Dissociative Ionization and Coulomb Explosion of CHBrCl2 in Intense Near-Infrared Femtosecond Laser Fields
Abstract
:1. Introduction
2. Experiment and Theory Calculation
3. Results and Discussion
3.1. Time-of-Flight Mass Spectrum and DC-Sliced Images
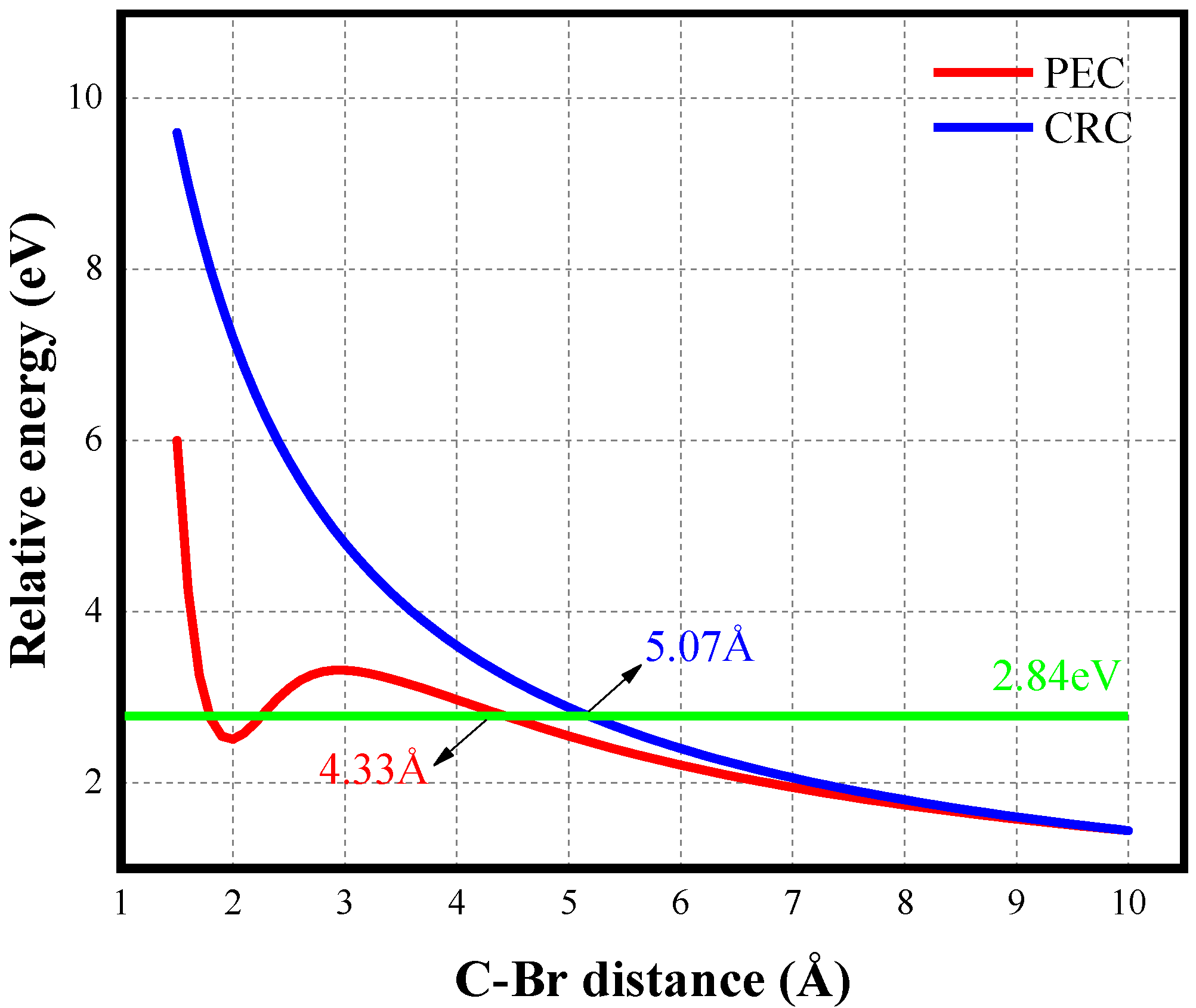
3.2. Dissociative Ionization of CHBrCl2 Molecules in 800 nm Femtosecond Laser Fields
- Channel (1) CHBrCl2 → CHBrCl2+ + e− → Cl+ + CHBrCl + e−
- Channel (2) CHBrCl2 → CHBrCl2+ + e− → Br+ + CHCl2 + e−
3.3. Coulomb Explosion of the Double-Charged Molecular Ion CHBrCl22+
- Channel (3) CHBrCl2 → CHBrCl22+ + 2e−→Br+ + CHCl2+ + 2e−
- Channel(4) CHBrCl2 → CHBrCl22+ + 2e− → Br+ + CHCl2+ + 2e−
- Channel(5) CHBrCl2 → CHBrCl22+ + 2e− → Br+ + CHCl2+ + 2e−
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cattaneo, L.; Pedrelli, L.; Bello, R.Y.; Palacios, A.; Keathley, P.D.; Martín, F.; Keller, U. Isolating Attosecond Electron Dynamics in Molecules where Nuclei Move Fast. Phys. Rev. Lett. 2022, 128, 063001. [Google Scholar] [CrossRef]
- Luo, S.; Liu, J.; Li, X.; Zhang, D.; Yu, X.; Ren, D.; Li, M.; Yang, Y.; Wang, Z.; Ma, P.; et al. Revealing Molecular Strong Field Autoionization Dynamics. Phys. Rev. Lett. 2021, 126, 103202. [Google Scholar] [CrossRef]
- Kaufman, B.; Rozgonyi, T.; Marquetand, P.; Weinacht, T. Competition between dynamic resonance and internal conversion in strong-field molecular ionization with chirped ultrafast laser pulses. Phys. Rev. A. 2021, 103, 023108. [Google Scholar] [CrossRef]
- Guo, Z.; Fang, Y.; Ge, P.; Yang, X.Y. Probing tunneling dynamics of dissociative H2 molecules using two-color bicircularly polarized fields. Phys. Rev. A 2021, 104, L051101. [Google Scholar] [CrossRef]
- Che, J.-Y.; Chen, C.; Wang, S.; Xin, G.-G.; Chen, Y.-J. Characterizing subcycle electron dynamics of polar molecules by asymmetry in photoelectron momentum distributions. Phys. Rev. A 2021, 104, 063104. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, P.; Ma, J.; Li, H.; Gong, X.; Wu, J. Correlated electron–nuclear dynamics of molecules in strong laser fields. J. Phys. B At. Mol. Opt. Phys. 2020, 53, 162001. [Google Scholar] [CrossRef]
- Keldysh, L. Ionization in the field of a strong electromagnetic wave. Sov. Phys. JETP 1965, 20, 1307–1314. [Google Scholar]
