New Achievements for the Treatment of Triple-Negative Breast Cancer
Abstract
:1. Introduction
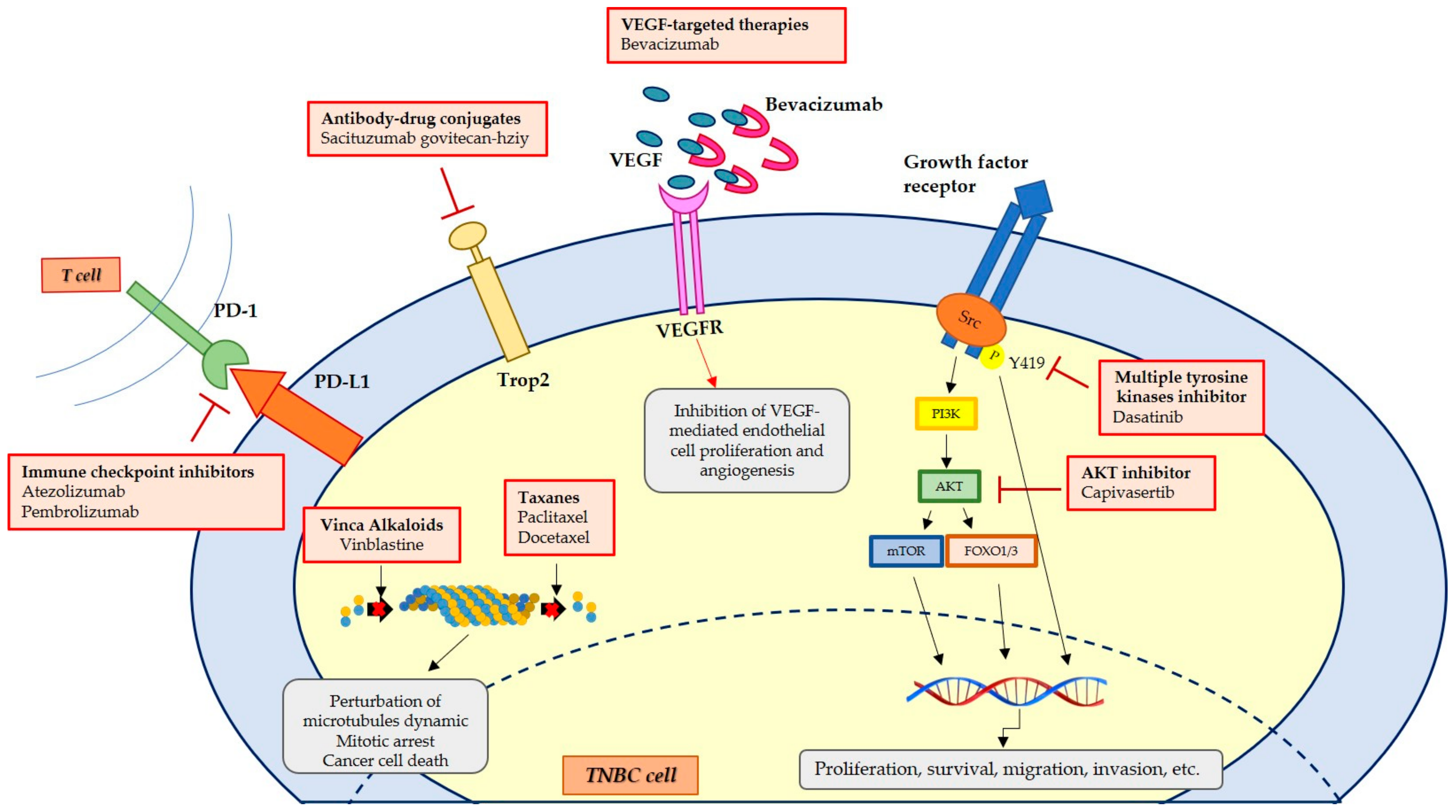
2. Therapeutic Treatments
3. Novel Approaches to Therapy in TNBC
3.1. Drug Repurposing for TNBC
3.2. Synthetic Compounds with Antiproliferative Activity in Preclinical Studies
3.2.1. Carbazoles Derivatives or Bioisosters
3.2.2. Indole Derivatives
3.2.3. Metal Complexes
3.3. Nanoformulations and Nanobiosensors
3.4. Nutraceuticals in the Prevention and Treatment of TNBC
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACAT-1 | Acyl-CoA:cholesterol acyl transferase-1 |
| ACT | Anthracycline, cyclophosphamide, and taxane |
| AMP | Cyclic adenosine monophosphate |
| ASNPs | Albumin-coated silver nanoparticles |
| BBOX1 | Gamma-butyrobetaine hydroxylase |
| BC | Breast cancer |
| BCSCs | Breast cancer stem cells |
| BL1 | Basal-like 1 |
| BL2 | Basal-like 2 |
| BM | Brain metastasis |
| CAR T | Chimeric antigen receptor–modified T-cell therapy |
| CDK | Cyclin-dependent kinases |
| Chk1 | Checkpoint kinase 1 |
| COVID-19 | Coronavirus disease |
| CSC | Cancer stem cells |
| CTLA-4 | Cytotoxic T-lymphocyte-associated protein 4 |
| 1,25D | 1α,25-Dihydroxy vitamin D3 |
| EGFR | Epidermal growth factor receptor |
| EMA | European Medicine Agency |
| EMT | Epithelial to mesenchymal transition |
| ER | Estrogen receptor |
| FAK | Focal adhesion kinase |
| FDA | Food and Drug Administration |
| HA | Hyaluronic acid |
| HAS2 | Hyaluronic acid synthase 2 |
| HER2 | Human epidermal growth factor receptor 2 |
| HT | Hdroxytyrosol |
| hTopo | Human topoisomerase |
| ICIs | Immune checkpoint inhibitors |
| IGF-1 | Insulin-like growth factor-1 |
| IGF-1R | Insulin-like growth factor receptor-type 1 |
| ITGAV | Integrin subunit alpha-V gene |
| Kif11 | Kinesin family member 11 |
| LAR | Luminal androgen receptor |
| lncRNAs | Long noncoding RNAs |
| MBC | Metaplastic breast carcinoma |
| miRs | Micro RNAs |
| MRI | Magnetic resonance imaging |
| MSL | Mesenchymal stem cell-like |
| mTNBC | Metastatic triple-negative breast cancer |
| NACT | Neoadjuvant chemotherapy |
| NEs | Nanoemulsions |
| NPs | Nanoparticles |
| NSCLC | Non-small cell lung cancer |
| OL | Oleuropein |
| OS | Overall survival |
| PAKT | Prevention through Activity in Kindergarten Trial |
| PARP | Poly(ADP-ribose) polymerase |
| PBC | Platinum-based chemotherapy |
| PCNA | Proliferating cell nuclear antigen |
| pCR | Pathologic response |
| PD-1 | Programmed death-1 |
| PD-L1 | Programmed death ligand 1 |
| PFS | Progression-free survival |
| PI3K | Phosphatidylinositol 3-kinase |
| PR | Progesterone receptor |
| ROS | Reactive oxygen species |
| RSV | Resveratrol |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SMA | Poly(styrene-co-maleic acid) |
| STAT3 | Signal transducer and activator of transcription-3 |
| TICs | Tumor initiating cells |
| TILs | Tumor-infiltrating lymphocytes |
| TKI | Tyrosine kinase inhibitor |
| TNBC | Triple-negative breast cancer |
| UPS | Ubiquitin-proteasome system |
| VDR | Vitamin D receptor |
| VEGFR | Vascular endothelial growth factor receptor |
| YAP | Yes-associated protein |
References
- Ghoncheh, M.; Pournamdar, Z.; Salehiniya, H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac. J. Cancer Prev. APJCP 2016, 17, 43–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houssein, E.H.; Emam, M.M.; Ali, A.A.; Suganthan, P.N. Deep and machine learning techniques for medical imaging-based breast cancer: A comprehensive review. Expert Syst. Appl. 2020, 167, 114161. [Google Scholar] [CrossRef]
- Britt, K.L.; Cuzick, J.; Phillips, K.A. Key steps for effective breast cancer prevention. Nat. Rev. Cancer 2020, 20, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Saturnino, C.; Barone, I.; Iacopetta, D.; Mariconda, A.; Sinicropi, M.S.; Rosano, C.; Campana, A.; Catalano, S.; Longo, P.; Ando, S. N-heterocyclic carbene complexes of silver and gold as novel tools against breast cancer progression. Future Med. Chem. 2016, 8, 2213–2229. [Google Scholar] [CrossRef] [PubMed]
- Vagia, E.; Mahalingam, D.; Cristofanilli, M. The landscape of targeted therapies in TNBC. Cancers 2020, 12, 916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burstein, M.D.; Tsimelzon, A.; Poage, G.M.; Covington, K.R.; Contreras, A.; Fuqua, S.A.; Savage, M.I.; Osborne, C.K.; Hilsenbeck, S.G.; Chang, J.C.; et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin. Cancer Res. 2015, 21, 1688–1698. [Google Scholar] [CrossRef] [Green Version]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Saturnino, C.; Bonomo, M.G.; Franchini, C.; Sinicropi, M.S. Schiff bases: Interesting scaffolds with promising antitumoral properties. Appl. Sci. 2021, 11, 1877. [Google Scholar] [CrossRef]
- Scrivano, L.; Parisi, O.I.; Iacopetta, D.; Ruffo, M.; Ceramella, J.; Sinicropi, M.S.; Puoci, F. Molecularly imprinted hydrogels for sustained release of sunitinib in breast cancer therapy. Polym. Adv. Technol. 2019, 30, 743–748. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Sinicropi, M.S.; Franchini, C. Diarylureas as antitumor agents. Appl. Sci. 2021, 11, 374. [Google Scholar] [CrossRef]
- Jenkins, S.; Kachur, M.E.; Rechache, K.; Wells, J.M.; Lipkowitz, S. Rare breast cancer subtypes. Curr. Oncol. Rep. 2021, 23, 1–14. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Jovanović, B.; Chen, X.; Estrada, M.V.; Johnson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of triple-negative breast cancer molecular subtypes: Implications for neoadjuvant chemotherapy selection. PLoS ONE 2016, 11, e0157368. [Google Scholar] [CrossRef] [PubMed]
- Bando, Y.; Kobayashi, T.; Miyakami, Y.; Sumida, S.; Kakimoto, T.; Saijo, Y.; Uehara, H. Triple-negative breast cancer and basal-like subtype: Pathology and targeted therapy. J. Med. Investig. 2021, 68, 213–219. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network (NCCN) Guidelines. Breast Cancer [about 4 Screens]; 2019. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx (accessed on 2 April 2022).
- Bao, B.; Prasad, A.S. Targeting CSC in a most aggressive subtype of breast cancer TNBC. Adv. Exp. Med. Biol. 2019, 1152, 311–334. [Google Scholar] [PubMed]
