Trace Metal Partitioning in the Salinity Gradient of the Highly Stratified Estuary: A Case Study in the Krka River Estuary (Croatia)
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
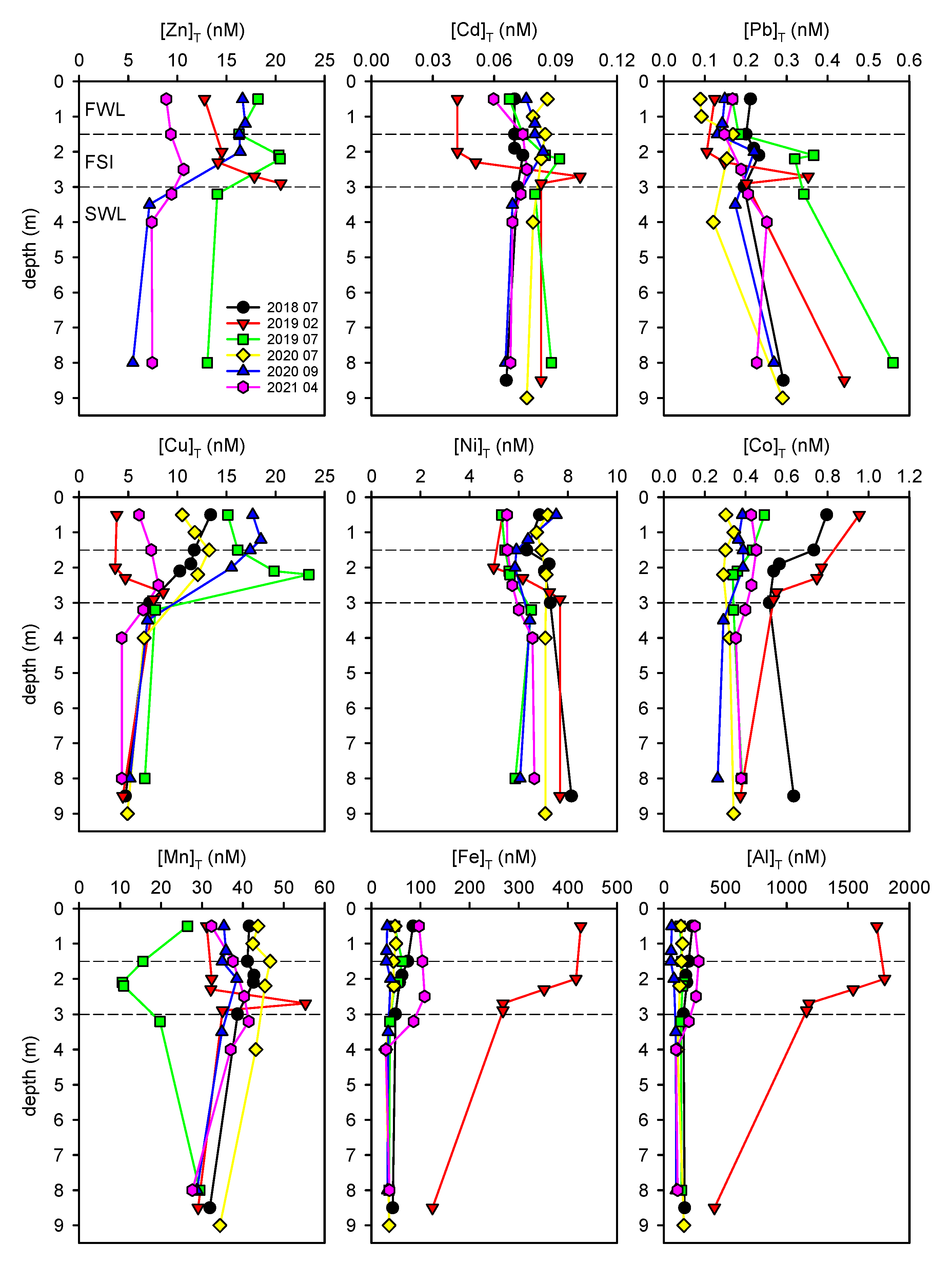
3.1. Total Metal Concentrations Distribution within the Water Column
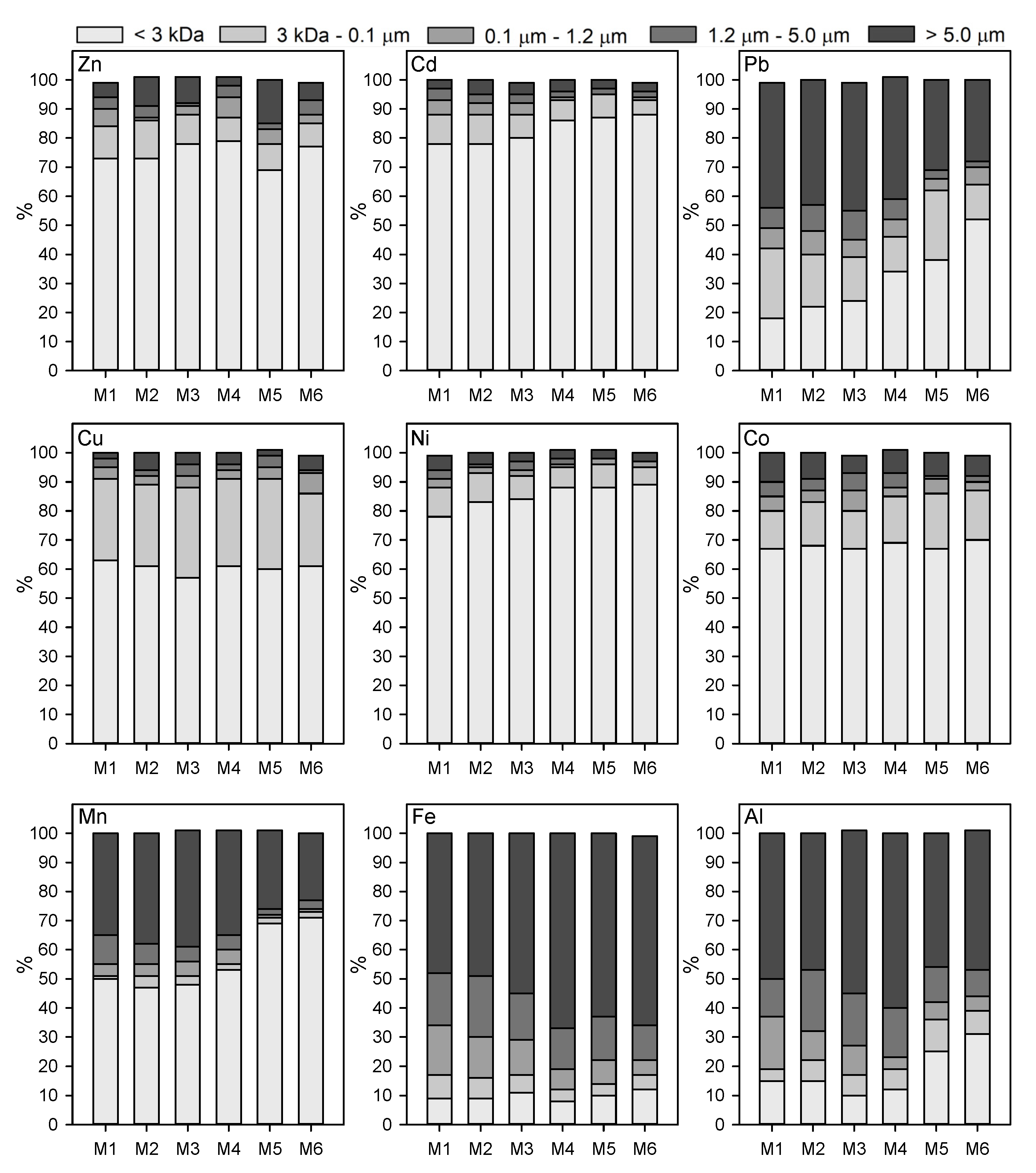
3.2. Trace Metal Partitioning into Different Size Fractions
3.3. Variation in Size Distribution between Stratified Layers
3.4. Significance of Colloidal Metal Fraction in the Dissolved Phase-Comparison with Other Coastal Areas
3.4.1. Metals with High Particulate Reactivity (Pb, Mn, Fe, Al)
3.4.2. Metals Associated with Dissolved Organic Matter (Cu, Co)
3.4.3. Metals with Small Colloidal and Particulate Fractions (Zn, Cd, Ni)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lead, J.R.; Wilkinson, K.J. Environmental Colloids and Particles: Current Knowledge and Future Developments. In Environmental Colloids and Particles: Behaviour, Separation and Characterisation; Wilkinson, K.J., Lead, J.R., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2007; pp. 1–15. [Google Scholar]
- Wells, M.L. Marine Colloids and Trace Metals. In Biogeochemistry of Marine Dissolved Organic Matter; Hansell, D.A., Carlson, C.A., Eds.; Academic Press: Cambridge, MA, USA, 2002; pp. 367–404. ISBN 9780124059405. [Google Scholar]
- Filella, M. Colloidal Properties of Submicron Particles in Natural Waters. In Environmental Colloids and Particles: Behaviour, Separation and Characterisation; Wilkinson, K.J., Lead, J.R., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2007; pp. 6, 17–93. ISBN 9780470024539. [Google Scholar]
- Everett, D.H. Manual of Symbols and Terminology for Physicochemical Quantities and Units, Appendix II: Definitions, Terminology and Symbols in Colloid and Surface Chemistry. Pure Appl. Chem. 1972, 31, 577–638. [Google Scholar] [CrossRef]
- Sañudo-Wilhelmy, S.A.; Rivera-Duarte, I.; Flegal, A.R. Distribution of colloidal trace metals in the San Francisco Bay estuary. Geochim. Cosmochim. Acta 1996, 60, 4933–4944. [Google Scholar] [CrossRef]
- Waeles, M.; Tanguy, V.; Lespes, G.; Riso, R.D. Behaviour of colloidal trace metals (Cu, Pb and Cd) in estuarine waters: An approach using frontal ultrafiltration (UF) and stripping chronopotentiometric methods (SCP). Estuarine Coast. Shelf Sci. 2008, 80, 538–544. [Google Scholar] [CrossRef]
- Santschi, P.H.; Lenhart, J.J.; Honeyman, B.D. Heterogeneous processes affecting trace contaminant distribution in estuaries: The role of natural organic matter. Mar. Chem. 1997, 58, 99–125. [Google Scholar] [CrossRef]
