Exploring Structure-Property Relationships in a Family of Ferrocene-Containing, Triphenylamine-Based Hybrid Organic Dyes
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. Synthesis
2.3. Experimental Details
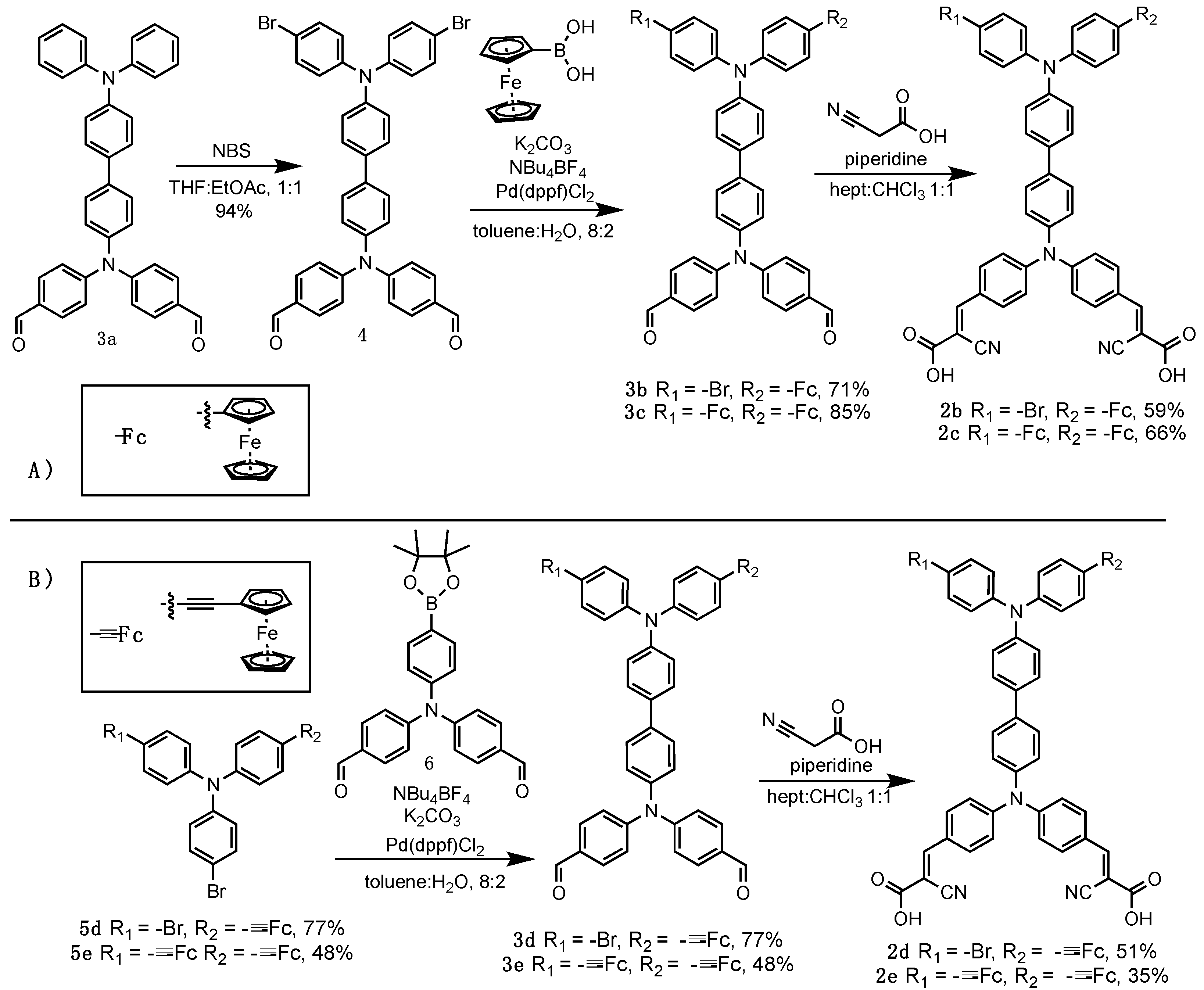
3. Results
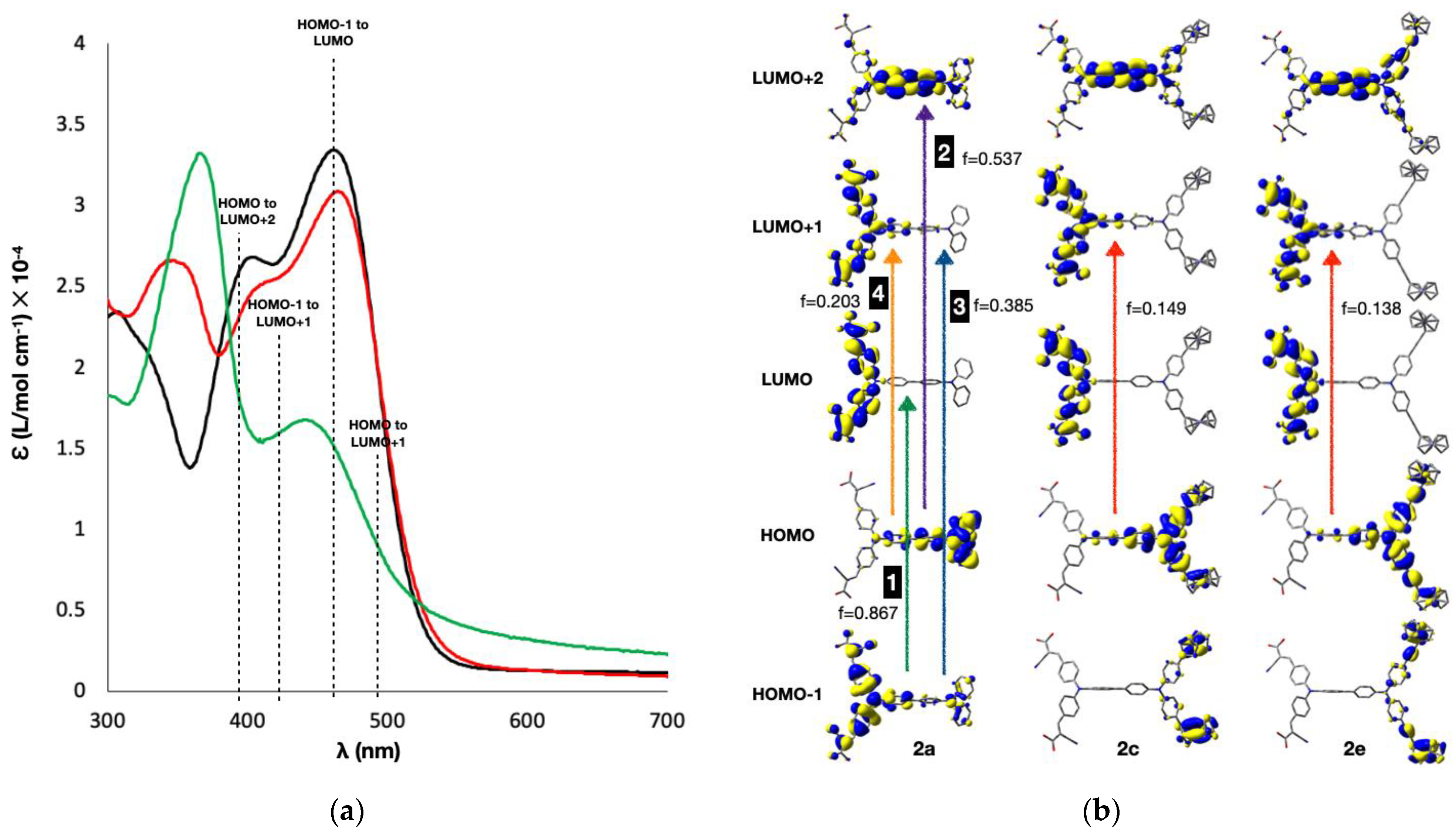
3.1. Optical Properties of the Dyes
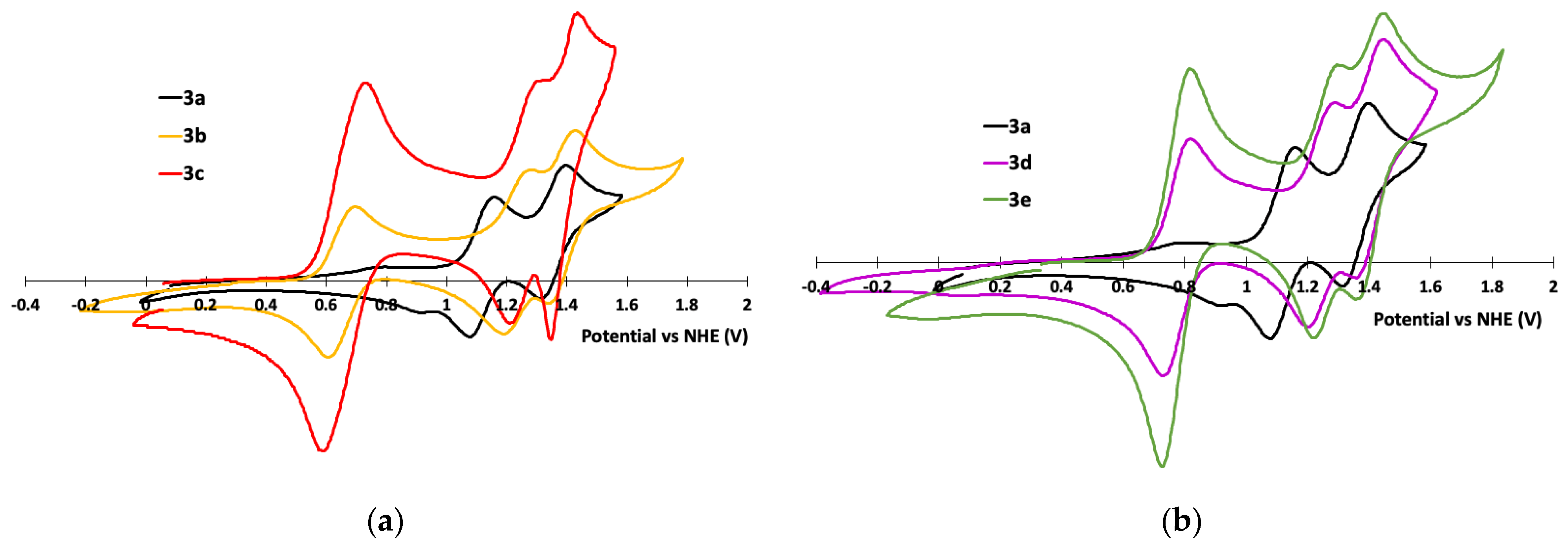
3.2. Electrochemical Properties
3.3. Device Performance
3.4. Spectroelectrochemistry
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Yang, C.; Yu, R.; Liu, C.; Li, H.; Zhang, S.; Hou, J. Achieving over 10% Efficiency in Poly(3-Hexylthiophene)-Based Organic Solar Cells via Solid Additives. ChemSusChem 2021, 14, 3607–3613. [Google Scholar] [CrossRef]
- Andersen, T.R.; Weyhe, A.T.; Tao, Q.; Zhao, F.; Qin, R.; Zhang, S.; Chen, H.; Yu, D. Novel Cost-Effective Acceptor: P3HT Based Organic Solar Cells Exhibiting the Highest Ever Reported Industrial Readiness Factor. Mater. Adv. 2020, 1, 658–665. [Google Scholar] [CrossRef]
- Fakharuddin, A.; Jose, R.; Brown, T.M.; Fabregat-Santiago, F.; Bisquert, J. A Perspective on the Production of Dye-Sensitized Solar Modules. Energy Environ. Sci. 2014, 7, 3952–3981. [Google Scholar] [CrossRef]
- Hardin, B.E.; Snaith, H.J.; McGehee, M.D. The Renaissance of Dye-Sensitized Solar Cells. Nat. Photonics 2012, 6, 162–169. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Grätzel, M. Dye-Sensitized Solar Cells. J. Photochem. Photobiol. C Photochem. Rev. 2003, 4, 145–153. [Google Scholar] [CrossRef]
