1. Introduction
We have previously published data on the parameters of the magnetic field or electromagnetic treatments that control the physical properties of water [
1,
2,
3,
4,
5,
6]. Changes in the physical properties of water can affect biological processes, i.e., they can exhibit biophysical effects. One of the models most sensitive to changes in the physical properties of solutions after magnetic field treatment is the respiratory burst of neutrophils [
5,
6]. The respiratory burst is manifested during phagocytosis or in response to soluble mediators (e.g., the bacterial peptide N-formyl-Met-Leu-Phe (fMLF) or the phorbol ester, phorbol 12-myristate 13-acetate (PMA)) by a rapid release of ROS. One of the most sensitive methods for detecting ROS is based on the measurement of chemiluminescence that occurs during the oxidation of luminol (luminol-enhanced chemiluminescence).
Using luminol-enhanced cellular chemiluminescence, we have previously shown the effect of weak and very weak combined magnetic fields (CMF) with static and variable components at the micro- and nanotesla scale directly on neutrophil suspensions and, indirectly, through aqueous solutions. The addition of a water sample pre-treated with CMF (60 µT static magnetic field (SMF) and 100 nT alternating magnetic field (AMF), 12.6 Hz) to a neutrophil suspension increased the production of ROS by approximately 70% [
7,
8]. This property, which was originally characteristic of a water sample pre-treated with CMF, was maintained after a series of dilutions that were performed in combination with mechanical treatment. Namely, a water sample pre-treated with CMF was mixed with deionized water at a ratio of 1:100 and shaken intensively by hand with an approximate frequency of about 4 Hz (21 strokes in 4.8 s) to produce the first centesimal dilution. All subsequent dilutions consisted of one part of the previous dilution and 99 parts of the solvent with intensive shaking performed identically after each successive step. However, if a similar dilution process was performed in a “magnetic vacuum” or without mechanical action, then the ability of the sample to increase the production of ROS did not differ from the control [
5,
6]. This indicates that a magnetic field with certain controlled parameters, as well as a mechanical effect, represent factors that determine the activity of a water sample.
When testing the effect of various substances (including HD forms of proteins and low molecular weight regulators of neutrophil functions) on such exposure to a magnetic field, we concluded that the result of CMF treatment to a certain extent could depend not only on the field parameters, but also on the way that these experiments were carried out. The production of ROS changed by orders of magnitude if various types of samples were subjected to CMF treatment simultaneously inside a controlled magnetic field generation device, possibly indicating a contactless interaction between the samples.
The possibility of such contactless or distant interaction between samples was initially treated by us with skepticism, but since such an assumption appeared, we decided to conduct experiments to confirm or disprove this phenomenon. It was clear that if the results of these experiments could not be reproduced, or they were consistently negative, then it would be pointless to continue studies in this field.
Phenomena similar to the ultra-weak photon emission ability of biological objects or the ability to interact distantly have been extensively studied and described earlier [
9,
10,
11,
12,
13]. However, it had not been possible to reach a consensus on this matter, since the effects in some cases were too unpredictable, not always reproducible, and could often be caused by other processes (e.g., absorption of atmospheric gases). Therefore, some researchers still doubt the existence of such effects, including distant interactions [
13]. Probably, this can happen if the researchers do not know or do not observe all the necessary conditions for the realization of these effects.
It has been previously shown that solutions obtained by repeated sequential dilution of an original substance with mechanical treatment (intensive hand shaking with an approximate frequency of about 4 Hz (21 strokes in about 4.8 s)) at each step, in particular, HD of Abs to IFNγ, are capable of emitting radiation in the IR range. More interestingly, the IFNγ solution shows lower emission intensity within 1600–1800 cm
−1 after contactless incubation with HD Abs to IFNγ. This may indicate lower population of the vibrational energy levels of water molecules in the analyzed samples [
14].