- Lv, Y.; Ma, X.; Du, Y.; Feng, J. Understanding patterns of brain metastasis in triple-negative breast cancer and exploring potential therapeutic targets. OncoTargets Ther. 2021, 14, 589–607. [Google Scholar] [CrossRef]
- Nakhjavani, M.; Samarasinghe, R.M.; Shigdar, S. Triple-negative breast cancer brain metastasis: An update on druggable targets, current clinical trials, and future treatment options. Drug Discov. Today 2022, 27, 1298–1314. [Google Scholar] [CrossRef]
- Moffa, G.; Galati, F.; Collalunga, E.; Rizzo, V.; Kripa, E.; D’Amati, G.; Pediconi, F. Can MRI Biomarkers Predict Triple-Negative Breast Cancer? Diagnostics 2020, 10, 1090. [Google Scholar] [CrossRef]
- Gupta, G.K.; Collier, A.L.; Lee, D.; Hoefer, R.A.; Zheleva, V.; Siewertsz van Reesema, L.L.; Tang-Tan, A.M.; Guye, M.L.; Chang, D.Z.; Winston, J.S.; et al. Perspectives on triple-negative breast cancer: Current treatment strategies, unmet needs, and potential targets for future therapies. Cancers 2020, 12, 2392. [Google Scholar] [CrossRef]
- Yin, L.; Duan, J.J.; Bian, X.W.; Yu, S.C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef]
- Kast, K.; Link, T.; Friedrich, K.; Petzold, A.; Niedostatek, A.; Schoffer, O.; Werner, C.; Klug, S.J.; Werner, A.; Gatzweiler, A.; et al. Impact of breast cancer subtypes and patterns of metastasis on outcome. Breast Cancer Res. Treat. 2015, 150, 621–629. [Google Scholar] [CrossRef]
- Papanicolas, L.E.; Gordon, D.L.; Wesselingh, S.L.; Rogers, G.B. Not just antibiotics: Is cancer chemotherapy driving antimicrobial resistance? Trends Microbiol. 2018, 26, 393–400. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug resistance (MDR): A widespread phenomenon in pharmacological therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef] [PubMed]
- Zolota, V.; Tzelepi, V.; Piperigkou, Z.; Kourea, H.; Papakonstantinou, E.; Argentou, Μ.I.; Karamanos, N.K. Epigenetic alterations in triple-negative breast cancer—The critical role of extracellular matrix. Cancers 2021, 13, 713. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Ling, Y.Y.; Zhong, Y.M.; Li, Z.Y.; Tan, C.P.; Mao, Z.W. Ferroptosis-enhanced cancer immunity by a ferroceneappended iridium(iii) diphosphine complex. Angew. Chem. Int. Ed. 2021, 134, e202115247. [Google Scholar]
- Olivo, E.; La Chimia, M.; Ceramella, J.; Catalano, A.; Chiaradonna, F.; Sinicropi, M.S.; Cuda, G.; Iacopetta, D.; Scumaci, D. Moving beyond the tip of the iceberg: DJ-1 implications in cancer metabolism. Cells 2022, 11, 1432. [Google Scholar] [CrossRef]
- Hossain, F.; Majumder, S.; David, J.; Miele, L. Precision medicine and triple-negative breast cancer: Current landscape and future directions. Cancers 2021, 13, 3739. [Google Scholar] [CrossRef]
- Finelli, F.; Giuzio, F.; Catalano, A.; Iacopetta, D.; Ceramella, J.; Sinicropi, M.S.; Capasso, A.; Saturnino, C. Target therapy in cancer treatment: mPGES-1 and PARP. Pharmacologyonline 2021, 3, 1167–1176. [Google Scholar]
- Garrido-Castro, A.C.; Lin, N.U.; Polyak, K. Insights into molecular classifications of triple-negative breast cancer: Improving patient selection for treatment. Cancer Discov. 2019, 9, 176–198. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.J.; Yan, L.; Zhang, J.; Zhang, W.D. STAT3 as a potential therapeutic target in triple negative breast cancer: A systematic review. J. Exp. Clin. Cancer Res. 2019, 38, 195. [Google Scholar] [CrossRef]
- Nakai, K.; Hung, M.-C.; Yamaguchi, H. A perspective on anti-EGFR therapies targeting triple-negative breast cancer. Am. J. Cancer Res. 2016, 6, 1609–1623. [Google Scholar]
- Liao, C.; Zhang, Y.; Fan, C.; Herring, L.E.; Liu, J.; Locasale, J.W.; Takada, M.; Zhou, J.; Zurlo, G.; Hu, L.; et al. Identification of BBOX1 as a therapeutic target in triple-negative breast cancer. Cancer Discov. 2020, 10, 1706–1721. [Google Scholar] [CrossRef]
- Quereda, V.; Bayle, S.; Vena, F.; Frydman, S.M.; Monastyrskyi, A.; Roush, W.R.; Duckett, D.R. Therapeutic targeting of CDK12/CDK13 in triple-negative breast cancer. Cancer Cell 2019, 36, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, A.; Lakshmanan, D.K.; Ravichandran, G.; Paul, S.; Manickam, S.; Kumar, P.V.; Thilagar, S. Regulatory mechanisms of heme regulatory protein BACH1: A potential therapeutic target for cancer. Med. Oncol. 2021, 38, 122. [Google Scholar] [CrossRef] [PubMed]
- Gaule, P.B.; Crown, J.; O’Donovan, N.; Duffy, M.J. cMET in triple negative breast cancer: Is it a therapeutic target for this subset of breast cancer patients? Expert Opin. Ther. Targets 2014, 18, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Cheuk, I.W.; Siu, M.T.; Ho, J.C.; Chen, J.; Shin, V.Y.; Kwong, A. ITGAV targeting as a therapeutic approach for treatment of metastatic breast cancer. Am. J. Cancer Res. 2020, 10, 211–223. [Google Scholar] [PubMed]
- Yang, C.-Q.; Liu, J.; Zhao, S.-Q.; Zhu, K.; Gong, Z.-Q.; Xu, R.; Lu, H.-M.; Zhou, R.-B.; Zhao, G.; Yin, D.-C.; et al. Recent treatment progress of triple negative breast cancer. Prog. Biophys. Mol. Biol. 2020, 151, 40–53. [Google Scholar]
- Rigiracciolo, D.C.; Nohata, N.; Lappano, R.; Cirillo, F.; Talia, M.; Scordamaglia, D.; Gutkind, J.S.; Maggiolini, M. IGF-1/IGF-1R/FAK/YAP Transduction Signaling Prompts Growth Effects in Triple-Negative Breast Cancer (TNBC) Cells. Cells 2020, 9, 2619. [Google Scholar] [CrossRef]
- Tan, A.R.; Swain, S.M. Therapeutic strategies for TNBC. Cancer J. 2008, 14, 343–351. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, M.; Tao, Z.; Cao, J.; Wang, L.; Hu, X. MiR-331-3p suppresses cell proliferation in TNBC cells by downregulating NRP2. Technol. Cancer Res. Treat. 2020, 19, 1533033820905824. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.; Yuan, J.; Li, X.; Ma, Y.; Wang, X.; Xu, B.; Li, X. LncRNA LINC00173 enhances triple-negative breast cancer progression by suppressing miR-490-3p expression. Biomed. Pharmacother. 2020, 125, 109987. [Google Scholar] [CrossRef]
- Zhou, Y.; Yamamoto, Y.; Takeshita, F.; Yamamoto, T.; Xiao, Z.; Ochiya, T. Delivery of miR-424-5p via Extracellular vesicles promotes the apoptosis of MDA-MB-231 TNBC cells in the tumor microenvironment. Int. J. Mol. Sci. 2021, 22, 844. [Google Scholar] [CrossRef]
- Jin, L.; Luo, C.; Wu, X.; Li, M.; Wu, S.; Feng, Y. LncRNA-HAGLR motivates triple negative breast cancer progression by regulation of WNT2 via sponging miR-335-3p. Aging 2021, 13, 19306. [Google Scholar] [CrossRef] [PubMed]
