Pulsed Electric Fields for Valorization of Platelets with No Therapeutic Value towards a High Biomedical Potential Product—A Proof of Concept
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Platelet Concentrates
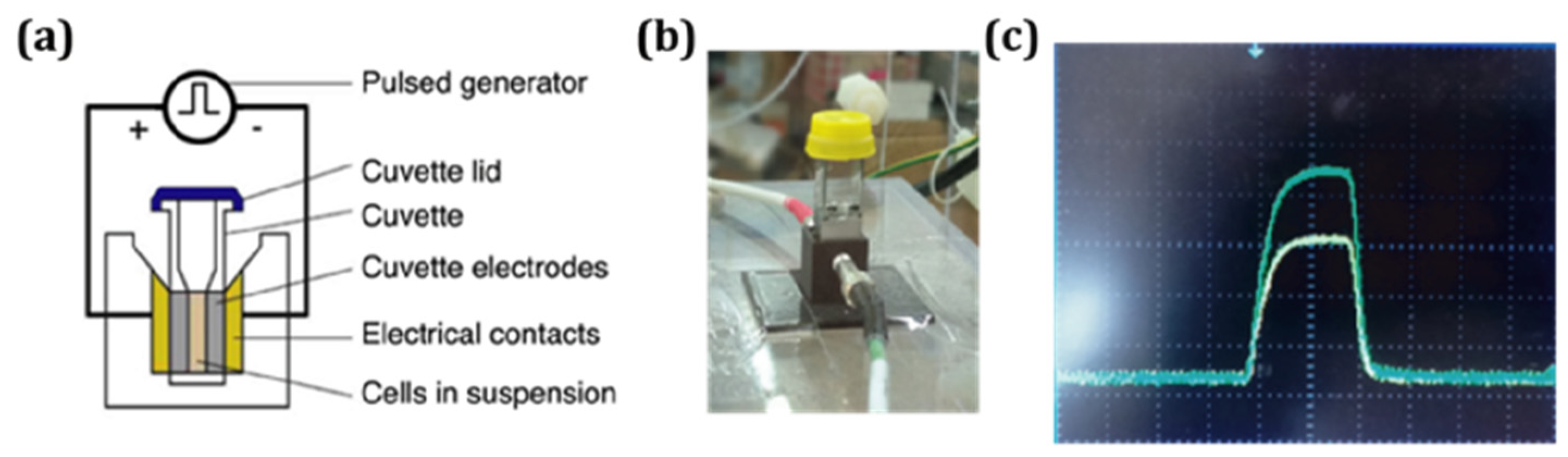
2.2. Pulse Electric Fields Application
2.3. Preparation of Platelet Lysates
2.4. Platelet Activation Measurement
2.5. Growth Factor Quantification
2.6. Human BM-MSC Culture
3. Results
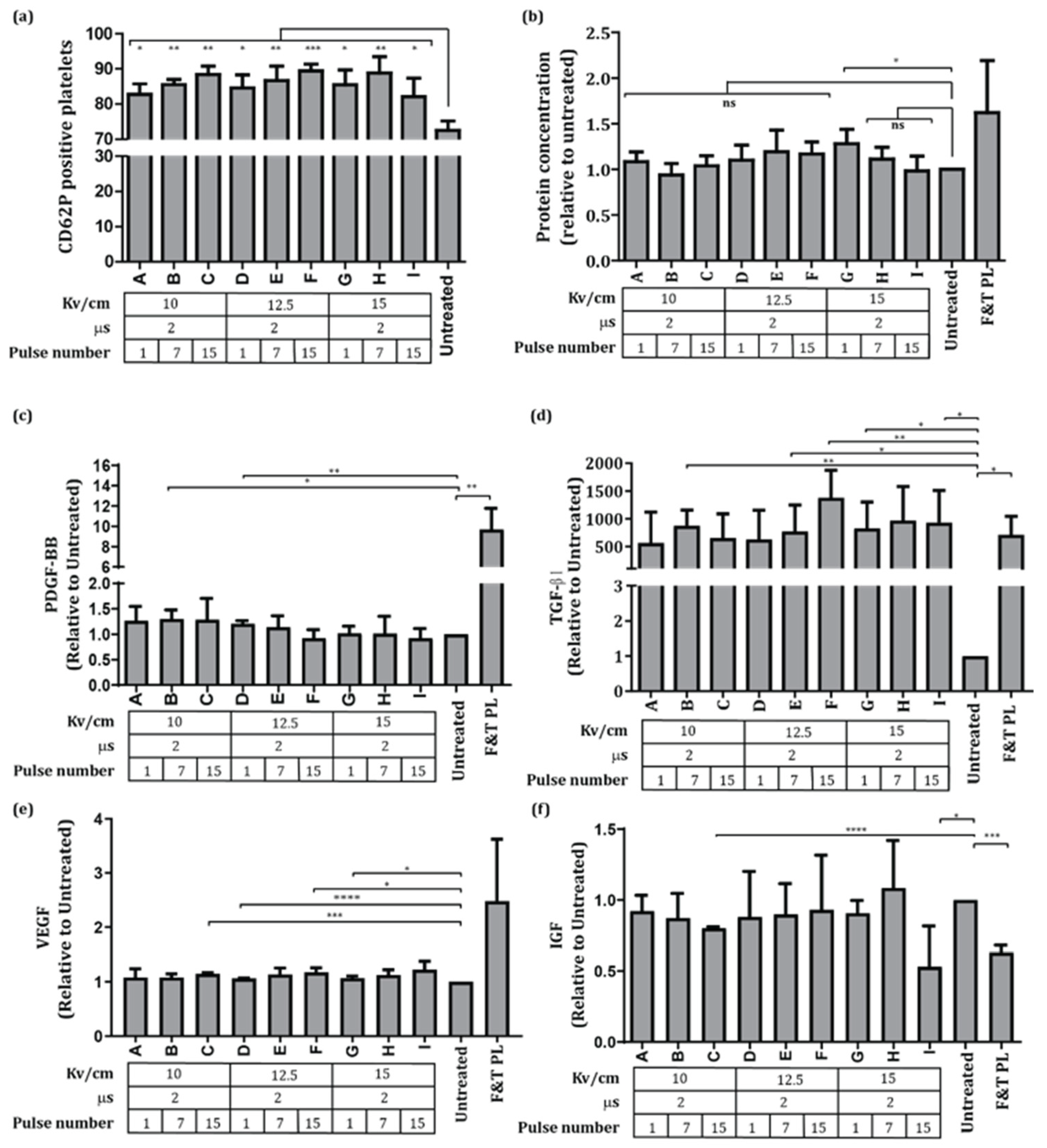
3.1. Effects of PEF on Platelet Activation and Growth Factor Release
3.2. BM-MSC Expansion on PR PEF Supplemented Media
3.3. BM-MSC Differentiation of Cells Expanded in PR PEF Supplemented Media
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dörnen, J.; Dittmar, T. The Role of MSCs and Cell Fusion in Tissue Regeneration. Int. J. Mol. Sci. 2020, 22, 10980. [Google Scholar] [CrossRef]
- Serra, M.; Cunha, B.; Peixoto, C.; Gomes-Alves, P.; Alves, P.M. Advancing manufacture of human mesenchymal stem cells therapies: Technological challenges in cell bioprocessing and characterization. Curr. Opin. Chem. Eng. 2018, 22, 226–235. [Google Scholar] [CrossRef]
- Bieback, K.; Fernandez-Muñoz, B.; Pati, S.; Schäfer, R. Gapsin the knowledge of human platelet lysate as a cell culture supplement for cell therapy: A joint publication from the AABB and the International Society for Cell & Gene Therapy. Transfusion 2019, 59, 3448–3460. [Google Scholar]
- Kouchakian, M.R.; Baghban, N.; Moniri, S.F.; Baghban, M.; Bakhshalizadeh, S.; Najafzadeh, V.; Safaei, Z.; Izanlou, S.; Khoradmehr, A.; Nabipour, I.; et al. The Clinical Trials of Mesenchymal Stromal Cells Therapy. Stem Cells Int. 2021, 2021, 1634782. [Google Scholar] [CrossRef] [PubMed]
- Karnieli, O.; Friedner, O.M.; Allickson, J.G.; Zhang, N.; Jung, S.; Fiorentini, D.; Abraham, E.; Eaker, S.S.; Chan, A.; Griffiths, S.; et al. A consensus introduction to serum replacements and serum-free media for cellular therapies. Cytotherapy 2017, 19, 155–169. [Google Scholar] [CrossRef]
- Pawitan, J.A. Platelet Rich Plasma in Xeno-Free Stem Cell Culture: The Impact of Platelet Count and Processing Method. Curr. Stem Cell Res. Ther. 2012, 7, 329–335. [Google Scholar] [CrossRef]
- Doucet, C.; Ernou, I.; Zhang, Y.; Llense, J.R.; Begot, L.; Holy, X.; Lataillade, J.J. Platelet lysates promote mesenchymal stem cell expansion: A safety substitute for animal serum in cell-based therapy applications. J. Cell. Physiol. 2005, 205, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Schallmoser, K.; Henschler, R.; Gabriel, C.; Koh, M.B.C.; Burnouf, T. Production and Quality Requirements of Human Platelet Lysate: A Position Statement from the Working Party on Cellular Therapies of the International Society of Blood Transfusion. Trends Biotechnol. 2019, 38, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Bieback, K. Platelet lysate as replacement for fetal bovine serum in mesenchymal stromal cell cultures. Transfus. Med. Hemother. 2013, 40, 326–335. [Google Scholar] [CrossRef] [Green Version]