The aim of this study was to determine the effect of incubation conditions, namely, the presence of weak and ultra-weak magnetic fields, on distant interactions between solutions of HD Abs to IFNγ and samples of IFNγ in nanomolar concentration or water. In addition, our goal was to test the distant effect of another HD sample, HD PMA, on the properties of solutions which were jointly incubated with it under different conditions: various magnetic fields, shielding between cuvettes, and equalizing the influence of atmospheric gases.
2. Materials and Methods
2.1. Generating a Controlled Magnetic Field
The experiments were carried out using relatively weak magnetic fields with values less than or comparable to the parameters of the geomagnetic field (GMF). The constant component of GMF is usually in the range of 30 to 65 μT, and the variable component corresponded to the intensity and frequency ranges close to the industrial levels ubiquitous in laboratory rooms (~50 Hz, ~50 nT).
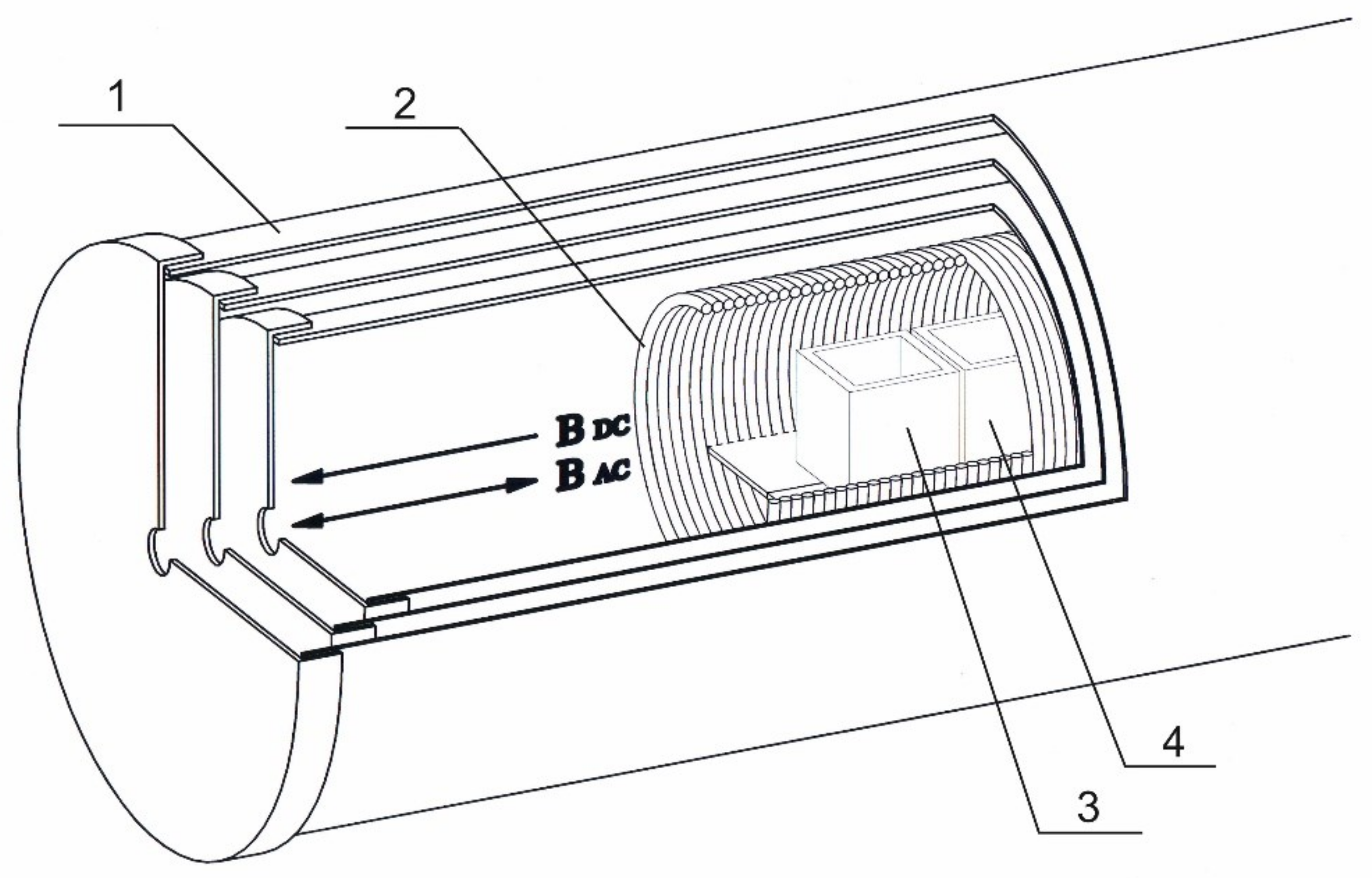
Therefore, to standardize experimental conditions and minimize the effect of environment, specialized research equipment was used—a device for generating a controlled magnetic field (
Figure 1).
