesame under Simulated Drought. Phyton 2022, 91, 4. [Google Scholar]
- Kheir, A.M.S.; Alrajhi, A.A.; Ghoneim, A.M.; Ali, E.F.; Magrashi, A.; Zoghdan, M.G.; Elnashar, A. Modeling deficit irrigation-based evapotranspiration optimizes wheat yield and water productivity in arid regions. Agric. Water Manag. 2021, 256, 107122. [Google Scholar] [CrossRef]
- Fuhlendorf, S.D.; Briske, D.D.; Smeins, F.E. Herbaceous vegetation change in variable rangeland environments: The relative contribution of grazing and climatic variability. Appl. Veg. Sci. 2001, 4, 177–188. [Google Scholar] [CrossRef]
- Gremer, J.R.; Bradford, J.B.; Munson, S.M.; Duniway, M.C. Desert grassland responses to climate and soil moisture suggest divergent vulnerabilities across the southwestern United States. Glob. Chang. Biol. 2015, 21, 4049–4062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, Y.; Han, H.Y.; Du, Y.; Zhang, Q.; Jiang, L.; Hui, D.F.; Wang, S.Q. Nonlinear responses of soil respiration to precipitation changes in a semiarid temperate steppe. Sci. Rep. 2017, 7, 45782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, M.X.; Song, J.; Zhou, Z.X.; Ru, J.Y. Asymmetric responses of plant community structure and composition to precipitation variabilities in a semi-arid steppe. Oecologia 2019, 191, 697–708. [Google Scholar] [CrossRef]
- Han, J.J.; Chen, J.Q.; Miao, Y.; Wang, S.Q. Multiple Resource Use Efficiency (mRUE): A New Concept for Ecosystem Production. Sci. Rep. 2016, 6, 37453. [Google Scholar] [CrossRef] [Green Version]
- Reader, R.J.; Jalili, A.; Grime, J.P.; Spencer, R.E.; Matthews, N. A comparative study of plasticity in seedling rooting depth in drying soil. J. Ecol. 1993, 81, 543–550. [Google Scholar] [CrossRef]
- Ludlow, M.M. Strategies of response to water stress. In Structural and Functional Responses to Environmental Stresses; Kreeb, K., Richter, H., Hinckley, T., Eds.; SPB Academic Publishers: The Hague, The Netherlands, 1989; pp. 269–281. [Google Scholar]
- Volaire, F.; Norton, M.R.; Lelièvre, F. Summer drought survival strategies and sustainability of perennial temperate forage grasses in Mediterranean areas. Crop Sci. 2009, 49, 2386–2392. [Google Scholar] [CrossRef]
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J.K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef] [Green Version]
- Eissenstat, D.M.; Yanai, R. The ecology of root lifespan. Adv. Ecol. Res. 1997, 27, 1–60. [Google Scholar]
- Facette, M.R.; McCully, M.E.; Canny, M.J. Responses of maize roots to drying-limits of viability. Plant Cell Environ. 1999, 22, 1559–1568. [Google Scholar] [CrossRef]
- Currie, D.J.; Mittelbach, G.G.; Cornell, H.V.; Field, R.; Guégan, J.F.; Hawkins, B.A.; Kaufman, D.M.; Kerr, J.T.; Oberdorff, T.; O’Brien, E.; et al. Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol. Lett. 2004, 7, 1121–1134. [Google Scholar] [CrossRef]
- Wright, S.J.; Kitajima, K.; Kraft, N.J.B.; Reich, P.B.; Wright, I.J.; Bunker, D.E.; Condit, R.; Dalling, J.W.; Davies, S.J.; Diaz, S.; et al. Functional traits and the growth-mortality trade-off in tropical trees. Ecology 2010, 91, 3664–3674. [Google Scholar] [CrossRef] [PubMed]