- Luhar, R.K.; Shah, R.J. Discard rate in blood transfusion service-A critical tool to support blood inventory management. Int. J. Med. Sci. Public Health 2020, 9, 426–430. [Google Scholar] [CrossRef]
- Lee, H.J.; Oh, S.H.; Jo, S.Y.; Kim, I.S. Platelet Inventory Management Program: Development and Practical Experience. Ann. Lab. Med. 2020, 41, 95–100. [Google Scholar] [CrossRef]
- Giusti, V.D.I.; D’Ascenzo, S.; Macchiarelli, G. In vitro evidence supporting applications of platelet derivatives in regenerative medicine. Blood Transfus. 2020, 18, 117–129. [Google Scholar] [PubMed]
- Lacoste, E.; Martineau, I.; Gagnon, G. Platelet Concentrates: Effects of Calcium and Thrombin on Endothelial Cell Proliferation and Growth Factor Release. J. Periodontol. 2003, 74, 10. [Google Scholar] [CrossRef] [PubMed]
- Strandberg, G.; Sellberg, F.; Sommar, P.; Ronaghi, M.; Lubenow, N.; Knutson, F.; Berglund, D. Standardizing the freeze-thaw preparation of growth factors from platelet lysate. Transfusion 2017, 57, 1058–1065. [Google Scholar] [CrossRef]
- Kocaoemer, A.; Kern, S.; Klüter, H.; Bieback, K. Human AB Serum and Thrombin-Activated Platelet-Rich Plasma Are Suitable Alternatives to Fetal Calf Serum for the Expansion of Mesenchymal Stem Cells from Adipose Tissue. Stem Cells 2007, 25, 1270–1278. [Google Scholar] [CrossRef] [Green Version]
- Clark, J.; Crean, S.; Reynolds, M.W. Topical bovine thrombin and adverse events: A review of the literature. Curr. Med. Res. Opin. 2008, 24, 2071–2087. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, D.C.; Weaver, J.C.; Davalos, R.V. Characterization of cell membrane permeability in vitro part I: Transport behavior induced by single-pulse electric fields. Technol. Cancer Res. Treat. 2018, 17, 1533033818792491. [Google Scholar] [CrossRef] [Green Version]
- Pakhomov, A.G.; Gianulis, E.; Vernier, P.T.; Semenov, I.; Xiao, S.; Pakhomova, O.N. Multiple nanosecond electric pulses increase the number but not the size of long-lived nanopores in the cell membrane. Biochim. Biophys. Acta-Biomembr. 2015, 1848, 958–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rego, D.; Redondo, L.; Casaleiro, S.; Sousa, A.P.; Abreu, S.; Serra, M.; Santo, V.E. Application of pulsed electric fields for the valorization of platelets with no therapeutic value for transfusion medicine. Technology 2019, 7, 40–45. [Google Scholar] [CrossRef]