A magnetically shielded chamber was comprised of three co-axial nested cylinders made of permalloy (1 mm thick), each covered with a removable lid with a hole in it for cable feed-through (the inner screen measured 22 cm in diameter and 42 cm in length) (
Figure 1). This enclosure provided a high level of GMF reduction—up to 10,000 times (a residual static magnetic field of no more than 20 nT), and also significantly reduced the electromagnetic environmental noise (down to several nT). The magnetic fields were directly measured with a Mag-03MS100 fluxgate magnetometer (Bartington Instruments, Witney, UK).
To create a weak CMF (static or alternating), a coil (solenoid) was placed inside the chamber. The solenoid (18 cm in diameter and 36 cm long) consisted of 720 turns of copper wire 1 mm in diameter and had a winding resistance of 7.5 ohms. The test samples were placed inside the solenoid along the central axis.
The solenoid was connected to a DC source to generate a static magnetic field (SMF). An alternating magnetic field (AMF) of required frequency and amplitude was generated using a digital and analog transducer (DAT) based on an L-791 card (L-Card Company, Moscow, Russia). Two frequencies of the variable component of CMF were used—12.6 Hz, which is equivalent to the ion cyclotron resonance (ICR) frequency of the hydrated hydronium ion (H
3O
+ (H
2O)
3) [
15], as well as 48.5 Hz, which is equivalent to the ICR frequency of the hydronium ion H
3O
+ [
2,
16]. These frequencies were calculated using the standard equation:
where
q and
m are ion charge and ion mass, and
BDC is induction of the static component of CMF (SMF of 60 μT).
To generate an AMF, an alternating current of sinusoidal waveform, described by the equations below, was passed through the solenoid:
where
—frequency in Hz.
Thus, the controlled magnetic field generation device could operate in one of the following modes:
Mode 1—CMF (SMF of 60 μT, AMF of 100 nT, which is formed according to the sinusoidal regime with a frequency of 12.6 Hz or 48.5 Hz)
Mode 2—SMF of 60 μT
Mode 3—“zero” magnetic field (“0”MF or “magnetic vacuum”, in this case, the current sources were disconnected from the solenoid, and the shielding was maintained; residual magnetic field ~<20 nT).
2.2. Sample Preparation
2.2.1. High Dilutions of Antibodies to IFNγ
Affinity-purified rabbit polyclonal Abs to human IFNγ (stock concentration of 2.5 mg/mL) were manufactured in accordance with the GMP requirements for active pharmaceutical ingredients. Technological processing consisted of sequential multiple dilutions of the original Abs to IFNγ in combination with a controlled intense mechanical treatment at each dilution step [
17]. For that purpose, sample tubes were shaken intensively by hand with controlled shaking frequency of about 4 Hz (21 strokes in about 4.8 s). An ethanol-water solution (25%
v/
v) was used for the preparation of all dilutions (1:100 at each step), except for the final one, for which purified water was used. The theoretical level of reduction in the concentration of the original Abs was at least 10
24 times. The solutions were prepared in 40 mL glass vials (Glastechnik Grafenroda, Geratal, Germany) under sterile conditions, protected from direct intense light, and stored for 6 days at room temperature with closed lids. The samples were prepared by OOO “NPF “MATERIA MEDICA HOLDING”, coded, and delivered in a form ready for research.