- Shaath, H.; Vishnubalaji, R.; Elango, R.; Khattak, S.; Alajez, N.M. Single-cell long noncoding RNA (LncRNA) transcriptome implicates MALAT1 in triple-negative breast cancer (TNBC) resistance to neoadjuvant chemotherapy. Cell Death Discov. 2021, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Chen, Y.; Zhang, D.; Wei, Y.; Li, Z.; Li, Q.; Xu, B. Homologous recombination deficiency (HRD) and BRCA 1/2 gene mutation for predicting the effect of platinum-based neoadjuvant chemotherapy of early-stage triple-negative breast cancer (TNBC): A systematic review and meta-analysis. J. Personaliz. Med. 2022, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, P.; Scatena, C.; Ghilli, M.; Bargagna, I.; Lorenzini, G.; Nicolini, A. Molecular mechanisms, biomarkers and emerging therapies for chemotherapy resistant TNBC. Int. J. Mol. Sci. 2022, 23, 1665. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, B.; Wei, X.; Guan, X.; Zhang, W. CXCL8 is a prognostic biomarker and correlated with TNBC brain metastasis and immune infiltration. Int. Immunopharmacol. 2022, 103, 108454. [Google Scholar] [CrossRef]
- López-Camacho, E.; Trilla-Fuertes, L.; Gámez-Pozo, A.; Dapía, I.; López-Vacas, R.; Zapater-Moros, A.; Lumbreras-Herrera, M.I.; Arias, P.; Zamora, P.; Fresno Vara, J.A.; et al. Synergistic effect of antimetabolic and chemotherapy drugs in triple-negative breast cancer. Biomed. Pharmacother. 2022, 149, 112844. [Google Scholar] [CrossRef]
- Yu, J.; Mu, Q.; Fung, M.; Xu, X.; Zhu, L.; Ho, R.J. Challenges and opportunities in metastatic breast cancer treatments: Nano-drug combinations delivered preferentially to metastatic cells may enhance therapeutic response. Pharmacol. Ther. 2022, 236, 108108. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, J.; Jiang, Y. Partial response after toripalimab plus anlotinib for advanced metaplastic breast carcinoma: A case report. Front. Endocrinol. 2022, 1, 810747. [Google Scholar] [CrossRef]
- Anderson, T.S.; Wooster, A.L.; Piersall, S.L.; Okpalanwaka, I.F.; Lowe, D.B. Disrupting cancer angiogenesis and immune checkpoint networks for improved tumor immunity. Seminars Canc. Biol. 2022, in press. [Google Scholar] [CrossRef]
- Bajbouj, K.; Qaisar, R.; Alshura, M.A.; Ibrahim, Z.; Alebaji, M.B.; Al Ani, A.W.; Janajrah, H.M.; Bilalaga, M.M.; Omara, A.I.; Abou Assaleh, R.S.; et al. Synergistic anti-angiogenic effect of combined VEGFR kinase inhibitors, lenvatinib, and regorafenib: A therapeutic potential for breast cancer. Int. J. Mol. Sci. 2022, 23, 4408. [Google Scholar] [CrossRef]
- Jourdan, J.P.; Bureau, R.; Rochais, C.; Dallemagne, P. Drug repositioning: A brief overview. J. Pharm. Pharmacol. 2020, 72, 1145–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, K.; Koval, A.; Xu, J.; Bodmer, A.; Katanaev, V.L. Towards the first targeted therapy for triple-negative breast cancer: Repositioning of clofazimine as a chemotherapy-compatible selective Wnt pathway inhibitor. Cancer Lett. 2019, 449, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Qiu, Y.; Lu, W.; Jiang, Y.; Wang, J. Immunotherapeutic interventions of triple negative breast cancer. J. Transl. Med. 2018, 16, 147. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Hu, Y.; Zhou, N.; Yao, C.; Wu, L.; Liu, L.; Chen, F. CAR T-cell therapy for triple-negative breast cancer: Where we are. Cancer Lett. 2020, 491, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Dees, S.; Ganesan, R.; Singh, S.; Grewal, I.S. Emerging CAR-T Cell Therapy for the treatment of triple-negative breast cancer. Mol. Cancer Ther. 2020, 19, 2409–2421. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Saturnino, C.; Pellegrino, M.; Mariconda, A.; Longo, P.; Sinicropi, M.S. COVID-19 at a glance: An up-to-date overview on variants, drug design and therapies. Viruses 2022, 14, 573. [Google Scholar]
- Catalano, A. COVID-19: Could irisin become the handyman myokine of the 21st century. Coronaviruses 2020, 1, 32–41. [Google Scholar] [CrossRef]
- Brown, J.M.; Wasson, M.C.D.; Marcato, P. Triple-negative breast cancer and the COVID-19 pandemic: Clinical management perspectives and potential consequences of infection. Cancers 2021, 13, 296. [Google Scholar] [CrossRef]
- Ryu, W.-J.; Sohn, J.H. Molecular targets and promising therapeutics of triple-negative breast cancer. Pharmaceuticals 2021, 14, 1008. [Google Scholar] [CrossRef]
- Jiang, Y.Z.; Liu, Y.; Xiao, Y.; Hu, X.; Jiang, L.; Zuo, W.J.; Ma, D.; Ding, J.; Zhu, X.; Zou, J.; et al. Molecular subtyping and genomic profiling expand precision medicine in refractory metastatic triple-negative breast cancer: The FUTURE trial. Cell Res. 2021, 31, 178–186. [Google Scholar] [CrossRef]
- Lesmana, R.; Hadi, E.J.; Goenawan, H. Is cancer immunotherapy more hype than hope? J. Med. Health 2020, 2, 6. [Google Scholar] [CrossRef]
- Kim, I.; Sanchez, K.; McArthur, H.L.; Page, D. Immunotherapy in triple-negative breast cancer: Present and future. Curr. Breast Cancer Rep. 2019, 11, 259–271. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Numan, A.; Maddiboyina, B.; Arora, S.; Riadi, Y.; Md, S.; Alhakamy, N.A.; Kesharwani, P. The emerging role of immune checkpoint inhibitors in the treatment of triple-negative breast cancer. Drug Discov. Today 2021, 26, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Berger, E.R.; Park, T.; Saridakis, A.; Golshan, M.; Greenup, R.A.; Ahuja, N. Immunotherapy treatment for triple negative breast cancer. Pharmaceuticals 2021, 14, 763. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef]
- Motzer, R.J.; Rini, B.I.; McDermott, D.F.; Redman, B.G.; Kuzel, T.M.; Harrison, M.R.; Vaishampayan, U.N.; Drabkin, H.A.; George, S.; Logan, T.F.; et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J. Clin. Oncol. 2015, 33, 1430–1437. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; Van Der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef] [Green Version]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef]
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: Mechanisms of action, efficacy, and limitations. Front. Oncol. 2018, 8, 86. [Google Scholar] [CrossRef]