- Nimmo, D.G.; Mac, N.R.; Cunningham, S.C.; Haslem, A.; Bennett, A.F. Vive la resistance: Reviving resistance for 21st century conservation. Trends Ecol. Evol. 2015, 30, 516–523. [Google Scholar] [CrossRef]
- Lake, P.S. Resistance, resilience and restoration. Ecol. Manag. Restor. 2013, 14, 20–24. [Google Scholar] [CrossRef]
- Rondeau, R.J.; Pearson, K.T.; Kelso, S. Vegetation Response in a Colorado Grassland-shrub Community to Extreme Drought: 1999–2010. Am. Midl. Nat. 2013, 170, 14–25. [Google Scholar] [CrossRef]
- Marine, Z.; Catherine, P.C.; Annette, M.B.; Marie, P.P.; Florence, V. What functional strategies drive drought survival and recovery of perennial species from upland grassland? Ann. Bot. 2015, 6, 1001–1015. [Google Scholar]
- Liu, Y.; Zhao, C.; Guo, J.; Zhang, L.; You, C. Short-term phosphorus addition augments the effects of nitrogen addition on soil respiration in a typical steppe. Sci. Total Environ. 2021, 761, 143211. [Google Scholar] [CrossRef]
- Miao, Y.; Liu, M.Z.; Xuan, J.; Xu, W. Effects of warming on soil respiration during the non-growing seasons in a semiarid temperate steppe. J. Plant Ecol. 2020, 13, 288–294. [Google Scholar] [CrossRef]
- Wang, D.; Huang, X.D.; Qiao, N.; Geng, Q.L.; Liu, Y.Z.; Song, H.Q.; Yang, Z.L.; Liu, C.; Wang, G. Effects of mowing and fertilization on soil quality in a semiarid grassland of North China. Land Degrad. Dev. 2020, 32, 1656–1666. [Google Scholar] [CrossRef]
- Zheng, J.; Cui, M.; Wang, C.; Wang, J.; Wang, S.L.; Sun, Z.J.; Ren, F.R.; Wan, S.Q.; Han, S.J. Elevated CO2, warming, N addition, and increased precipitation affect different aspects of the arbuscular mycorrhizal fungal community. Sci. Total Environ. 2022, 806, 150522. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhao, C.; Zhang, L.; Han, Y.; Cao, R.; Liu, Y.; Sun, S. Water table decline alters arthropod community structure by shifting plant communities and leaf nutrients in a Tibetan peatland. Sci. Total Environ. 2022, 814, 151944. [Google Scholar] [CrossRef] [PubMed]
- Morecroft, M.D.; Masters, G.J.; Brown, V.K.; Clarke, I.P.; Taylor, M.E.; Whitehouse, A.T. Changing precipitation patterns alter plant community dynamics and succession in an ex-arable grassland. Funct. Ecol. 2010, 18, 648–655. [Google Scholar] [CrossRef]
- Dostálek, J.; Frantík, T. Response of dry grassland vegetation to fluctuations in weather conditions: A 9-year case study in Prague (Czech Republic). Biologia 2011, 66, 837–847. [Google Scholar] [CrossRef]
- Zhang, L.H.; Wang, J.F.; Zhao, R.F.; Guo, Y.F.; Hao, L.Y. Aboveground net primary productivity and soil respiration display different responses to precipitation changes in desert grassland. J. Plant Ecol. 2022, 15, 57–70. [Google Scholar] [CrossRef]
- Mackie, K.A.; Zeiter, M.; Bloor, J.M.G.; Stampfli, A. Plant functional groups mediate drought resistance and recovery in a multisite grassland experiment. J. Ecol. 2019, 107, 937–949. [Google Scholar] [CrossRef] [Green Version]