- Cindric, A.-M.; Garnier, C.; Oursel, B.; Pižeta, I.; Omanović, D. Evidencing the natural and anthropogenic processes controlling trace metals dynamic in a highly stratified estuary: The Krka River estuary (Adriatic, Croatia). Mar. Pollut. Bull. 2015, 94, 199–216. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Chen, M.; Guo, L.; Wang, W.-X. Size partitioning and mixing behavior of trace metals and dissolved organic matter in a South China estuary. Sci. Total Environ. 2017, 603–604, 434–444. [Google Scholar] [CrossRef]
- Tang, D.; Warnken, K.W.; Santschi, P.H. Distribution and partitioning of trace metals (Cd, Cu, Ni, Pb, Zn) in Galveston Bay waters. Mar. Chem. 2002, 78, 29–45. [Google Scholar] [CrossRef]
- Braungardt, C.B.; Howell, K.A.; Tappin, A.D.; Achterberg, E.P. Temporal variability in dynamic and colloidal metal fractions determined by high resolution in situ measurements in a UK estuary. Chemosphere 2011, 84, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Tercier-Waeber, M.L.; Stoll, S.; Slaveykova, V.I. Trace Metal Behavior in Surface Waters: Emphasis on Dynamic Spéciation, Sorption Processes and Bioavailability. Arch. Sci. 2012, 65, 119–142. [Google Scholar]
- Tanguy, V.; Waeles, M.; Gigault, J.; Cabon, J.-Y.; Quentel, F.; Riso, R.D. The removal of colloidal lead during estuarine mixing: Seasonal variations and importance of iron oxides and humic substances. Mar. Freshw. Res. 2011, 62, 329–341. [Google Scholar] [CrossRef]
- Oursel, B.; Garnier, C.; Durrieu, G.; Mounier, S.; Omanović, D.; Lucas, Y. Dynamics and fates of trace metals chronically input in a Mediterranean coastal zone impacted by a large urban area. Mar. Pollut. Bull. 2013, 69, 137–149. [Google Scholar] [CrossRef]
- Waeles, M.; Tanguy, V.; Riso, R.D. On the control of copper colloidal distribution by humic substances in the Penzé estuary. Chemosphere 2015, 119, 1176–1184. [Google Scholar] [CrossRef]
- De Souza Machado, A.A.; Spencer, K.; Kloas, W.; Toffolon, M.; Zarfl, C. Metal fate and effects in estuaries: A review and conceptual model for better understanding of toxicity. Sci. Total Environ. 2016, 541, 268–281. [Google Scholar] [CrossRef]
- Cobelo-García, A.; Prego, R. Land inputs, behaviour and contamination levels of copper in a ria estuary (NW Spain). Mar. Environ. Res. 2003, 56, 403–422. [Google Scholar] [CrossRef]