- Shadbad, M.A.; Safaei, S.; Brunetti, O.; Derakhshani, A.; Lotfinejad, P.; Mokhtarzadeh, A.; Hemmat, N.; Racanelli, V.; Solimando, A.G.; Argentiero, A. A systematic review on the therapeutic potentiality of PD-L1-inhibiting microRNAs for triple-negative breast cancer: Toward single-cell sequencing-guided biomimetic delivery. Genes 2021, 12, 1206. [Google Scholar] [CrossRef]
- Schettini, F.; Giuliano, M.; De Placido, S.; Arpino, G. Nab-paclitaxel for the treatment of triple-negative breast cancer: Rationale, clinical data and future perspectives. Cancer Treat. Rev. 2016, 50, 129–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenmotsu, H.; Tanigawara, Y. Pharmacokinetics, dynamics and toxicity of docetaxel: Why the japanese dose differs from the western dose. Cancer Sci. 2015, 106, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.; Greenwood, H.; Dudley, P.; Crafter, C.; Yu, D.-H.; Zhang, J.; Li, J.; Gao, B.; Ji, Q.; Maynard, J.; et al. Preclinical pharmacology of AZD5363, an inhibitor of AKT: Pharmacodynamics, antitumor activity, and correlation of monotherapy activity with genetic background. Mol. Cancer Ther. 2012, 11, 873–887. [Google Scholar] [CrossRef] [Green Version]
- Robertson, J.F.; Coleman, R.E.; Cheung, K.-L.; Evans, A.; Holcombe, C.; Skene, A.; Rea, D.; Ahmed, S.; Jahan, A.; Horgan, K.; et al. Proliferation and AKT Activity Biomarker Analyses after Capivasertib (AZD5363) Treatment of Patients with ER+ Invasive Breast Cancer (STAKT). Clin. Cancer Res. 2020, 26, 1574–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A. Vincristine and vinblastine: A review. IJMPS 2016, 6, 23–30. [Google Scholar]
- Dhamodharan, R.; Jordan, M.A.; Thrower, D.; Wilson, L.; Wadsworth, P. Vinblastine suppresses dynamics of individual microtubules in living interphase cells. Mol. Biol. Cell 1995, 6, 1215–1229. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Kim, K. Anti-cancer natural products and their bioactive compounds inducing ER stress-mediated apoptosis: A review. Nutrients 2018, 10, 1021. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, S.; Verma, S.S.; Aggarwal, S.; Gupta, S.C. Drug repurposing for breast cancer therapy: Old weapon for new battle. Semin. Cancer Biol. 2021, 68, 8–20. [Google Scholar] [CrossRef]
- Malik, J.A.; Ahmed, S.; Jan, B.; Bender, O.; Al Hagbani, T.; Alqarni, A.; Anwar, S. Drugs repurposed: An advanced step towards the treatment of breast cancer and associated challenges. Biomed. Pharmacother. 2022, 145, 112375. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Pellegrino, M.; Aquaro, S.; Franchini, C.; Sinicropi, M.S. Diarylureas: Repositioning from antitumor to antimicrobials or multi-target agents against new pandemics. Antibiotics 2021, 10, 92. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Ávalos-Moreno, M.; López-Tejada, A.; Blaya-Cánovas, J.L.; Cara-Lupiañez, F.E.; González-González, A.; Lorente, J.A.; Sánchez-Rovira, P.; Granados-Principal, S. Drug repurposing for triple-negative breast cancer. J. Pers. Med. 2020, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.W.; Sood, A.K. Molecular pathways: Beta-adrenergic signaling in cancer. Clin. Cancer Res. 2012, 18, 1201–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, W.Y.; He, R.H.; Zhang, J.; He, Y.J.; Wan, Z.; Zhou, C.F.; Tang, Y.J.; Li, Z.; McLeod, H.L.; Liu, J. β-blockers inhibit the viability of breast cancer cells by regulating the ERK/COX-2 signaling pathway and the drug response is affected by ADRB2 single-nucleotide polymorphisms. Oncol. Rep. 2019, 41, 341–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talarico, G.; Orecchioni, S.; Dallaglio, K.; Reggiani, F.; Mancuso, P.; Calleri, A.; Gregato, G.; Labanca, V.; Rossi, T.; Noonan, D.M. Aspirin and atenolol enhance metformin activity against breast cancer by targeting both neoplastic and microenvironment cells. Sci. Rep. 2016, 6, 18673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, X.; Yin, C.; Zhang, Y.; Guo, G.; Zhao, C.; Wang, O.; Xiang, Y.; Zhang, X.; Liang, G. Osthole inhibits triple negative breast cancer cells by suppressing STAT3. J. Exp. Clin. Cancer Res. 2018, 37, 322. [Google Scholar] [CrossRef]
- Stella Sravanthi, V.; Palaka, B.K.; Venkatesan, R.; Ampasala, D.R.; Periyasamy, L. Identification of novel inhibitors of signal transducer and activator of transcription 3 over signal transducer and activator of transcription 1 for the treatment of breast cancer by in-silico and in-vitro approach. Process Biochem. 2019, 82, 153–166. [Google Scholar]
- Mercurio, A.; Adriani, G.; Catalano, A.; Carocci, A.; Rao, L.; Lentini, G.; Cavalluzzi, M.M.; Franchini, C.; Vacca, A.; Corbo, F. A mini-review on thalidomide: Chemistry, mechanisms of action, therapeutic potential and anti-angiogenic properties in multiple myeloma. Curr. Med. Chem. 2017, 24, 2736–2744. [Google Scholar] [CrossRef]
- Iacopetta, D.; Carocci, A.; Sinicropi, M.S.; Catalano, A.; Lentini, G.; Ceramella, J.; Curcio, R.; Caroleo, M.C. Old drug scaffold, new activity: Thalidomide-correlated compounds exert different effects on breast cancer cell growth and progression. ChemMedChem 2017, 12, 381–389. [Google Scholar] [CrossRef]
- Oh, E.; Kim, Y.J.; An, H.; Sung, D.; Cho, T.M.; Farrand, L.; Jang, S.; Seo, J.H.; Kim, J.Y. Flubendazole elicits anti-metastatic effects in triple-negative breast cancer via STAT3 inhibition. Int. J. Cancer 2018, 143, 1978–1993. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.C.; Chao, T.K.; Chang, C.C.; Yo, Y.T.; Yu, M.H.; Lai, H.C. Drug screening identifies niclosamide as an inhibitor of breast cancer stem-like cells. PLoS ONE 2013, 8, e74538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Zhang, Y. Teaching an old dog new tricks: Drug discovery by repositioning natural products and their derivatives. Drug Discov. Today 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Caruso, A.; Chiret, A.S.V.; Lancelot, J.C.; Sinicropi, M.S.; Garofalo, A.; Rault, S. Efficient and simple synthesis of 6-aryl-1,4-dimethyl-9H-carbazoles. Molecules 2008, 13, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Saturnino, C.; Caruso, A.; Iacopetta, D.; Rosano, C.; Ceramella, J.; Muia, N.; Mariconda, A.; Bonomo, M.G.; Ponassi, M.; Rosace, G.; et al. Inhibition of human topoisomerase II by N,N,N-trimethylethanammonium iodide alkylcarbazole derivatives. ChemMedChem 2018, 13, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, D.; Rosano, C.; Puoci, F.; Parisi, O.I.; Saturnino, C.; Caruso, A.; Longo, P.; Ceramella, J.; Malzert-Freon, A.; Dallemagne, P.; et al. Multifaceted properties of 1,4-dimethylcarbazoles: Focus on trimethoxybenzamide and trimethoxyphenylurea derivatives as novel human topoisomerase II inhibitors. Eur. J. Pharm. Sci. 2017, 96, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Ceramella, J.; Caruso, A.; Occhiuzzi, M.A.; Iacopetta, D.; Barbarossa, A.; Rizzuti, B.; Dallemagne, P.; Rault, S.; El-Kashef, H.; Saturnino, C.; et al. Benzothienoquinazolinones as new multi-target scaffolds: Dual inhibition of human Topoisomerase I and tubulin polymerization. Eur. J. Med. Chem. 2019, 181, 111583. [Google Scholar] [CrossRef] [PubMed]
