Effects of Biochar Production Methods and Biomass Types on Lead Removal from Aqueous Solution
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
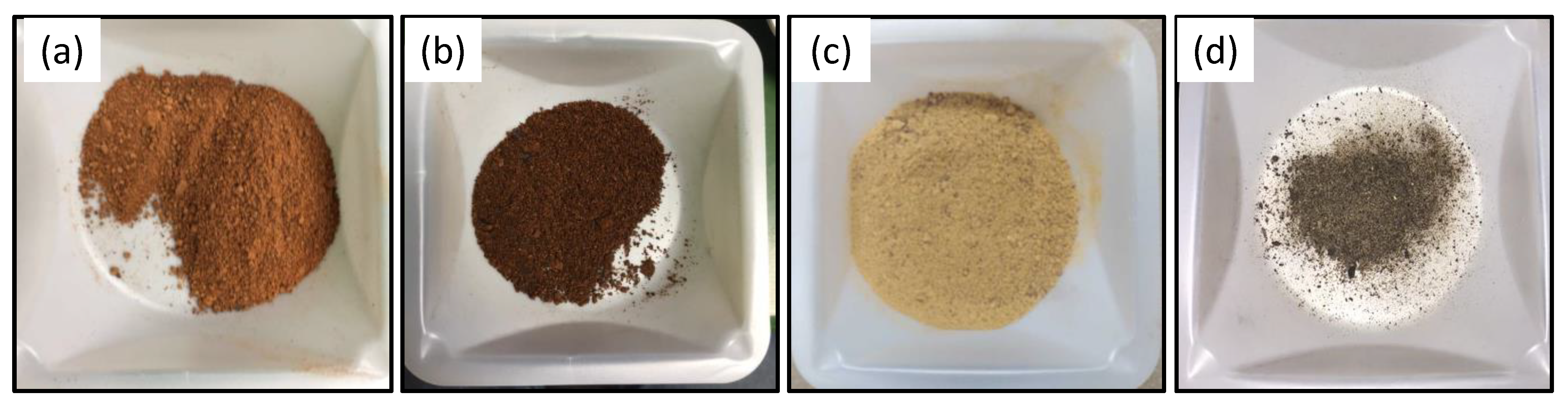
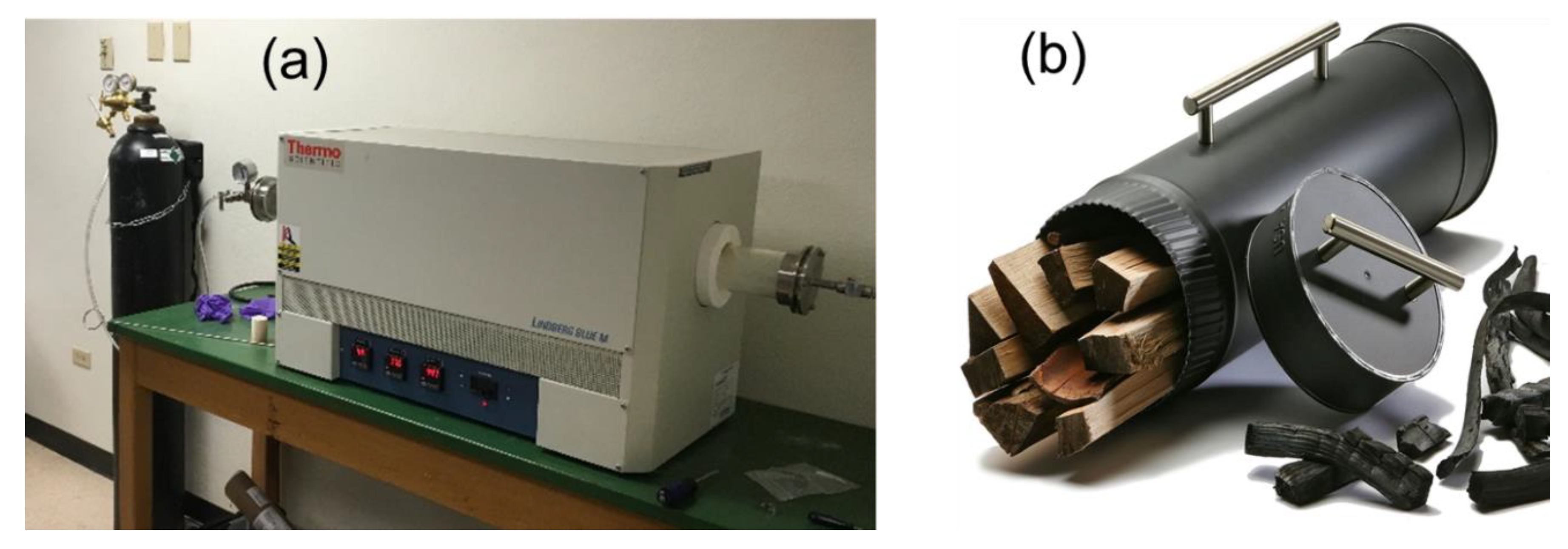
2.1. Biochar Production
2.2. Biochar Characterization
2.3. Lead Adsorption
3. Results and Discussion
3.1. Biochar Yield and Properties
3.2. FTIR Analysis
3.3. Lead Adsorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Q.; Yang, N.; Li, Y.; Ren, B.; Ding, X.; Bian, H.; Yao, X. Total concentrations and sources of heavy metal pollution in global river and lake water bodies from 1972 to 2017. Glob. Ecol. Conserv. 2020, 22, e00925. [Google Scholar] [CrossRef]
- Naseem, R.; Tahir, S.S. Removal of Pb (II) from aqueous/acidic solutions by using bentonite as an adsorbent. Water Res. 2001, 35, 3982–3986. [Google Scholar] [CrossRef]
- Karunanayake, A.G.; Todd, O.A.; Crowley, M.; Ricchetti, L.; Pittman, C.U.; Anderson, R.; Mohan, D.; Mlsna, T. Lead and cadmium remediation using magnetized and nonmagnetized biochar from Douglas fir. Chem. Eng. J. 2018, 331, 480–491. [Google Scholar] [CrossRef]
- Bellinger, D.C. Lead Contamination in Flint—An Abject Failure to Protect Public Health. N. Engl. J. Med. 2016, 374, 1101–1103. [Google Scholar] [CrossRef] [PubMed]
- Renner, R. Out of Plumb: When Water Treatment Causes Lead Contamination. Environ. Health Perspect. 2009, 117, A542–A547. [Google Scholar] [CrossRef] [Green Version]
- Schock, M.R.; Cantor, A.F.; Triantafyllidou, S.; Desantis, M.K.; Scheckel, K.G. Importance of Pipe Deposits to Lead and Copper Rule Compliance. J. AWWA 2014, 106, E336–E349. [Google Scholar] [CrossRef]
- Roy, S. Lead Results from Tap Water Sampling in Flint, MI during the Flint Water Crisis. 2015. Available online: http://flintwaterstudy.org/2015/12/complete-dataset-lead-results-in-tap-water-for-271-flint-samples/ (accessed on 3 May 2022).
- Inyang, M.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.R.; Pullammanappallil, P.; Cao, X. Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresour. Technol. 2012, 110, 50–56. [Google Scholar] [CrossRef]
- Mohan, D.; Singh, K.P. Single-and multi-component adsorption of cadmium and zinc using activated carbon derived from bagasse—An agricultural waste. Water Res. 2002, 36, 2304–2318. [Google Scholar] [CrossRef]
- Holan, Z.R.; Volesky, B. Biosorption of lead and nickel by biomass of marine algae. Biotechnol. Bioeng. 1994, 43, 1001–1009. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, A.; Anastopoulos, I. Adsorptive removal of bisphenol A (BPA) from aqueous solution: A review. Chemosphere 2017, 168, 885–902. [Google Scholar] [CrossRef]