- Májeková, M.; Martínková, J.; Hájek, T. Grassland plants show no relationship between leaf drought tolerance and soil moisture affinity, but rapidly adjust to changes in soil moisture. Funct. Ecol. 2019, 33, 774–785. [Google Scholar] [CrossRef]
- Song, H.H. Correlations among Plant Functional Traits in a Temperate Typical Steppe of Northern China. Master’s Thesis, Henan University, Kaifeng, China, 2018. (In Chinese with English abstract). [Google Scholar]
- Yang, H.J.; Li, Y.; Wu, M.Y.; Liu, W.; Zhang, Z.; Li, L.H.; Wan, S.Q. Plant community responses to nitrogen addition and increased precipitation: The importance of water availability and species traits. Glob. Chang. Biol. 2011, 17, 2936–2944. [Google Scholar] [CrossRef]
- Wang, J.M.; Song, H.Y.; Chen, J.Y.; Zhang, J.; Li, S.H.; Tao, J.P.; Liu, J.C. Response strategies of Lolium perenne L. to karst heterogeneous habitats under drought stress. Acta Ecol. Sin. 2020, 40, 4566–4572. [Google Scholar]
- Zhou, Z.X.; Zhang, L.W.; Liu, Y.Z.; Zhang, K.P.; Wang, W.R.; Zhu, J.K.; Chai, S.J.; Zhang, H.Y.; Miao, Y. Contrasting effects of nitrogen addition on vegetative phenology in dry and wet years in a temperate steppe on the Mongolian Plateau. Front. Plant Sci. 2022, 13, 10–11. [Google Scholar] [CrossRef]



| Coverage | Abundance | |||
|---|---|---|---|---|
| C | D | C | D | |
| Heteropappus altaicus | 0.96 ± 0.30 a | 0.43 ± 0.20 a | 12.83 ± 2.40 a | 4.00 ± 2.40 b |
| Potentilla bifurca | 2.82 ± 0.70 a | 2.24 ± 0.50 a | 11.20 ± 2.19 a | 13.00 ± 4.23 a |
| Artemisia scoparia | 0.56 ± 0.40 a | 0.56 ± 0.20 a | 9.75 ± 5.50 a | 13.80 ± 4.90 a |
| Artemisia frigida | 7.50 ± 1.00 a | 8.33 ± 2.00 a | 10.00 ± 1.70 a | 7.50 ± 1.60 a |
| Dontostemon dentatus | 0.01 * | 0.09 * | 1.00 ± 0.00 b | 2.00 ± 0.00 a |
| Melissilus ruthenicus | 1.18 ± 0.40 a | 0.78 ± 0.30 a | 2.33 ± 0.90 a | 2.60 ± 0.60 a |
| Agropyron cristatum | 2.97 ± 1.30 a | 1.00 ± 0.50 a | 125.67 ± 50.00 a | 39.00 ± 15.60 a |
| Stipa kiylovii | 4.58 ± 0.20 a | 12.18 ± 2.60 b | 9.20 ± 2.60 a | 16.50 ± 2.60 a |
| Cleistogenes squarrosa | 2.38 ± 0.70 a | 1.92 ± 0.40 a | 18.33 ± 3.40 a | 16.83 ± 2.80 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, Y.; Zhou, Z.; Jiang, M.; Song, H.; Yan, X.; Liu, P.; Ji, M.; Han, S.; Chen, A.; Wang, D. Resistance and Resilience of Nine Plant Species to Drought in Inner Mongolia Temperate Grasslands of Northern China. Appl. Sci. 2022, 12, 4967. https://doi.org/10.3390/app12104967
Miao Y, Zhou Z, Jiang M, Song H, Yan X, Liu P, Ji M, Han S, Chen A, Wang D. Resistance and Resilience of Nine Plant Species to Drought in Inner Mongolia Temperate Grasslands of Northern China. Applied Sciences. 2022; 12(10):4967. https://doi.org/10.3390/app12104967
Chicago/Turabian StyleMiao, Yuan, Zhenxing Zhou, Meiguang Jiang, Huanhuan Song, Xinyu Yan, Panpan Liu, Minglu Ji, Shijie Han, Anqun Chen, and Dong Wang. 2022. "Resistance and Resilience of Nine Plant Species to Drought in Inner Mongolia Temperate Grasslands of Northern China" Applied Sciences 12, no. 10: 4967. https://doi.org/10.3390/app12104967