- Sinicropi, M.S.; Tavani, C.; Rosano, C.; Ceramella, J.; Iacopetta, D.; Barbarossa, A.; Bianchi, L.; Benzi, A.; Maccagno, M.; Ponassi, M.; et al. A nitrocarbazole as a new microtubule-targeting agent in breast cancer treatment. Appl. Sci. 2021, 11, 9139. [Google Scholar] [CrossRef]
- Caruso, A.; Lancelot, J.; El-Kashef, H.; Sinicropi, M.S.; Legay, R.; Lesnard, A.; Rault, S. A rapid and versatile synthesis of novel pyrimido[5,4-b]carbazoles. Tetrahedron 2009, 65, 10400–10405. [Google Scholar] [CrossRef]
- Vlaar, C.P.; Castillo-Pichardo, L.; Medina, J.I.; Marrero-Serra, C.M.; Velez, E.; Ramos, Z.; Hernández, E. Design, synthesis and biological evaluation of new carbazole derivatives as anti-cancer and anti-migratory agents. Bioorg. Med. Chem. 2018, 26, 884–890. [Google Scholar] [CrossRef]
- Hou, S.; Yi, Y.W.; Kang, H.J.; Zhang, L.; Kim, H.J.; Kong, Y.; Liu, Y.; Wang, K.; Kong, H.-S.; Grindrod, S. Novel carbazole inhibits phospho-STAT3 through induction of protein–tyrosine phosphatase PTPN6. J. Med. Chem. 2014, 57, 6342–6353. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Xu, M.; Yang, C.; Yao, Y.; Liang, L.N.; Chung, P.E.D.; Long, Q.; Zacksenhaus, E.; He, Z.; Liu, S.; et al. Novel racemosin B derivatives as new therapeutic agents for aggressive breast cancer. Bioorg. Med. Chem. 2018, 26, 6096–6104. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Saturnino, C.; Sinicropi, M.S. A comprehensive review on pyranoindole-containing agents. Curr. Med. Chem. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Sun, X.; Ma, Y.; Cheng, Y.; Ma, Q.; Jing, W.; Qu, S.; Liu, L. Design, synthesis and biological evaluation of novel 1,3,4,9-tetrahydropyrano[3,4-b]indoles as potential treatment of triple negative breast cancer by suppressing PI3K/AKT/mTOR pathway. Bioorg. Med. Chem. 2022, 55, 116594. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-M.; Kim, S.H.; Jung, Y.-S.; Kwak, J.-H. Synthesis and biological evaluation of (S)-2-(substituted arylmethyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide analogs and their synergistic effect against PTEN-deficient MDA-MB-468 cells. Pharmaceuticals 2021, 14, 974. [Google Scholar] [CrossRef]
- Gautam, Y.; Das, S.; Khan, H.; Pathak, N.; Iqbal, H.; Yadav, P.; Sirohi, V.K.; Khan, S.; Raghuvanshi, D.S.; Dwivedi, A.; et al. Design, synthesis and broad spectrum antibreast cancer activity of diarylindoles via induction of apoptosis in aggressive breast cancer cells. Bioorg. Med. Chem. 2021, 42, 116252. [Google Scholar] [CrossRef]
- Guerra, F.S.; Dias, F.R.F.; Cunha, A.C.; Fernandes, P.D. Benzo[f]indole-4,9-dione derivatives effectively inhibit the growth of triple-negative breast cancer. Molecules 2021, 26, 4414. [Google Scholar] [CrossRef]
- Catalano, A.; Sinicropi, M.S.; Iacopetta, D.; Ceramella, J.; Mariconda, A.; Rosano, C.; Scali, E.; Saturnino, C.; Longo, P. A review on the advancements in the field of metal complexes with Schiff bases as antiproliferative agents. Appl. Sci. 2021, 11, 6027. [Google Scholar] [CrossRef]
- Ielo, I.; Iacopetta, D.; Saturnino, C.; Longo, P.; Galletta, M.; Drommi, D.; Rosace, G.; Sinicropi, M.S.; Plutino, M.R. Gold derivatives development as prospective anticancer drugs for breast cancer treatment. Appl. Sci. 2021, 11, 2089. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, H.; Guo, Y.; Wang, K.; Wang, X.; Guo, Z. Towards rational design of RAD51-targeting prodrugs: Platinum IV–artesunate conjugates with enhanced cytotoxicity against BRCA-proficient ovarian and breast cancer cells. Chem. Commun. 2018, 54, 11717–11720. [Google Scholar] [CrossRef]
- Jin, S.; Muhammad, N.; Sun, Y.; Tan, Y.; Yuan, H.; Song, D.; Guo, Z.; Wang, X. Multispecific Platinum(IV) complex deters breast cancer via interposing inflammation and immunosuppression as an inhibitor of COX-2 and PD-L1. Angew. Chem. Int. Ed. 2020, 59, 23313–23321. [Google Scholar] [CrossRef]
- Caporale, A.; Palma, G.; Mariconda, A.; Del Vecchio, V.; Iacopetta, D.; Parisi, O.I.; Sinicropi, M.S.; Puoci, F.; Arra, C.; Longo, P.; et al. Synthesis and antitumor activity of new group 3 metallocene complexes. Molecules 2017, 22, 526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abid, M.; Shamsi, F.; Azam, A. Ruthenium complexes: An emerging ground to the development of metallopharmaceuticals for cancer therapy. Min. Rev. Med. Chem. 2016, 16, 772–786. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.; Nicolò, F.; Lo Schiavo, S.; Sinicropi, M.S.; Tresoldi, G. Synthesis and spectroscopic properties of di-2-pyridyl sulfide(dps)compounds. Crystal structure of [Ru(dps)2Cl2]. J. Chem. Soc. Dalton Trans. 1995, 1, 17–24. [Google Scholar] [CrossRef]
- Ceramella, J.; Mariconda, A.; Iacopetta, D.; Saturnino, C.; Barbarossa, A.; Caruso, A.; Rosano, C.; Sinicropi, M.S.; Longo, P. From coins to cancer therapy: Gold, silver and copper complexes targeting human topoisomerases. Bioorg. Med. Chem. Lett. 2020, 30, 126905. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-B.; Jia, Y.-X.; Zhu, P.-C.; Chew, R.J.; Li, Y.; Tan, N.S.; Leung, P.-H. Highly selective anti-cancer properties of ester functionalized enantiopure dinuclear gold (I)-diphosphine. Eur. J. Med. Chem. 2015, 98, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Ortega, E.; Zamora, A.; Basu, U.; Lippmann, P.; Rodríguez, V.; Janiak, C.; Ott, I.; Ruiz, J. An Erlotinib gold(I) conjugate for combating triple-negative breast cancer. J. Inorg. Biochem. 2020, 203, 110910. [Google Scholar] [CrossRef] [PubMed]