- Dewitt, M.J.; Levis, R.J. Calculating the Keldysh adiabaticity parameter for atomic, diatomic, and polyatomic molecules. Chem. Phys. Lett. 1998, 108, 7739–7742. [Google Scholar] [CrossRef] [Green Version]
- Levis, R.J.; DeWitt, M.J. Photoexcitation, ionization, and dissociation of molecules using intense near-infrared radiation of femtosecond duration. J. Phys. Chem. A 1999, 103, 6493–6507. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Yang, Y. Multi-ionization of the Cl2 molecule in the near-infrared femtosecond laser field. RSC Adv. 2020, 10, 332–337. [Google Scholar] [CrossRef] [Green Version]
- Pei, M.; Yang, Y.; Zhang, J.; Sun, Z. Dehydrogenation involved Coulomb explosion of molecular C2H4 FBr in an intense laser field. Chem. Phys. Lett. 2018, 697, 53–60. [Google Scholar] [CrossRef]
- Corrales, M.; González-Vázquez, J.; De Nalda, R.; Bañares, L. Coulomb explosion imaging for the visualization of a conical intersection. J. Phys. Chem. Lett. 2018, 10, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Pitzer, M.; Kunitski, M.; Johnson, A.S.; Jahnke, T.; Sann, H.; Sturm, F.; Schmidt, L.P.H.H.; Schmidt-Böcking, H.; Dörner, R.; stohner, J.; et al. Direct determination of absolute molecular stereochemistry in gas phase by Coulomb explosion imaging. Science 2013, 341, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, W.B.; Lee, Y.R.; Lin, S.M. Photodissociation of CH2BrCl at 248 and 193 nm investigated by translational spectroscopy. Chem. Phys. Lett. 1994, 227, 467–471. [Google Scholar] [CrossRef]
- Person, M.D.; Kash, P.W.; Butler, L.J. Nonadiabaticity and the competition between alpha and beta bond fission upon 1[n,π*(C=O)] excitation in acetyl- and bromoacetyl chloride. J. Chem. Phys. 1992, 97, 355–373. [Google Scholar] [CrossRef]
- Che, D.-C.; Nakamura, M.; Chang, H.-P.; Lin, K.-C.; Kasai, T.; Aquilanti, V.; Palazzetti, F.; Lin, K.-C. UV Photodissociation of Halothane in a Focused Molecular Beam: Space-Speed Slice Imaging of Competitive Bond Breaking into Spin–Orbit-Selected Chlorine and Bromine Atoms. J. Phys. Chem. A 2020, 124, 5288–5296. [Google Scholar] [CrossRef]
- Liu, B.; Li, Z.; Sun, H.; Sun, Z. Dissociative Ionization of Molecular CF2Br2 under 800 and 400 nm Intense Femtosecond Laser Fields. Appl. Sci. 2021, 11, 1704. [Google Scholar] [CrossRef]