All solutions, including ethanol solutions, were prepared using deionized water (resistivity of >18.0 MΩ·cm) obtained using a Milli-Q Integral 5 water purification system (Merck, Darmstadt, Germany). Water was stored in 1 L laboratory glass jars with tight-fitting lids.
2.2.2. High Dilutions of PMA
The HD PMA samples were prepared by the authors of this article themselves according to the technology described above for HD Abs to IFNγ. For this, a PMA solution at an initial concentration of 20 nM (Sigma-Aldrich, Saint Louis, MO, USA) was used. Solutions were prepared under sterile conditions, protected from direct intense light, and stored at room temperature. The samples were analyzed on the day they were prepared.
2.3. Isolation of Murine Peritoneal Neutrophils
Balb/c male mice weighing 22–25 g were obtained from the nursery of the Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry of the Russian Academy of Sciences. Mice were intraperitoneally injected with 150 µL of suspension of opsonized zymosan with a concentration of 5 mg/mL (Zymozan A from Saccharomyces cerevisiae, Sigma-Aldrich, Saint Louis, MO, USA). Using opsonized zymosan as a chemoattractant minimizes induced level of ROS production by neutrophils. After 12 h, the animals were euthanized by cervical dislocation, and their peritoneal cavities were washed out with 4 mL of cooled calcium-free Hanks solution. The peritoneal cell suspension was mixed by pipetting and centrifuged for 5 min at 600×
g. Neutrophils were isolated according to a standard procedure as described in [
7]. For use in a chemiluminescence assay, a neutrophil suspension with viability of at least 98% was diluted with a modified Hanks medium (138 mM NaCl, 6 mM KCl, 1 mM MgSO
4, 1 mM Na
2HPO
4, 5 mM NaHCO
3, 5.5 mM glucose, 1 mM CaCl
2, and 10 mM HEPES, pH 7,4; all obtained from Sigma-Aldrich, Saint Louis, MO, USA).
The animal studies were conducted in accordance with the Guidelines for Ethical Conduct in the Care and Use of Animals and approved by the institutional animal care and use committee (protocol number 57.30.12.2011) at the Institute of Cell Biophysics.
2.4. Experiments with HD Abs to IFNγ
To assess the distant effect of the donor on the acceptor, HD Abs to IFNγ sample (donor) was incubated at room temperature (23–24 °C) for 0, 20, or 60 min in the presence of acceptor samples: water or a 10 ng/mL aqueous solution of IFNγ (provided by OOO “NPF “Materia Medica Holding”, Moscow, Russia). Each 18 mL sample was placed in an identical cubic optical glass cuvette (Hellma Analytics, Mullheim, Germany, Cat. No. 704-001-30-10) and incubated in a controlled magnetic field generation device under conditions of CMF. The cuvettes were located adjacent to one another, as shown in
Figure 1.
During control measurements, only the acceptor was incubated in a controlled magnetic field generation device (in the absence of a donor) at identical settings (the same as in the presence of a donor).
All experiments were carried out in 6 independent repetitions. During the incubation (after 0, 20, or 60 min), an aliquot was taken from the cuvette with the acceptor sample, which was then used to assess the activity of solutions by cell chemiluminescence assay.
2.5. Experiments with HD PMA
2.5.1. Studying the Effect of Shielding on the Efficiency of Distant Interaction
An HD PMA (donor sample) was incubated together with 0.5% ethanol (acceptor sample) at room temperature (23–24 °C) for 60 min. As in
Section 2.4, 18 mL of donor or acceptor sample were poured into a separate identical cubic optical glass cuvette, which was then placed in a controlled magnetic field generation device (depicted in
Figure 1).