- Gwenzi, W.; Chaukura, N.; Noubactep, C.; Mukome, F.N.D. Biochar-based water treatment systems as a potential low-cost and sustainable technology for clean water provision. J. Environ. Manag. 2017, 197, 732–749. [Google Scholar] [CrossRef]
- Owsianiak, M.; Lindhjem, H.; Cornelissen, G.; Hale, S.E.; Sørmo, E.; Sparrevik, M. Environmental and economic impacts of biochar production and agricultural use in six developing and middle-income countries. Sci. Total Environ. 2021, 755, 142455. [Google Scholar] [CrossRef]
- García-Vargas, M.C.; Contreras, M.D.M.; Castro, E. Avocado-derived biomass as a source of bioenergy and bioproducts. Appl. Sci. 2020, 10, 8195. [Google Scholar] [CrossRef]
- Teigiserova, D.A.; Tiruta-Barna, L.; Ahmadi, A.; Hamelin, L.; Thomsen, M. A step closer to circular bioeconomy for citrus peel waste: A review of yields and technologies for sustainable management of essential oils. J. Environ. Manag. 2021, 280, 111832. [Google Scholar] [CrossRef] [PubMed]
- Warguła, Ł.; Wieczorek, B.; Kukla, M.; Krawiec, P.; Szewczyk, J.W. The problem of removing seaweed from the beaches: Review of methods and machines. Water 2021, 13, 736. [Google Scholar] [CrossRef]
- Hoslett, J.; Ghazal, H.; Ahmad, D.; Jouhara, H. Removal of copper ions from aqueous solution using low temperature biochar derived from the pyrolysis of municipal solid waste. Sci. Total Environ. 2019, 673, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Mireles, S.; Parsons, J.; Trad, T.; Cheng, C.-L.; Kang, J. Lead removal from aqueous solutions using biochars derived from corn stover, orange peel, and pistachio shell. Int. J. Environ. Sci. Technol. 2019, 16, 5817–5826. [Google Scholar] [CrossRef]
- Karunanithi, R.; Ok, Y.S.; Dharmarajan, R.; Ahmad, M.; Seshadri, B.; Bolan, N.; Naidu, R. Sorption, kinetics and thermodynamics of phosphate sorption onto soybean stover derived biochar. Environ. Technol. Innov. 2017, 8, 113–125. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, J.-Y.; Cho, T.-S.; Choi, J.W. Influence of Pyrolysis Temperature on Physicochemical Properties of Biochar Obtained from the Fast Pyrolysis of Pitch Pine (Pinus rigida). Bioresour. Technol. 2012, 118, 158–162. [Google Scholar] [CrossRef]
- Sun, J.; He, F.; Pan, Y.; Zhang, Z. Effects of Pyrolysis Temperature and Residence Time on Physicochemical Properties of Different Biochar Types. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2016, 67, 12–22. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, P.; Yuan, X.; Li, Y.; Han, L. Effect of Pyrolysis Temperature and Correlation Analysis on the Yield and Physicochemical Properties of Crop Residue Biochar. Bioresour. Technol. 2020, 296, 122318. [Google Scholar] [CrossRef] [PubMed]