- Redondo, L.M.; Silva, J.F. Repetitive high-voltage solid-state marx modulator design for various load conditions. IEEE Trans. Plasma Sci. 2009, 37, 1632–1637. [Google Scholar] [CrossRef]
- Michelson, A.D.; Barnard, M.R.; Krueger, L.A.; Frelinger, A.L.; Furman, M.I. Evaluation of Platelet Function by Flow Cytometry. Methods 2000, 270, 259–270. [Google Scholar] [CrossRef]
- Cunha, B.; Peixoto, C.; Silva, M.M.; Carrondo, M.J.T.; Serra, M.; Alves, P.M. Filtration methodologies for the clarification and concentration of human mesenchymal stem cells. J. Memb. Sci. 2015, 478, 117–129. [Google Scholar] [CrossRef]
- Kicken, H.; Roest, M.; Henskens, Y.M.C.; de Laat, B. Application of an optimized flow cytometry- based quantification of Platelet Activation (PACT): Monitoring platelet activation in platelet concentrates. PLoS ONE 2017, 12, e0172265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler, C.G.; Van Sloun, R.; Gonzalez, S.; Whitney, K.E.; DePhillipo, N.N.; Kennedy, M.I.; Dornan, G.J.; Evans, T.A.; Huard, J.; LaPrade, R.F. Characterization of Growth Factors, Cytokines, and Chemokines in Bone Marrow Concentrate and Platelet-Rich Plasma A Prospective Analysis. Am. J. Sports Med. 2019, 47, 2174–2187. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, H.; Kantarci, A.; Deady, J.; Hasturk, H.; Liu, H.; Alshahat, M.; Van Dyke, T.E. Platelet-rich plasma: Growth factors and pro- and anti-inflammatory properties. J. Periodontol. 2017, 78, 661–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazari, M.; Ni, N.C.; Lüdke, A.; Li, S.H.; Guo, J.; Weisel, R.D.; Li, R.K. Mast cells promote proliferation and migration and inhibit differentiation of mesenchymal stem cells through PDGF. J. Mol. Cell. Cardiol. 2016, 94, 32–42. [Google Scholar] [CrossRef]
- Grafe, I.; Alexander, S.; Peterson, J.R.; Snider, T.N.; Levi, B.; Lee, B.; Mishina, Y. TGF-β Family Signaling in Mesenchymal Differentiation. Cold Spring Harb. Perspect. Biol. 2018, 10, a022202. [Google Scholar] [CrossRef]
- Brown, P.T.; Squire, M.W.; Li, W. Characterization and evaluation of mesenchymal stem cells derived from human embryonic stem cells and bone marrow. Cell Tissue Res. 2014, 358, 149–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pons, J.; Huang, Y.; Arakawa-Hoyt, J.; Washko, D.; Takagawa, J.; Ye, J.; Grossman, W.; Su, H. Biochemical and Biophysical Research Communications VEGF improves survival of mesenchymal stem cells in infarcted hearts. Biochem. Biophys. Res. Commun. 2008, 376, 419–422. [Google Scholar] [CrossRef]