Incubations were performed at 4 different settings:
The cuvettes with the donor or the acceptor were placed into the device separately (to avoid the distant effect between them).
The cuvettes were adjacent to each other (as indicated in
Figure 1)
The cuvettes were adjacent to each other, but each cuvette was completely wrapped in aluminum foil
The cuvettes were adjacent to each other, but each cuvette was wrapped in aluminum foil, except for the side which was in closest contact between two cuvettes.
All experiments were carried out in 6 independent repetitions. After the incubation (60 min), an aliquot was taken from the cuvette with the donor or from the cuvette with the acceptor sample, which was then used to assess the activity of solutions by cell chemiluminescence assay.
2.5.2. Studying the Effect of Magnetic Field Parameters on the Efficiency of Distant Interaction
An HD PMA (donor sample) was incubated together with water (acceptor sample) at room temperature (23–24 °C) for 0 and 60 min. As in
Section 2.4, the donor or acceptor sample (18 mL) was poured into a separate identical cubic optical glass cuvette. Then, the cuvettes were placed adjacent to each other in a controlled magnetic field generation device (depicted in
Figure 1). Several such incubations were performed at different parameters of magnetic field:
CMF (combination of SMF of 60 μT and AMF of 100 nT, which is formed according to the sinusoidal regime at 48.5 Hz)
CMF (combination of SMF of 60 μT and AMF of 100 nT, which is formed according to the sinusoidal regime at 12.6 Hz)
SMF of 60 μT
“Magnetic vacuum” (~<20 nT)
Additionally, as a control, incubation of some samples was carried out in GMF, outside a controlled magnetic field generation device. In this case, the cuvettes were placed on a laboratory bench either adjacent to each other or at least 2 m away from each other as well as from any ferromagnetic materials. The magnetic field parameters during this experiment were as follows: the AMF component with an amplitude and frequency of approximately 50 nT and 50 Hz, respectively; the SMF component was slightly less than 45 µT.
All experiments were carried out in 6 independent repetitions. After a 60-min incubation, an aliquot was taken from the cuvette with the acceptor sample, which was then used to assess the activity of solutions by cell chemiluminescence assay.
2.6. Testing the Activity of Solutions by Cell Chemiluminescence Assay
After the incubation steps described above, a sample aliquot was added to a concentrated Hanks solution (at a ratio of 8 to 1) to obtain an isotonic solution. A suspension of neutrophils was added to the resulting solution to obtain the final concentration of 106 cells/mL in 0.25 mL. Next, the cells were incubated with the sample at 37.0 ± 0.2 °C for 40 min in round-bottomed polystyrene cuvettes (1.2 cm in diameter and 5.5 cm in length, Sarstedt, Numbrecht, Germany) under GMF conditions (with SMF of ~45 μT). Constant temperature was maintained by means of a UH 4 circulating bath (MLW, Medingen, Germany).
After incubation, cell chemiluminescence intensity was measured. For this, luminol solution (Enzo Life Sciences, Lausen, Switzerland) at the final concentration of 0.35 mM as well as fMLF, an activator of ROS generation by neutrophils (Sigma-Aldrich, Saint Louis, MO, USA), at a final concentration of 2 µM were added to all samples immediately before measurement. To register the chemiluminescence kinetics, the cuvette was immediately placed in a chemiluminometer (DISoft LLC, Moscow, Russia). Representative kinetic curves are shown in
Figure 2.
PowerGraph software (DISoft LLC, Moscow, Russia) was used to estimate the maximum intensity of chemiluminescence. The results are presented in % relative to the amplitude of chemiluminescent response in the control taken as 100% as well as in absolute values.
2.7. Statistical Analysis
Statistical analysis was carried out using the environment for statistical calculations R version 4.0.2. (R Foundation for Statistical Computing, Vienna, Austria). Data visualization was performed using MS Excel. Data are presented as arithmetic means ± SD. The normality of distribution was assessed by the Shapiro–Wilk test, and the homogeneity of variances was assessed by the Bartlett test.