- Ceramella, J.; Mariconda, A.; Sirignano, M.; Iacopetta, D.; Rosano, C.; Catalano, A.; Saturnino, C.; Sinicropi, M.S.; Longo, P. Novel Au carbene complexes as promising multi-target agents in breast cancer treatment. Pharmaceuticals 2022, 15, 507. [Google Scholar] [CrossRef]
- Iacopetta, D.; Rosano, C.; Sirignano, M.; Mariconda, A.; Ceramella, J.; Ponassi, M.; Saturnino, C.; Sinicropi, M.S.; Longo, P. Is the way to fight cancer paved with gold? Metal-based carbene complexes with multiple and fascinating biological features. Pharmaceuticals 2020, 13, 91. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Rosano, C.; Mariconda, A.; Pellegrino, M.; Sirignano, M.; Saturnino, C.; Catalano, A.; Aquaro, S.; Longo, P.; et al. N-Heterocyclic carbene-gold(I) complexes targeting actin polymerization. Appl. Sci. 2021, 11, 5626. [Google Scholar] [CrossRef]
- Silva, D.E.S.; Becceneri, A.B.; Santiago, J.V.; Neto, J.A.G.; Ellena, J.; Cominetti, M.R.; Pereira, J.C.M.; Hannon, M.J.; Netto, A.V. Silver (I) complexes of 3-methoxy-4-hydroxybenzaldehyde thiosemicarbazones and triphenylphosphine: Structural, cytotoxicity, and apoptotic studies. Dalton Transact. 2020, 49, 16474–16487. [Google Scholar] [CrossRef]
- Şahin, N.; Şahin-Bölükbaşı, S.; Tahir, M.N.; Arıcı, C.; Çevik, E.; Gürbüz, N.; Özdemïr, I.; Cummings, B.S. Synthesis, characterization and anticancer activity of allyl substituted N-Heterocyclic carbene silver (I) complexes. J. Mol. Struct. 2019, 1179, 92–99. [Google Scholar] [CrossRef]
- Abdul Halim, S.N.A.; Nordin, F.J.; Mohd Abd Razak, M.R.; Mohd Sofyan, N.R.F.; Abdul Halim, S.N.; Rajab, N.F.; Sarip, R. Synthesis, characterization, and evaluation of silver(I) complexes with mixed-ligands of thiosemicarbazones and diphenyl (p-tolyl) phosphine as biological agents. J. Coord. Chem. 2019, 72, 879–893. [Google Scholar] [CrossRef]
- Şahin, N.; Şahin-Bölükbaşı, S.; Marşan, H. Synthesis and antitumor activity of new silver(I)-N-heterocyclic carbene complexes. J. Coord. Chem. 2019, 72, 3602–3613. [Google Scholar] [CrossRef]
- Guadagno, L.; Raimondo, M.; Longo, R.; Sarno, M.; Iuliano, M.; Mariconda, A.; Saturnino, C.; Ceramella, J.; Iacopetta, D.; Sinicropi, M.S. Development and characterization of antitumoral electrospun polycaprolactone/functionalized Fe3O4 hybrid membranes. Mater. Today Chem. 2020, 17, 100309. [Google Scholar] [CrossRef]
- Keihan Shokooh, M.; Emami, F.; Jeong, J.H.; Yook, S. Bio-inspired and smart nanoparticles for triple negative breast cancer microenvironment. Pharmaceutics 2021, 13, 287. [Google Scholar] [CrossRef]
- Miller-Kleinhenz, J.M.; Bozeman, E.N.; Yang, L. Targeted nanoparticles for image-guided treatment of triple-negative breast cancer: Clinical significance and technological advances. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 797–816. [Google Scholar] [CrossRef] [Green Version]
- Abumanhal-Masarweh, H.; Da Silva, D.; Poley, M.; Zinger, A.; Goldman, E.; Krinsky, N.; Kleiner, R.; Shenbach, G.; Schroeder, J.E.; Shklover, J.; et al. Tailoring the lipid composition of nanoparticles modulates their cellular uptake and affects the viability of triple negative breast cancer cells. J. Control. Release 2019, 307, 331–341. [Google Scholar] [CrossRef]
- Azizi, M.; Ghourchian, H.; Yazdian, F.; Bagherifam, S.; Bekhradnia, S.; Nyström, B. Anti-cancerous effect of albumin coated silver nanoparticles on MDA-MB 231 human breast cancer cell line. Sci. Rep. 2017, 7, 5178. [Google Scholar] [CrossRef]
- Surapaneni, S.K.; Bashir, S.; Tikoo, K. Gold nanoparticles-induced cytotoxicity in triple negative breast cancer involves different epigenetic alterations depending upon the surface charge. Sci. Rep. 2018, 8, 12295. [Google Scholar] [CrossRef]
- Bahman, F.; Pittalà, V.; Haider, M.; Greish, K. Enhanced anticancer activity of nanoformulation of dasatinib against triple-negative breast cancer. J. Personal. Med. 2021, 11, 559. [Google Scholar] [CrossRef]
- Ceramella, J.; Groo, A.C.; Iacopetta, D.; Séguy, L.; Mariconda, A.; Puoci, F.; Saturnino, C.; Leroy, F.; Since, M.; Longo, P.; et al. A winning strategy to improve the anticancer properties of Cisplatin and Quercetin based on the nanoemulsions formulation. J. Drug Deliv. Sci. Technol. 2021, 66, 102907. [Google Scholar] [CrossRef]
- Saraiva, S.M.; Gutiérrez-Lovera, C.; Martínez-Val, J.; Lores, S.; Bouzo, B.L.; Díez-Villares, S.; Alijas, S.; Pensado-López, A.; Vázquez-Ríos, A.J.; Sánchez, L.; et al. Edelfosine nanoemulsions inhibit tumor growth of triple negative breast cancer in zebrafish xenograft model. Sci. Rep. 2021, 11, 9873. [Google Scholar] [CrossRef] [PubMed]
- Shashni, B.; Nagasaki, Y. Nitroxide radical-containing nanoparticles attenuate tumorigenic potential of triple negative breast cancer. Biomaterials 2018, 178, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, Q.; Lin, G.; Shi, Y.; Gu, Z.; Ding, T. Combination of using prodrug-modified cationic liposome nanocomplexes and a potentiating strategy via targeted co-delivery of gemcitabine and docetaxel for CD44-overexpressed triple negative breast cancer therapy. Acta Biomater. 2017, 62, 257–272. [Google Scholar] [CrossRef]
- Chamorro-Garcia, A.; Merkoçi, A. Nanobiosensors in diagnostics. Nanobiomedicine 2016, 3, 1–26. [Google Scholar] [CrossRef]
- Dass, S.; Tan, K.; Rajan, R.S.; Mokhtar, N.; Adzmi, E.M.; Rahman, W.W.A.; Din, T.T.; Balakrishnan, V. Triple negative breast cancer: A review of present and future diagnostic modalities. Medicina 2021, 57, 62. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Nikokar, I.; Zokaei, M.; Bozorgzadeh, E. Design, development and evaluation of microRNA-199a-5p detecting electrochemical nanobiosensor with diagnostic application in Triple Negative Breast Cancer. Talanta 2018, 189, 592–598. [Google Scholar] [CrossRef]
- Scaria, B.; Sood, S.; Raad, C.; Khanafer, J.; Jayachandiran, R.; Pupulin, A.; Grewal, S.; Okoko, M.; Arora, M.; Miles, L.; et al. Natural Health Products (NHP’s) and Natural Compounds as Therapeutic Agents for the Treatment of Cancer; Mechanisms of Anti-Cancer Activity of Natural Compounds and Overall Trends. Int. J. Mol. Sci. 2020, 21, 8480. [Google Scholar] [CrossRef]
- Augimeri, G.; Montalto, F.I.; Giordano, C.; Barone, I.; Lanzino, M.; Catalano, S.; Andò, S.; De Amicis, F.; Bonofiglio, D. Nutraceuticals in the Mediterranean diet: Potential avenues for breast cancer treatment. Nutrients 2021, 13, 2557. [Google Scholar] [CrossRef]