- Longobardi, L.; O’Rear, L.; Aakula, S.; Johnstone, B.; Shimer, K.; Chytil, A.; Horton, W.A.; Moses, H.L.; Spagnoli, A. Effect of IGF-I in the Chondrogenesis of Bone Marrow Mesenchymal Stem Cells in the Presence or Absence of TGF-β Signaling. J. Bone Miner. Res. 2006, 21, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Frelinger, A.L., III; Gerrits, A.J.; Neculaes, V.B.; Gremmel, T.; Torres, A.S.; Caiafa, A.; Carmichael, S.L.; Michelson, A.D. Tunable activation of therapeutic platelet-rich plasma by pulse electric field: Differential effects on clot formation, growth factor release, and platelet morphology. PLoS ONE 2018, 13, e0203557. [Google Scholar] [CrossRef] [PubMed]
- Neculaes, B.; Frelinger, A.L., III; Gerrits, A.J.; Gremmel, T.; Forde, E.E.; Klopman, S.; Carmichael, S.L.; Michelson, A.D. Activation of platelet-rich plasma by pulse electric fields: Voltage, pulse width and calcium concentration can be used to control and tune the release of growth factors, serotonin and hemoglobin. PLoS ONE 2021, 16, e0249209. [Google Scholar] [CrossRef] [PubMed]
- Frelinger, A.L., III; Gerrits, A.J.; Garner, A.L.; Torres, A.S.; Caiafa, A.; Morton, C.A.; Berny-Lang, M.A.; Carmichael, S.L.; Neculaes, V.B.; Michelson, A.D. Modification of pulsed electric field conditions results in distinct activation profiles of platelet-rich plasma. PLoS ONE 2016, 11, e0160933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garner, A.L.; Frelinger, A.L., III; Gerrits, A.J.; Gremmel, T.; Forde, E.E.; Carmichael, S.L.; Michelson, A.D.; Neculaes, V.B. Using extracellular calcium concentration and electric pulse conditions to tune platelet-rich plasma growth factor release and clotting. Med. Hypotheses 2019, 125, 100–105. [Google Scholar] [CrossRef]
- Smith, K.C.; Weaver, J.C. Microdosimetry for conventional and supra-electroporation in cells with organelles. Biochem. Biophys. Res. Commun. 2006, 341, 1266–1276. [Google Scholar]
- Hanna, H.; Denzi, A.; Liberti, M.; André, F.M.; Mir, L.M. Electropermeabilization of Inner and Outer Cell Membranes with Microsecond Pulsed Electric Fields: Quantitative Study with Calcium Ions. Sci. Rep. 2017, 7, 13079. [Google Scholar] [CrossRef]
- Napotnik, D.M.T.B.; Wu, Y.; Gundersen, M.A.; Vernier, P.T. Nanosecond Electric Pulses Cause Mitochondrial Membrane Permeabilization in Jurkat Cells. Bioelectromagnetics 2012, 264, 257–264. [Google Scholar] [CrossRef]
- Rols, M.P.; Teissié, J. Electropermeabilization of mammalian cells to macromolecules: Control by pulse duration. Biophys. J. 1998, 75, 1415–1423. [Google Scholar] [CrossRef] [Green Version]