- Muller, F. Interactions of copper, lead and cadmium with the dissolved, colloidal and particulate components of estuarine and coastal waters. Mar. Chem. 1996, 52, 245–268. [Google Scholar] [CrossRef]
- Jiann, K.-T.; Wen, L.-S.; Santschi, P.H. Trace metal (Cd, Cu, Ni and Pb) partitioning, affinities and removal in the Danshuei River estuary, a macro-tidal, temporally anoxic estuary in Taiwan. Mar. Chem. 2005, 96, 293–313. [Google Scholar] [CrossRef]
- Batchelli, S.; Muller, F.L.; Baalousha, M.; Lead, J.R. Size fractionation and optical properties of colloids in an organic-rich estuary (Thurso, UK). Mar. Chem. 2009, 113, 227–237. [Google Scholar] [CrossRef]
- Fang, Z.; Wang, W.-X. Size speciation of dissolved trace metals in hydrothermal plumes on the Southwest Indian Ridge. Sci. Total Environ. 2021, 771, 145367. [Google Scholar] [CrossRef]
- Lu, Y.; Gao, X.; Chen, C.-T.A. Separation and determination of colloidal trace metals in seawater by cross-flow ultrafiltration, liquid-liquid extraction and ICP-MS. Mar. Chem. 2019, 215, 103685. [Google Scholar] [CrossRef]
- Schlosser, C.; Streu, P.; Croot, P.L. Vivaspin ultrafiltration: A new approach for high resolution measurements of colloidal and soluble iron species. Limnol. Oceanogr. Methods 2013, 11, 187–201. [Google Scholar] [CrossRef]
- Lu, Y.; Gao, X.; Song, J.; Chen, C.-T.A.; Chu, J. Colloidal toxic trace metals in urban riverine and estuarine waters of Yantai City, southern coast of North Yellow Sea. Sci. Total Environ. 2019, 717, 135265. [Google Scholar] [CrossRef]
- Scribe, P.; Fillaux, J.; Laureillard, J.; Denant, V.; Saliot, A. Fatty acids as biomarkers of planktonic inputs in the stratified estuary of the Krka River, Adriatic Sea: Relationship with pigments. Mar. Chem. 1991, 32, 299–312. [Google Scholar] [CrossRef]
- Cukrov, N.; Frančišković-Bilinski, S.; Mikac, N.; Roje, V. Natural and Anthropogenic Influences Recorded in Sediments from the Krka River Estuary (Eastern Adriatic Coast), Evaluated by Statistical Methods. Fresenius Environ. Bull. 2008, 17, 855–863. [Google Scholar]
- Pađan, J.; Marcinek, S.; Cindrić, A.-M.; Layglon, N.; Lenoble, V.; Salaün, P.; Garnier, C.; Omanović, D. Improved voltammetric methodology for chromium redox speciation in estuarine waters. Anal. Chim. Acta 2019, 1089, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Pađan, J.; Marcinek, S.; Cindrić, A.-M.; Layglon, N.; Garnier, C.; Salaün, P.; Cobelo-Garcia, A.; Omanović, D. Determination of sub-picomolar levels of platinum in the pristine Krka River estuary (Croatia) using improved voltammetric methodology. Environ. Chem. 2020, 17, 77. [Google Scholar] [CrossRef]