Comparison of values at different time points within the same group was carried out using paired Student’s t-test. To compare unrelated groups, Student’s t-test was used (Welch’s t-test in the absence of homogeneity of variances). The Holm’s procedure was used to adjust p-values for multiple comparisons. Differences were considered statistically significant at p < 0.05.
4. Discussion
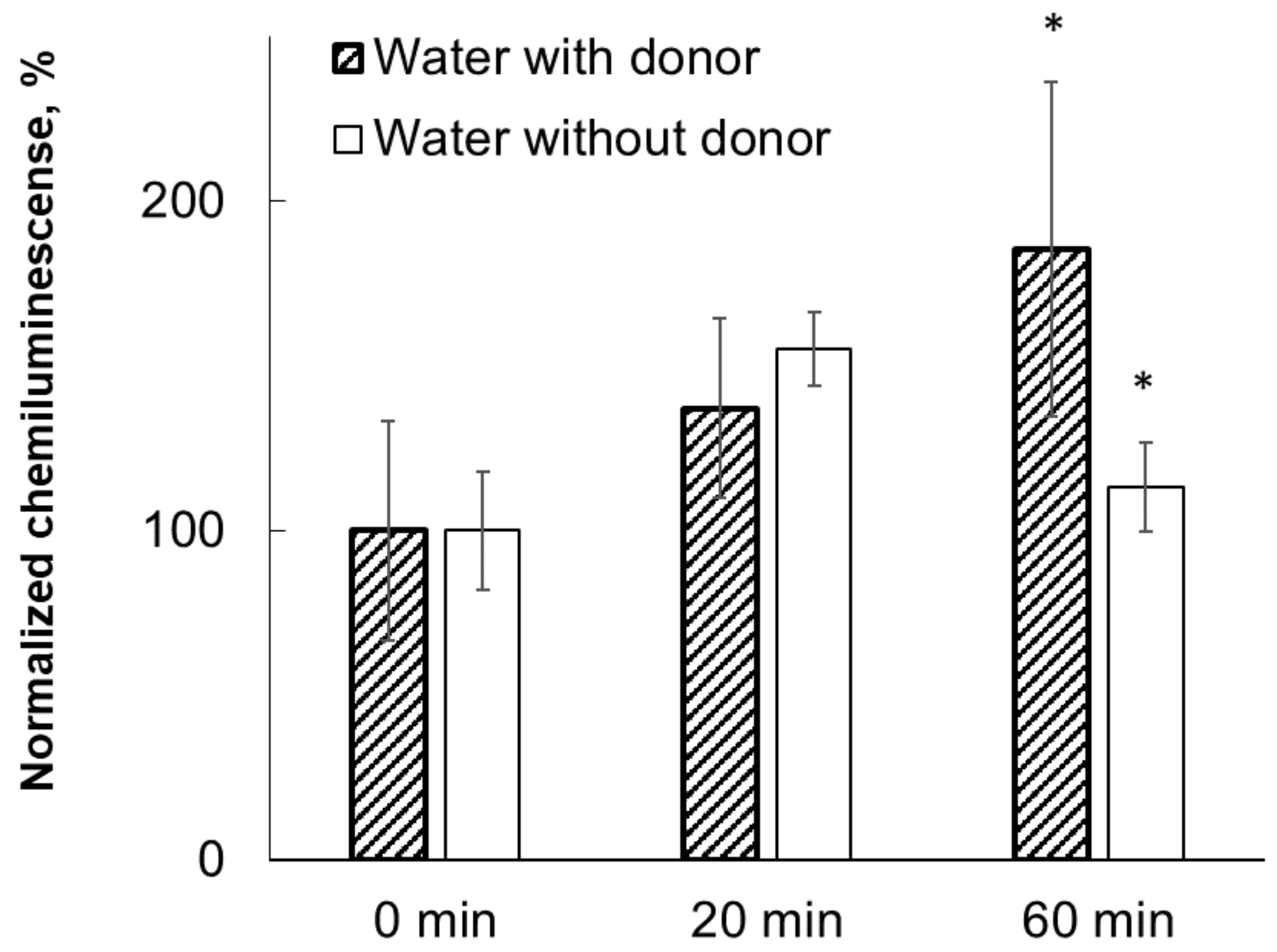
Preliminary results obtained in the first set of experiments clearly demonstrate the existence of distant interactions between water samples and HD Abs to IFNγ under CMF conditions, since the presence of HD Abs to IFNγ near water modifies its activity. This was done in comparison with the magnetic field treatment of water samples located separately from HD Abs to IFNγ.