- Liu, B.; Yang, Y.; Sun, H.; Sun, Z. Dissociative Ionization and Coulomb Explosion of Molecular Bromocyclopropane in an Intense Femtosecond Laser Field. Molecules 2018, 23, 3096. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Li, M.; Nibarger, J.P.; Gibson, G.N. Single and double ionization of diatomic molecules in strong laser fields. Phys. Rev. A 1998, 58, R4271–R4274. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Brandhorst, K.; Grunenberg, J. How strong is it? The interpretation of force and compliance constants as bond strength descriptors. Chem. Soc. Rev. 2008, 37, 1558–1567. [Google Scholar] [CrossRef]
- Rassolov, V.A.; Ratner, M.A.; Pople, J.A.; Redfern, P.C.; Curtiss, L.A. 6-31G* basis set for third-row atoms. J. Comput. Chem. 2001, 22, 976–984. [Google Scholar] [CrossRef]
- Curtiss, L.A.; Redfern, P.C.; Raghavachari, K. Gaussian-4 theory. J. Chem. Phys. 2007, 126, 084108. [Google Scholar] [CrossRef] [PubMed]
- Kállay, M.; Gauss, J. Calculation of excited-state properties using general coupled-cluster and configuration-interaction models. J. Chem. Phys. 2004, 121, 9257–9269. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Cvitaš, T.; Klasinc, L.; Güsten, H. Photoelectron spectra of some halogenomethanes. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1981, 77, 2049–2058. [Google Scholar] [CrossRef]
- Wu, C.; Yang, Y.; Wu, Z.; Chen, B.; Dong, H.; Liu, X.; Deng, Y.; Liu, H.; Liu, Y.; Gong, Q. Coulomb explosion of nitrogen and oxygen molecules through non-Coulombic states. Phys. Chem. Chem. Phys. 2011, 13, 18398. [Google Scholar] [CrossRef] [PubMed]







| Channel | EAP (eV) | KERs (eV) | ET (eV) | EAV (eV) |
|---|---|---|---|---|
| (1) | 17.57 | 0.25 | 0.32 | 1.03 |
| (2) | 15.65 | 0.18 | 0.35 | 1.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Yang, Y. Dissociative Ionization and Coulomb Explosion of CHBrCl2 in Intense Near-Infrared Femtosecond Laser Fields. Appl. Sci. 2022, 12, 5014. https://doi.org/10.3390/app12105014
Liu B, Yang Y. Dissociative Ionization and Coulomb Explosion of CHBrCl2 in Intense Near-Infrared Femtosecond Laser Fields. Applied Sciences. 2022; 12(10):5014. https://doi.org/10.3390/app12105014
Chicago/Turabian StyleLiu, Botong, and Yan Yang. 2022. "Dissociative Ionization and Coulomb Explosion of CHBrCl2 in Intense Near-Infrared Femtosecond Laser Fields" Applied Sciences 12, no. 10: 5014. https://doi.org/10.3390/app12105014