- Fava, R.A.; Casey, T.T.; Wilcox, J.; Pelton, R.W.; Moses, H.L.; Nanney, L.B. Synthesis of transforming growth factor-beta 1 by megakaryocytes and its localization to megakaryocyte and platelet alpha-granules. Blood 1990, 76, 1946–1955. [Google Scholar] [CrossRef] [Green Version]
- Mcclain, A.K.; Mccarrel, T.M. The effect of four different freezing conditions and time in frozen storage on the concentration of commonly measured growth factors and enzymes in equine platelet-rich plasma over six months. BMC Vet. Res. 2019, 4, 292. [Google Scholar] [CrossRef] [PubMed]
- Al-Saqi, S.H.; Saliem, M.; Asikainen, S.; Quezada, H.C.; Ekblad, Å.; Hovatta, O.; Le Blanc, K.; Jonasson, A.F.; Götherström, C. Defined serum-free media for in vitro expansion of adipose-derived mesenchymal stem cells. Cytotherapy 2014, 16, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirsch, M.; Rach, J.; Handke, W.; Seltsam, A.; Pepelanova, I.; Strauß, S.; Vogt, P.; Scheper, T.; Lavrentieva, A. Comparative Analysis of Mesenchymal Stem Cell Cultivation in Fetal Calf Serum, Human Serum, and Platelet Lysate in 2D and 3D Systems. Front. Bioeng. Biotechnol. 2021, 8, 1499. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.; McDonald, D.; Nicholson, L.; Godthardt, K.; Knobel, S.; Dickinson, A.M.; Filby, A.; Wang, X.N. Global phenotypic characterisation of human platelet lysate expanded MSCs by high-throughput flow cytometry. Sci. Rep. 2018, 8, 3907. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvador, D.; Almeida, H.; Rego, D.; Mendonça, P.; Sousa, A.P.; Serra, M.; Redondo, L. Pulsed Electric Fields for Valorization of Platelets with No Therapeutic Value towards a High Biomedical Potential Product—A Proof of Concept. Appl. Sci. 2022, 12, 5773. https://doi.org/10.3390/app12125773
Salvador D, Almeida H, Rego D, Mendonça P, Sousa AP, Serra M, Redondo L. Pulsed Electric Fields for Valorization of Platelets with No Therapeutic Value towards a High Biomedical Potential Product—A Proof of Concept. Applied Sciences. 2022; 12(12):5773. https://doi.org/10.3390/app12125773
Chicago/Turabian StyleSalvador, Daniela, Henrique Almeida, Duarte Rego, Pedro Mendonça, Ana Paula Sousa, Margarida Serra, and Luis Redondo. 2022. "Pulsed Electric Fields for Valorization of Platelets with No Therapeutic Value towards a High Biomedical Potential Product—A Proof of Concept" Applied Sciences 12, no. 12: 5773. https://doi.org/10.3390/app12125773
APA StyleSalvador, D., Almeida, H., Rego, D., Mendonça, P., Sousa, A. P., Serra, M., & Redondo, L. (2022). Pulsed Electric Fields for Valorization of Platelets with No Therapeutic Value towards a High Biomedical Potential Product—A Proof of Concept. Applied Sciences, 12(12), 5773. https://doi.org/10.3390/app12125773