- Cindrić, A.-M.; Marcinek, S.; Garnier, C.; Salaün, P.; Cukrov, N.; Oursel, B.; Lenoble, V.; Omanović, D. Evaluation of diffusive gradients in thin films (DGT) technique for speciation of trace metals in estuarine waters—A multimethodological approach. Sci. Total Environ. 2020, 721, 137784. [Google Scholar] [CrossRef]
- Marcinek, S.; Santinelli, C.; Cindrić, A.-M.; Evangelista, V.; Gonnelli, M.; Layglon, N.; Mounier, S.; Lenoble, V.; Omanović, D. Dissolved organic matter dynamics in the pristine Krka River estuary (Croatia). Mar. Chem. 2020, 225, 103848. [Google Scholar] [CrossRef]
- Carić, H.; Cukrov, N.; Omanović, D. Nautical Tourism in Marine Protected Areas (MPAs): Evaluating an Impact of Copper Emission from Antifouling Coating. Sustainability 2021, 13, 11897. [Google Scholar] [CrossRef]
- Viličić, D.; Legović, T.; Žutić, V. Vertical distribution of phytoplankton in a stratified estuary. Aquat. Sci. 1989, 51, 31–46. [Google Scholar] [CrossRef]
- Sempéré, R.; Cauwet, G. Occurrence of organic colloids in the stratified estuary of the Krka River (Croatia). Estuarine Coast. Shelf Sci. 1995, 40, 105–114. [Google Scholar] [CrossRef]
- Bilinski, H. Mercury distribution in the water column of the stratified Krka river estuary (Croatia): Importance of natural organic matter and of strong winds. Water Res. 2000, 34, 2001–2010. [Google Scholar] [CrossRef]
- Louis, Y.; Garnier, C.; Lenoble, V.; Mounier, S.; Cukrov, N.; Omanović, D.; Pižeta, I. Kinetic and equilibrium studies of copper-dissolved organic matter complexation in water column of the stratified Krka River estuary (Croatia). Mar. Chem. 2009, 114, 110–119. [Google Scholar] [CrossRef]
- Karanfil, T.; Erdogan, I.; Schlautman, M.A. Selecting Filter Membranes for measuring DOC and UV254. J. Am. Water Work. Assoc. 2003, 95, 86–100. [Google Scholar] [CrossRef]
- Klun, K.; Falnoga, I.; Mazej, D.; Šket, P.; Faganeli, J. Colloidal Organic Matter and Metal(loid)s in Coastal Waters (Gulf of Trieste, Northern Adriatic Sea). Aquat. Geochem. 2019, 25, 179–194. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Graeber, D.; Bro, R. Fluorescence spectroscopy and multi-way techniques. PARAFAC. Anal. Methods 2013, 5, 6557–6566. [Google Scholar] [CrossRef] [Green Version]
- Murphy, K.R.; Stedmon, C.A.; Wenig, P.; Bro, R. OpenFluor—An online spectral library of auto-fluorescence by organic compounds in the environment. Anal. Methods 2013, 6, 658–661. [Google Scholar] [CrossRef] [Green Version]