- Mukome, F.N.; Parikh, S.J. Chemical, Physical, and Surface characterization of Biochar. In Biochar: Production, Characterization, and Applications; Ok, Y.S., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 68–96. [Google Scholar]
- Masís-Meléndez, F.; Segura-Chavarría, D.; García-González, C.A.; Quesada-Kimsey, J.; Villagra-Mendoza, K. Variability of physical and chemical properties of TLUD stove derived biochars. Appl. Sci. 2020, 10, 507. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Camps-Arbestain, M.; Lehmann, J. Biochar pH, electrical conductivity and liming potential. In Biochar: A Guide to Analytical Methods, Taylor and Francis; Singh, B., Camps-Arbestain, M., Lehmann, J., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 23–38. [Google Scholar]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, B.; Yao, Y.; Fang, J.; Zhang, M.; Zhou, Y.; Chen, H.; Yang, L. Effects of Feedstock Type, Production Method, and Pyrolysis Temperature on Biochar and Hydrochar Properties. Chem. Eng. J. 2014, 240, 574–578. [Google Scholar] [CrossRef]
- Li, X.; Shen, Q.; Zhang, D.; Mei, X.; Ran, W.; Xu, Y.; Yu, G. Functional Groups Determine Biochar Properties (PH and EC) as Studied by Two-Dimensional 13C NMR Correlation Spectroscopy. PLoS ONE 2013, 8, e65949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jouhara, H.; Ahmad, D.; van den Boogaert, I.; Katsou, E.; Simons, S.; Spencer, N. Pyrolysis of Domestic Based Feedstock at Temperatures up to 300 °C. Therm. Sci. Eng. Prog. 2018, 5, 117–143. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef] [Green Version]
- Roberts, D.A.; Paul, N.A.; Dworjanyn, S.A.; Bird, M.I.; de Nys, R. Biochar from commercially cultivated seaweed for soil amelioration. Sci. Rep. 2015, 5, 9665. [Google Scholar] [CrossRef] [Green Version]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [Green Version]
- Elnour, A.Y.; Alghyamah, A.A.; Shaikh, H.M.; Poulose, A.M.; Al-Zahrani, S.M.; Anis, A.; Al-Wabel, M.I. Effect of Pyrolysis Temperature on Biochar Microstructural Evolution, Physicochemical Characteristics, and Its Influence on Biochar/Polypropylene Composites. Appl. Sci. 2019, 9, 1149. [Google Scholar] [CrossRef] [Green Version]
- Habte, L.; Shiferaw, N.; Mulatu, D.; Thenepalli, T.; Chilakala, R.; Ahn, J.W. Synthesis of Nano-Calcium Oxide from Waste Eggshell by Sol-Gel Method. Sustainability 2019, 11, 3196. [Google Scholar] [CrossRef] [Green Version]