This phenomenon of distant interaction of samples with each other was even more pronounced when instead of water, an IFNγ solution was jointly incubated with HD Abs to IFNγ. However, it should be noted that in these experiments, the activity of the samples was compared with only one time point—the value at “0 minutes” of joint incubation of samples. Additionally, the effects of other factors such as the absorption of carbon dioxide from the atmosphere could have influenced the obtained results. In this regard, in the main group of experiments we adjusted the experimental conditions: we unified the incubation time of experimental and control samples and used a comparison of samples incubated under different conditions of magnetic field and with shielding. In all experiments, we used the same incubation time (60 min) and also used samples with tightly closed lids to reduce possible absorption effects. It should be noted that after these adjustments, the distant effect of HD preparations was reliably detected.
It should also be clarified that the GMF mode (incubation outside the device) differs from the SMF not only by a slightly lower CMF (45 μT), but also by the presence of relatively low-intensity variable fields (mainly at an industrial frequency of 50 Hz, ~50 nT), which are shielded in the device. For the studies described in this paper, we opted for CMF (combination of SMF (60 μT) and AMF (100 nT), which is formed according to the sinusoidal regime at 12.6 Hz), because the stability of the measurement results when applying this field is higher compared to the much more efficient but less stable CMF mode, where the modulation is carried out according to the sinusoidal regime at 48.5 Hz.
The results obtained in our studies agree with the previously described emission of HD samples in the IR range, as well as changes in the parameters of the hydrogen bond network in aqueous solutions of IFNγ after joint incubation with HD Abs to IFNγ [
14,
22]. It has been shown that the samples of HD Abs to IFNγ themselves not only emit in the IR range, but may contribute to a change in the IR emission spectra of the IFNγ solution after its joint incubation with HD Abs to IFNγ [
14]. Another study, performed using a similar model, shows a change in the parameters of the THz spectrum of the IFNγ solution which was incubated in the presence of HD Abs to IFNγ. These changes were quantitatively interpreted after the measurement results were approximated by an equation describing the imaginary component of the permittivity of the water surrounding the protein [
22]. Thus, at the physical level, the ability of HD samples to have a distant effect was also shown; however, the detailed mechanism of this effect needs to be defined in future studies.
The physical (quantum) nature of the distant interaction of samples is clearly shown in
Figure 5, where placing donor and acceptor samples side by side (columns 3 and 4) leads to an approximately twofold increase in the chemiluminescence intensity compared to similar samples separated from each other (columns 1 and 2). Moreover, it should be noted that there is not much difference in the magnitude of the effect between the pair of the samples either completely wrapped (columns 5 and 6) or not wrapped in aluminum foil (columns 3 and 4). In the absence of foil (columns 3 and 4,
Figure 5), the effect is much more pronounced (equally for the donor and acceptor), and when the cuvettes are wrapped in foil on all sides except the contact side of the cuvettes, the effect is much less pronounced and manifests itself differently for the donor and the acceptor (columns 7 and 8). To explain this effect, it can be assumed that aluminum foil shields the electrical component of electromagnetic radiation in the radio frequency range, in contrast to permalloy (the outer shield of the device used in the experiments), which is a magnetic shield mainly for SMF and low-frequency AMF. In this regard, it can be assumed that the result obtained by us in experiments with shielding (changes in the distant effect when foil was partially wrapped around each cuvette) indicates the need for the presence of not only CMF with optimal parameters for implementing the distant effect, but also possibly the simultaneous presence of the electrical component of the electromagnetic field (EMF). Seemingly, in addition to the magnetic field, we discovered another factor that controls the distant interactions—EMF. This hypothesis also explains the differences in the relative values in pairs of samples 3 and 4, as well as 7 and 8 (
Figure 5), where partial shielding with aluminum foil, in all likelihood, can to a certain extent shield the external electromagnetic effect on the samples. This is, no doubt, an important result that requires a special detailed study in the future.