- Omanović, D.; Kwokal, Ž.; Goodwin, A.; Lawrence, A.; Banks, C.E.; Compton, R.G.; Komorsky-Lovrić, Š. Trace metal detection in Šibenik Bay, Croatia: Cadmium, lead and copper with anodic stripping voltammetry and manganese via sonoelectrochemistry. A case study. J. Iran. Chem. Soc. 2006, 3, 128–139. [Google Scholar] [CrossRef]
- Penezić, A.; Milinković, A.; Alempijević, S.B.; Žužul, S.; Frka, S. Atmospheric deposition of biologically relevant trace metals in the eastern Adriatic coastal area. Chemosphere 2021, 283, 131178. [Google Scholar] [CrossRef]
- Wells, M.L.; Smith, G.J.; Bruland, K. The distribution of colloidal and particulate bioactive metals in Narragansett Bay, RI. Mar. Chem. 2000, 71, 143–163. [Google Scholar] [CrossRef]
- Tovar-Sánchez, A.; Sañudo-Wilhelmy, S.A.; Flegal, A. Temporal and spatial variations in the biogeochemical cycling of cobalt in two urban estuaries: Hudson River Estuary and San Francisco Bay. Estuarine Coast. Shelf Sci. 2004, 60, 717–728. [Google Scholar] [CrossRef] [Green Version]
- Takata, H.; Aono, T.; Tagami, K.; Uchida, S. Processes controlling cobalt distribution in two temperate estuaries, Sagami Bay and Wakasa Bay, Japan. Estuarine Coast. Shelf Sci. 2010, 89, 294–305. [Google Scholar] [CrossRef]
- Rozan, T.F.; Lassman, M.E.; Ridge, D.P.; Luther, G.W. Evidence for iron, copper and zinc complexation as multinuclear sulphide clusters in oxic rivers. Nature 2000, 406, 879–882. [Google Scholar] [CrossRef]
- Svensen, C.; Viličić, D.; Wassmann, P.; Arashkevich, E.; Ratkova, T. Plankton distribution and vertical flux of biogenic matter during high summer stratification in the Krka estuary (Eastern Adriatic). Estuarine Coast. Shelf Sci. 2007, 71, 381–390. [Google Scholar] [CrossRef]
- Cetinić, I.; Viličić, D.; Burić, Z.; Olujić, G. Phytoplankton Seasonality in a Highly Stratified Karstic Estuary (Krka, Adriatic Sea). Hydrobiologia 2006, 555, 31–40. [Google Scholar] [CrossRef]
- Guerzoni, S.; Chester, R.; Dulac, F.; Herut, B.; Loÿe-Pilot, M.-D.; Measures, C.; Migon, C.; Molinaroli, E.; Moulin, C.; Rossini, P.; et al. The role of atmospheric deposition in the biogeochemistry of the Mediterranean Sea. Prog. Oceanogr. 1999, 44, 147–190. [Google Scholar] [CrossRef]
- Benoit, G.; Oktay-Marshall, S.; Cantu, A.; Hood, E.; Coleman, C.; Corapcioglu, M.; Santschi, P. Partitioning of Cu, Pb, Ag, Zn, Fe, Al, and Mn between filter-retained particles, colloids, and solution in six Texas estuaries. Mar. Chem. 1994, 45, 307–336. [Google Scholar] [CrossRef]