- Vargas, A.M.M.; Cazetta, A.L.; Kunita, M.H.; Silva, T.L.; Almeida, V.C. Adsorption of Methylene Blue on Activated Carbon Produced from Flamboyant Pods (Delonix regia): Study of Adsorption Isotherms and Kinetic Models. Chem. Eng. J. 2011, 168, 722–730. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Rupérez, P. FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocoll. 2011, 25, 1514–1520. [Google Scholar] [CrossRef]
- Buss, W.; Jansson, S.; Mašek, O. Unexplored potential of novel biochar-ash composites for use as organo-mineral fertilizers. J. Clean. Prod. 2019, 208, 960–967. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Zhao, Y.; Sima, J.; Zhao, L.; Mašek, O.; Cao, X. Indispensable role of biochar-inherent mineral constituents in its environmental applications: A review. Bioresour. Technol. 2017, 241, 887–899. [Google Scholar] [CrossRef] [Green Version]


| Tube Furnace | BioCharlie Log | |||||||
|---|---|---|---|---|---|---|---|---|
| Properties | Avocado Seed (AST) 2 | Avocado Peel (APT) | Grapefruit Peel (GPT) | Brown Seaweed (BST) | Avocado Seed (ASB) 2 | Avocado Peel (APB) | Grapefruit Peel (GPB) | Brown Seaweed (BSB) |
| Yield (%) | 42.50 | 56.18 | 47.57 | 63.15 | 36.65 | 48.65 | 41.72 | 51.12 |
| pH | 7.66 | 9.40 | 9.04 | 7.94 | 8.85 | 9.65 | 8.30 | 6.88 |
| EC (µs/cm) 1 | 125 | 272 | 855 | 1534 | 4466 | 3330 | 2400 | 2057 |
| Moisture (%) | 1.67 | 3.46 | 1.02 | N/A | 3.67 | 5.45 | 1.15 | 0.86 |
| Volatile Matter (%) | 28.17 | 23.85 | 30.00 | N/A | 33.87 | 58.71 | 35.52 | 8.27 |
| Ash Content (%) | 34.38 | 28.81 | 23.10 | N/A | 17.57 | 6.25 | 17.33 | 68.06 |
| Fixed Matter (%) | 35.78 | 43.88 | 45.88 | N/A | 44.89 | 29.59 | 46.00 | 22.81 |
| C (%) | 60.33 | 54.21 | 53.50 | 27.87 | N/A | N/A | N/A | N/A |
| N (%) | 1.38 | 1.02 | 1.59 | 1.6 | N/A | N/A | N/A | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granados, P.; Mireles, S.; Pereira, E.; Cheng, C.-L.; Kang, J.J. Effects of Biochar Production Methods and Biomass Types on Lead Removal from Aqueous Solution. Appl. Sci. 2022, 12, 5040. https://doi.org/10.3390/app12105040
Granados P, Mireles S, Pereira E, Cheng C-L, Kang JJ. Effects of Biochar Production Methods and Biomass Types on Lead Removal from Aqueous Solution. Applied Sciences. 2022; 12(10):5040. https://doi.org/10.3390/app12105040
Chicago/Turabian StyleGranados, Paola, Sergio Mireles, Engil Pereira, Chu-Lin Cheng, and James Jihoon Kang. 2022. "Effects of Biochar Production Methods and Biomass Types on Lead Removal from Aqueous Solution" Applied Sciences 12, no. 10: 5040. https://doi.org/10.3390/app12105040
APA StyleGranados, P., Mireles, S., Pereira, E., Cheng, C.-L., & Kang, J. J. (2022). Effects of Biochar Production Methods and Biomass Types on Lead Removal from Aqueous Solution. Applied Sciences, 12(10), 5040. https://doi.org/10.3390/app12105040