Thus, the results obtained in this work about the features of physical interactions between donor and acceptor samples are consistent with the results of a number of studies [
3,
4]. For example in [
4], the characteristics of vanishing voltage fluctuations on condenser plates filled with water were measured at impulse excitation of the contour. Preliminary cell exposure for several minutes to a microwave radiation field at 36 GHz inhibited a 47 Hz peak, and such a water state was retained after switching off the microwave field for dozens of minutes or hours, depending on the radiation power [
4]. Another study demonstrated that the aqueous solution remembers the effect of microwave radiation (42 GHz). In these experiments the effect manifested itself in a change in the opening frequency of Ca(
2+)-dependent K
+ channels in the presence of water after radio exposure [
3]. Thus, it has been shown that not only the electromagnetic but also the magnetic component has its own biophysical effect.
In our studies [
23,
24,
25], reproduced by other laboratories [
26,
27,
28,
29], it was shown that the effect of a weak CMF on aqueous solutions of amino acids (ionic current reaction) directly depends on the presence and direction of the electric field (in this case, set by the inter-electrode potential difference). This potential difference determines the energy transfer and its direction in the physical and chemical system under study. By analogy with the results of these experiments, it can be assumed that radio frequency EMF, in the case of the distant effect discussed here, is necessary for a more efficient polarization of the medium, and this, in turn, is a necessary condition for a finer control action of CMF. All this together creates necessary background for distant interaction (exchange of photons) between HD samples, protein solutions, and water.
It has already been found that EMF in the single-digit GHz range polarizes protein macromolecules in an aqueous solution [
30,
31]. Interest in the combined action of magnetic fields and radio frequency EMF on biological objects increased in connection with the study of the biological action of magnetic fields, taking into account the hyperfine interaction between nuclei and electrons in the implementation of the mechanism of radical pairs (in this case, frequencies in the range of 1–15 MHz can be active) [
32,
33]. It should be noted that the number of indoor sources of radio frequency EMF, including in laboratories, has increased significantly over the past two decades, as mobile phones, Wi-Fi, and other wireless technologies have become an integral part of our life. A large number of radio transmitters creates a constant multi-spectral radio signal environment, exposing experimental objects to low-power electromagnetic waves. In this situation, taking into account the data obtained by us, further studies will require a thorough control of the background electromagnetic environment and, possibly, the artificial formation of an optimal combination of electromagnetic parameters of different frequency ranges. Thus, in this paper we show that the use of an experimental device which allows the control and setting of the parameters of the magnetic field affects the results of the experiments (it increases the reproducibility and the magnitude of the effect).
In conclusion, it should be noted that, not surprisingly, our screening study of the possibility of distant interactions between HD preparations, protein solutions, and water turned out to be very productive. The existence of a distant effect was demonstrated. We showed that the distant effect can depend on accompanying processes, such as the influence of absorbing atmospheric gases into pure water, as well as on the interaction of magnetic and electromagnetic factors that possibly control the ultra-weak photon emission by solutions and, as a result, distant interaction. All the obtained data seem to us to be very promising for further in-depth studies.