- Martin, J.-M.; Dai, M.-H.; Cauwet, G. Significance of colloids in the biogeochemical cycling of organic carbon and trace metals in the Venice Lagoon (Italy). Limnol. Oceanogr. 1995, 40, 119–131. [Google Scholar] [CrossRef] [Green Version]
- Dulaquais, G.; Waeles, M.; Breitenstein, J.; Knoery, J.; Riso, R. Links between size fractionation, chemical speciation of dissolved copper and chemical speciation of dissolved organic matter in the Loire estuary. Environ. Chem. 2020, 17, 385. [Google Scholar] [CrossRef]
- Dai, M.-H.; Martin, J.-M. First data on trace metal level and behaviour in two major Arctic river-estuarine systems (Ob and Yenisey) and in the adjacent Kara Sea, Russia. Earth Planet. Sci. Lett. 1995, 131, 127–141. [Google Scholar] [CrossRef]
- Sedlak, D.L.; Phinney, J.T.; Bedsworth, W.W. Strongly Complexed Cu and Ni in Wastewater Effluents and Surface Runoff. Environ. Sci. Technol. 1997, 31, 3010–3016. [Google Scholar] [CrossRef]
- Berg, C.M.V.D.; Merks, A.G.; Duursma, E.K. Organic complexation and its control of the dissolved concentrations of copper and zinc in the Scheldt estuary. Estuarine Coast. Shelf Sci. 1987, 24, 785–797. [Google Scholar] [CrossRef]
- Buck, K.N.; Ross, J.R.; Flegal, A.R.; Bruland, K.W. A review of total dissolved copper and its chemical speciation in San Francisco Bay, California. Environ. Res. 2007, 105, 5–19. [Google Scholar] [CrossRef]
- Whitby, H.; Hollibaugh, J.T.; van den Berg, C.M.G. Chemical Speciation of Copper in a Salt Marsh Estuary and Bioavailability to Thaumarchaeota. Front. Mar. Sci. 2017, 4, 178. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.; Warnken, K.W.; Santschi, P.H. Organic complexation of copper in surface waters of Galveston Bay. Limnol. Oceanogr. 2001, 46, 321–330. [Google Scholar] [CrossRef]
- Coble, P.G. Marine Optical Biogeochemistry: The Chemistry of Ocean Color. Chem. Rev. 2007, 107, 402–418. [Google Scholar] [CrossRef]
- Yamashita, Y.; Tanoue, E. Chemical characterization of protein-like fluorophores in DOM in relation to aromatic amino acids. Mar. Chem. 2003, 82, 255–271. [Google Scholar] [CrossRef]
- Laglera, L.M.; Berg, C.M.V.D. Copper complexation by thiol compounds in estuarine waters. Mar. Chem. 2003, 82, 71–89. [Google Scholar] [CrossRef]
- Ciglenečki, I.; Ćosović, B.; Vojvodić, V.; Plavsic, M.; Furić, K.; Minacci, A.; Baldi, F. The role of reduced sulfur species in the coalescence of polysaccharides in the Adriatic Sea. Mar. Chem. 2000, 71, 233–249. [Google Scholar] [CrossRef]