- Ning, Z.; Fu, Y.; Tian, H. Improvement of Dye-Sensitized Solar Cells: What We Know and What We Need to Know. Energy Environ. Sci. 2010, 3, 1170–1181. [Google Scholar] [CrossRef]
- Ye, M.; Wen, X.; Wang, M.; Iocozzia, J.; Zhang, N.; Lin, C.; Lin, Z. Recent Advances in Dye-Sensitized Solar Cells: From Photoanodes, Sensitizers and Electrolytes to Counter Electrodes. Mater. Today 2015, 18, 155–162. [Google Scholar] [CrossRef]
- O’Regan, B.; Graetzel, M. A Low-Cost, High-Efficiency Solar Cell Based on Dye-Sensitized Colloidal TiO2 Films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Mishra, A.; Fischer, M.K.R.; Bäuerle, P. Metal-Free Organic Dyes for Dye-Sensitized Solar Cells: From Structure: Property Relationships to Design Rules. Angew. Chemie Int. Ed. 2009, 48, 2474–2499. [Google Scholar] [CrossRef]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Grätzel, M. Dye-Sensitized Solar Cells with 13% Efficiency Achieved through the Molecular Engineering of Porphyrin Sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Yella, A.; Mai, C.L.; Zakeeruddin, S.M.; Chang, S.N.; Hsieh, C.H.; Yeh, C.Y.; Grätzel, M. Molecular Engineering of Push-Pull Porphyrin Dyes for Highly Efficient Dye-Sensitized Solar Cells: The Role of Benzene Spacers. Angew. Chemie Int. Ed. 2014, 53, 2973–2977. [Google Scholar] [CrossRef] [PubMed]
- Lekesi, L.P.; Koao, L.F.; Motloung, S.V.; Motaung, T.E.; Malevu, T. Developments on Perovskite Solar Cells (PSCs): A Critical Review. Appl. Sci. 2022, 12, 672. [Google Scholar] [CrossRef]