- De Maio, A.C.; Basile, G.; Iacopetta, D.; Catalano, A.; Ceramella, J.; Cafaro, D.; Saturnino, C.; Sinicropi, M.S. The significant role of nutraceutical compounds in ulcerative colitis treatment. Curr. Med. Chem. 2022, 29, 4216–4234. [Google Scholar]
- Mock, C.D.; Jordan, B.C.; Selvam, C. Recent advances of curcumin and its analogues in breast cancer prevention and treatment. RSC Adv. 2015, 5, 75575–75588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iacopetta, D.; Catalano, A.; Ceramella, J.; Saturnino, C.; Salvagno, L.; Ielo, I.; Drommi, D.; Scali, E.; Plutino, M.R.; Rosace, G.; et al. The different facets of triclocarban: A review. Molecules 2021, 26, 2811. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Iacopetta, D.; Rosato, A.; Salvagno, L.; Ceramella, J.; Longo, F.; Sinicropi, M.S.; Franchini, C. Searching for small molecules as antibacterials: Non-cytotoxic diarylureas analogues of triclocarban. Antibiotics 2021, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.; Ding, Y.; Zhang, Y.; Zhou, Y.; Li, M.; Wang, C. Curcumin suppresses proliferation and migration of MDA-MB-231 breast cancer cells through autophagy-dependent Akt degradation. PLoS ONE 2016, 11, e0146553. [Google Scholar] [CrossRef] [Green Version]
- Narvaez, C.J.; Grebenc, D.; Balinth, S.; Welsh, J.E. Vitamin D regulation of HAS2, hyaluronan synthesis and metabolism in triple negative breast cancer cells. J. Steroid Biochem. Mol. Biol. 2020, 201, 105688. [Google Scholar] [CrossRef]
- Vassallo, A.; Finelli, F.; Bonomo, M.G.; Giuzio, F.; Capasso, A.; Salzano, G.; Ceramella, J.; Catalano, A.; Sinicropi, M.S.; Saturnino, C. Vitamin D in the prevention, development and therapy of oncological diseases. Pharmacologyonline 2021, 2, 267–276. [Google Scholar]
- Shityakov, S.; Bigdelian, E.; Hussein, A.A.; Hussain, M.B.; Tripathi, Y.C.; Khan, M.U.; Shariati, M.A. Phytochemical and pharmacological attributes of piperine: A bioactive ingredient of black pepper. Eur. J. Med. Chem. 2019, 176, 149–161. [Google Scholar] [CrossRef] [Green Version]
- Greenshields, A.L.; Doucette, C.D.; Sutton, K.M.; Madera, L.; Annan, H.; Yaffe, P.B.; Knickle, A.F.; Dong, Z.; Hoskin, D.W. Piperine inhibits the growth and motility of triple-negative breast cancer cells. Cancer Lett. 2015, 357, 129–140. [Google Scholar] [CrossRef]
- Cao, D.; Zhu, G.Y.; Lu, Y.; Yang, A.; Chen, D.; Huang, H.J.; Peng, S.X.; Chen, L.W.; Li, Y.W. Luteolin suppresses epithelial mesenchymal transition and migration of triple-negative breast cancer cells by inhibiting YAP/TAZ activity. Biomed. Pharmacother. 2020, 129, 110462. [Google Scholar] [CrossRef]
- Cook, M.T.; Liang, Y.; Besch-Williford, C.; Hyder, S.M. Luteolin inhibits lung metastasis, cell migration, and viability of triple-negative breast cancer cells. Breast Cancer Targets Ther. 2017, 9, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Kuang, G.; Wan, J.; Zhang, X.; Li, H.; Gong, X.; Li, H. Luteolin suppresses the metastasis of triple-negative breast cancer by reversing epithelial-to-mesenchymal transition via downregulation of β-catenin expression. Oncol. Rep. 2017, 37, 895–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iacopetta, D.; Lappano, R.; Mariconda, A.; Ceramella, J.; Sinicropi, M.S.; Saturnino, C.; Talia, M.; Cirillo, F.; Martinelli, F.; Puoci, F. Newly synthesized imino-derivatives analogues of resveratrol exert inhibitory effects in breast tumor cells. Int. J. Mol. Sci. 2020, 21, 7797. [Google Scholar] [CrossRef] [PubMed]
- Chimento, A.; Santarsiero, A.; Iacopetta, D.; Ceramella, J.; De Luca, A.; Infantino, V.; Parisi, O.; Avena, P.; Bonomo, M.; Saturnino, C.; et al. A phenylacetamide resveratrol derivative exerts inhibitory effects on breast cancer cell growth. Int. J. Mol. Sci. 2021, 22, 5255. [Google Scholar] [CrossRef]
- Liang, Z.J.; Wan, Y.; Zhu, D.D.; Wang, M.X.; Jiang, H.M.; Huang, D.L.; Luo, L.F.; Chen, M.J.; Yang, W.P.; Li, H.M.; et al. Resveratrol mediates the apoptosis of triple negative breast cancer cells by reducing POLD1 expression. Front. Oncol. 2021, 11, 569295. [Google Scholar] [CrossRef]
- Yar Saglam, A.S.; Kayhan, H.; Alp, E.; Onen, H.I. Resveratrol enhances the sensitivity of FL118 in triple-negative breast cancer cell lines via suppressing epithelial to mesenchymal transition. Mol. Biol. Rep. 2021, 48, 475–489. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Khan, I.A.; Ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef] [PubMed]
- Nettore, I.C.; Rocca, C.; Mancino, G.; Albano, L.; Amelio, D.; Grande, F.; Puoci, F.; Pasqua, T.; Desiderio, S.; Mazza, R.; et al. Quercetin and its derivative Q2 modulate chromatin dynamics in adipogenesis and Q2 prevents obesity and metabolic disorders in rats. J. Nutr. Biochem. 2019, 69, 151–162. [Google Scholar] [CrossRef]
- Umar, S.M.; Patra, S.; Kashyap, A.; Dev, A., Jr.; Kumar, L.; Prasad, C.P. Quercetin impairs HuR-driven progression and migration of triple negative breast cancer (TNBC) cells. Nutr. Cancer 2022, 74, 1497–1510. [Google Scholar] [CrossRef]
- Chen, W.J.; Tsai, J.H.; Hsu, L.S.; Lin, C.L.; Hong, H.M.; Pan, M.H. Quercetin blocks the aggressive phenotype of triple-negative breast cancer by inhibiting IGF1/IGF1R-mediated EMT program. J. Food Drug Anal. 2021, 29, 98–112. [Google Scholar] [CrossRef]
- Lu, H.-Y.; Zhu, J.-S.; Zhang, Z.; Shen, W.-J.; Jiang, S.; Long, Y.-F.; Wu, B.; Ding, T.; Huan, F.; Wang, S.-L. Hydroxytyrosol and oleuropein inhibit migration and invasion of MDA-MB-231 Triple-negative breast cancer cell via induction of autophagy. Anticancer Agents Med. Chem. 2019, 19, 1983–1990. [Google Scholar] [CrossRef]
- Messeha, S.S.; Zarmouh, N.O.; Asiri, A.; Soliman, K.F.A. Gene expression alterations associated with oleuropein-induced antiproliferative effects and S-phase cell cycle arrest in triple-negative breast cancer cells. Nutrients 2020, 12, 3755. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Saeed, F.; Gilani, S.A.; Shariati, M.A.; Imran, A.; Afzaal, M.; Atif, M.; Tufail, T.; Anjum, F.M. Fisetin: An anticancer perspective. Food Sci. Nutr. 2021, 9, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Sur, S.; Ray, R.B. Bitter Melon (Momordica charantia), a nutraceutical approach for cancer prevention and therapy. Cancers 2020, 12, 2064. [Google Scholar] [CrossRef]