- Wen, L.-S.; Santschi, P.; Gill, G.; Paternostro, C. Estuarine trace metal distributions in Galveston Bay: Importance of colloidal forms in the speciation of the dissolved phase. Mar. Chem. 1999, 63, 185–212. [Google Scholar] [CrossRef]
- de Carvalho, L.M.; Hollister, A.P.; Trindade, C.; Gledhill, M.; Koschinsky, A. Distribution and size fractionation of nickel and cobalt species along the Amazon estuary and mixing plume. Mar. Chem. 2021, 236, 104019. [Google Scholar] [CrossRef]
- Ndung’u, K.; Franks, R.P.; Bruland, K.W.; Flegal, A. Organic complexation and total dissolved trace metal analysis in estuarine waters: Comparison of solvent-extraction graphite furnace atomic absorption spectrometric and chelating resin flow injection inductively coupled plasma-mass spectrometric analysis. Anal. Chim. Acta 2003, 481, 127–138. [Google Scholar] [CrossRef]

| Zn | Cd | Pb | Cu | Ni | Co | Mn | Fe | Al |
|---|---|---|---|---|---|---|---|---|
| (A) Average % of particulate fraction in total fraction | ||||||||
| 15% | 9% | 51% | 11% | 7% | 17% | 42% | 84% | 75% |
| (B) Average % of colloidal fraction in total fraction | ||||||||
| 10% | 8% | 17% | 29% | 8% | 15% | 2% | 6% | 7% |
| (C) Average % of colloidal fraction in dissolved fraction | ||||||||
| 12% | 9% | 37% | 32% | 9% | 18% | 4% | 37% | 31% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcinek, S.; Cindrić, A.M.; Pađan, J.; Omanović, D. Trace Metal Partitioning in the Salinity Gradient of the Highly Stratified Estuary: A Case Study in the Krka River Estuary (Croatia). Appl. Sci. 2022, 12, 5816. https://doi.org/10.3390/app12125816
Marcinek S, Cindrić AM, Pađan J, Omanović D. Trace Metal Partitioning in the Salinity Gradient of the Highly Stratified Estuary: A Case Study in the Krka River Estuary (Croatia). Applied Sciences. 2022; 12(12):5816. https://doi.org/10.3390/app12125816
Chicago/Turabian StyleMarcinek, Saša, Ana Marija Cindrić, Jasmin Pađan, and Dario Omanović. 2022. "Trace Metal Partitioning in the Salinity Gradient of the Highly Stratified Estuary: A Case Study in the Krka River Estuary (Croatia)" Applied Sciences 12, no. 12: 5816. https://doi.org/10.3390/app12125816
APA StyleMarcinek, S., Cindrić, A. M., Pađan, J., & Omanović, D. (2022). Trace Metal Partitioning in the Salinity Gradient of the Highly Stratified Estuary: A Case Study in the Krka River Estuary (Croatia). Applied Sciences, 12(12), 5816. https://doi.org/10.3390/app12125816