- Yun, S.; Qin, Y.; Uhl, A.R.; Vlachopoulos, N.; Yin, M.; Li, D.; Han, X.; Hagfeldt, A. New-Generation Integrated Devices Based on Dye-Sensitized and Perovskite Solar Cells. Energy Environ. Sci. 2018, 11, 476–526. [Google Scholar] [CrossRef]
- Ferrere, S. New Photosensitizers Based upon [Fe(L)2(CN)2] and [Fe(L)3] (L = Substituted 2,2’-Bipyridine): Yields for the Photosensitization of TiO2 and Effects on the Band Selectivity. Chem. Mater. 2000, 12, 1083–1089. [Google Scholar] [CrossRef]
- Ferrere, S.; Gregg, B.G. Photosensitization of TiO2 by [FeII(2,2′-Bipyridine-4,4′-Dicarboxylic Acid)2(CN)2]: Band Selective Electron Injection from Ultra-Short-Lived Excited States. J. Am. Chem. Soc. 1998, 120, 843–844. [Google Scholar] [CrossRef]
- Monat, J.E.; McCusker, J.K. Femtosecond Excited-State Dynamics of an Iron(II) Polypyridyl Solar Cell Sensitizer Model. J. Am. Chem. Soc. 2000, 122, 4092–4097. [Google Scholar] [CrossRef]
- Yang, M.; Thompson, D.W.; Meyer, G.J. Charge-Transfer Studies of Iron Cyano Compounds Bound to Nanocrystalline TiO2 Surfaces. Inorg. Chem. 2002, 41, 1254–1262. [Google Scholar] [CrossRef]
- Duchanois, T.; Etienne, T.; Cebrián, C.; Liu, L.; Monari, A.; Beley, M.; Assfeld, X.; Haacke, S.; Gros, P.C. An Iron-Based Photosensitizer with Extended Excited-State Lifetime: Photophysical and Photovoltaic Properties. Eur. J. Inorg. Chem. 2015, 2015, 2469–2477. [Google Scholar] [CrossRef]
- Harlang, T.C.B.; Liu, Y.; Gordivska, O.; Fredin, L.A.; Ponseca, C.S.; Huang, P.; Chábera, P.; Kjaer, K.S.; Mateos, H.; Uhlig, J.; et al. Iron Sensitizer Converts Light to Electrons with 92% Yield. Nat. Chem. 2015, 7, 883–889. [Google Scholar] [CrossRef]