- Rasul, A.; Riaz, A.; Wei, W.; Sarfraz, I.; Hassan, M.; Li, J.; Asif, F.; Adem, Ş.; Bukhari, S.A.; Asrar, M.; et al. Mangifera indica extracts as novel PKM2 inhibitors for treatment of triple negative breast cancer. BioMed Res. Int. 2021, 2021, 5514669. [Google Scholar] [CrossRef] [PubMed]
- Caruso, A.; Barbarossa, A.; Tassone, A.; Ceramella, J.; Carocci, A.; Catalano, A.; Basile, G.; Fazio, A.; Iacopetta, D.; Franchini, C.; et al. Pomegranate: Nutraceutical with promising benefits on human health. Appl. Sci. 2020, 10, 6915. [Google Scholar] [CrossRef]
- Fazio, A.; Iacopetta, D.; La Torre, C.; Ceramella, J.; Muia, N.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Finding solutions for agricultural wastes: Antioxidant and antitumor properties of pomegranate Akko peel extracts and beta-glucan recovery. Food Funct. 2018, 9, 6618–6631. [Google Scholar] [CrossRef] [PubMed]
- Yurasakpong, L.; Apisawetakan, S.; Pranweerapaiboon, K.; Sobhon, P.; Chaithirayanon, K. Holothuria scabra extract induces cell apoptosis and suppresses Warburg effect by down-regulating Akt/mTOR/HIF-1 axis in MDA-MB-231 breast cancer cells. Nutr. Cancer 2021, 73, 1964–1975. [Google Scholar] [CrossRef]
- Schmiech, M.; Lang, S.J.; Ulrich, J.; Werner, K.; Rashan, L.J.; Syrovets, T.; Simmet, T. Comparative investigation of frankincense nutraceuticals: Correlation of boswellic and lupeolic acid contents with cytokine release inhibition and toxicity against triple-negative breast cancer cells. Nutrients 2019, 11, 2341. [Google Scholar] [CrossRef] [Green Version]
- Nigra, A.D.; Teodoro, A.J.; Gil, G.A. A decade of research on coffee as an anticarcinogenic beverage. Oxidative Med. Cell. Longev. 2021, 2021, 4420479. [Google Scholar] [CrossRef]
- Vassallo, A.; Bonomo, M.G.; Finelli, F.; Salzano, G.; Catalano, A.; Sinicropi, M.S.; Capasso, A.; Saturnino, C. Chemopreventive molecules of coffee and beneficial metabolic effects. Pharmacologyonline 2021, 2, 249–257. [Google Scholar]
- Zhao, H.; Mei, K.; Yang, L.; Liu, X.; Xie, L. Green tea consumption and risk of esophageal cancer: A systematic review and dose-response meta-analysis. Nutrition 2021, 87–88, 111197. [Google Scholar] [CrossRef] [PubMed]
- Bonomo, M.G.; Catalano, A.; Finelli, F.; Giuzio, F.; Iacopetta, D.; Ceramella, J.; Sinicropi, M.S.; Capasso, A.; Saturnino, C. Nutraceutical functions of green tea. Pharmacologyonline 2021, 3, 1156–1166. [Google Scholar]
- Nehlig, A.; Reix, N.; Arbogast, P.; Mathelin, C. Coffee consumption and breast cancer risk: A narrative review in the general population and in different subtypes of breast cancer. Eur. J. Nutr. 2021, 60, 1197–1235. [Google Scholar] [CrossRef] [PubMed]
- Alicandro, G.; Tavani, A.; La Vecchia, C. Coffee and cancer risk: A summary overview. Eur. J. Canc. Prevent. 2017, 26, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, Z.; Feng, G.; Ji, X.; Tan, Y.; Li, H.; Gunter, M.J.; Xiang, Y. Tea drinking and risk of cancer incidence: A meta-analysis of prospective cohort studies and evidence evaluation. Adv. Nutr. 2021, 12, 402–412. [Google Scholar] [CrossRef]
- Pauwels, E.K.J.; Volterrani, D. Coffee consumption and cancer risk; an assessment of the health implications based on recent knowledge. Med. Princ. Pract. 2021, 30, 401–411. [Google Scholar] [CrossRef]
- Tundis, R.; Iacopetta, D.; Sinicropi, M.S.; Bonesi, M.; Leporini, M.; Passalacqua, N.G.; Ceramella, J.; Menichini, F.; Loizzo, M.R. Assessment of antioxidant, antitumor and pro-apoptotic e_ects of Salvia fruticose Mill. subsp. thomasii (Lacaita) Brullo, Guglielmo, Pavone & Terrasi (Lamiaceae). Food Chem. Toxicol. 2017, 106, 155–164. [Google Scholar]
- Ceramella, J.; Loizzo, M.R.; Iacopetta, D.; Bonesi, M.; Sicari, V.; Pellicano, T.M.; Saturnino, C.; Malzert-Freon, A.; Tundis, R.; Sinicropi, M.S. Anchusa azurea Mill. (Boraginaceae) aerial parts methanol extract interfering with cytoskeleton organization induces programmed cancer cells death. Food Funct. 2019, 10, 4280–4290. [Google Scholar] [CrossRef]
- Varghese, E.; Samuel, S.M.; Abotaleb, M.; Cheema, S.; Mamtani, R.; Büsselberg, D. The “Yin and Yang” of natural compounds in anticancer therapy of triple-negative breast cancers. Cancers 2018, 10, 346. [Google Scholar] [CrossRef] [Green Version]

| Structure | Name | Original Indication | Ref |
|---|---|---|---|
 | Propranolol | Hypertension | [85] |
 | ICI-118,551 | Hypertension | [85] |
 | Atenolol | Hypertension | [86] |
 | Osthole | Osteoporosis | [87] |
 | Risedronate sodium | Osteoporosis | [88] |
 | Zoledronic acid | Osteoporosis | [88] |
 | 1 | Thalidomide analogues | [90] |
 | 2 | Thalidomide analogues | [90] |
 | Flubendazole | Anthelmintic | [91] |
 | Niclosamide | Anthelmintic | [92] |
| Structure | Compound | Ref |
|---|---|---|
 | 3 | [95] |
 | Ellipticine | [96] |
 | 4 | [96] |
 | 5 | [96] |
 | 6 | [97] |
 | 7 | [97] |
 | 8 | [100] |
 | 9 | [100] |
 | 10 | [100] |
 | 11 | [100] |
 | 12 | [101] |
 | 13 | [102] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catalano, A.; Iacopetta, D.; Ceramella, J.; Mariconda, A.; Rosano, C.; Scumaci, D.; Saturnino, C.; Longo, P.; Sinicropi, M.S. New Achievements for the Treatment of Triple-Negative Breast Cancer. Appl. Sci. 2022, 12, 5554. https://doi.org/10.3390/app12115554
Catalano A, Iacopetta D, Ceramella J, Mariconda A, Rosano C, Scumaci D, Saturnino C, Longo P, Sinicropi MS. New Achievements for the Treatment of Triple-Negative Breast Cancer. Applied Sciences. 2022; 12(11):5554. https://doi.org/10.3390/app12115554
Chicago/Turabian StyleCatalano, Alessia, Domenico Iacopetta, Jessica Ceramella, Annaluisa Mariconda, Camillo Rosano, Domenica Scumaci, Carmela Saturnino, Pasquale Longo, and Maria Stefania Sinicropi. 2022. "New Achievements for the Treatment of Triple-Negative Breast Cancer" Applied Sciences 12, no. 11: 5554. https://doi.org/10.3390/app12115554
APA StyleCatalano, A., Iacopetta, D., Ceramella, J., Mariconda, A., Rosano, C., Scumaci, D., Saturnino, C., Longo, P., & Sinicropi, M. S. (2022). New Achievements for the Treatment of Triple-Negative Breast Cancer. Applied Sciences, 12(11), 5554. https://doi.org/10.3390/app12115554


