- Duchanois, T.; Liu, L.; Pastore, M.; Monari, A.; Cebrián, C.; Trolez, Y.; Darari, M.; Magra, K.; Francés-Monerris, A.; Domenichini, E.; et al. NHC-Based Iron Sensitizers for DSSCs. Inorganics 2018, 6, 63. [Google Scholar] [CrossRef]
- Becker, M.; Housecroft, C.E.; Constable, E.C. Electrolyte Tuning in Iron(II)-Based Dye-Sensitized Solar Cells: Different Ionic Liquids and I2 Concentrations. Materials 2021, 14, 3053. [Google Scholar] [CrossRef] [PubMed]
- Marri, A.R.; Marchini, E.; Cabanes, V.D.; Argazzi, R.; Pastore, M.; Caramori, S.; Bignozzi, C.A.; Gros, P.C. A Series of Iron(II)-NHC Sensitizers with Remarkable Power Conversion Efficiency in Photoelectrochemical Cells. Chem. Eur. J. 2021, 27, 16260–16269. [Google Scholar] [CrossRef]
- Brunel, D.; Noirbent, G.; Dumur, F. Ferrocene: An Unrivaled Electroactive Building Block for the Design of Push-Pull Dyes with near-Infrared and Infrared Absorptions. Dyes Pigments 2019, 170, 107611. [Google Scholar] [CrossRef]
- Patil, Y.; Misra, R.; Singhal, R.; Sharma, G.D. Ferrocene-Diketopyrrolopyrrole Based Non-Fullerene Acceptors for Bulk Heterojunction Polymer Solar Cells. J. Mater. Chem. A 2017, 5, 13625–13633. [Google Scholar] [CrossRef]
- Nar, I.; Atsay, A.; Altındal, A.; Hamuryudan, E.; Koçak, M.B.; Gül, A. Ferrocenyl Phthalocyanine as Donor in Non-Poly(3-Hexylthiophen-2,5-Diyl) Bulk Heterojunction Solar Cell. Chem. Eur. J. 2018, 24, 6946–6949. [Google Scholar] [CrossRef] [PubMed]
- Patil, Y.; Misra, R.; Singh, M.K.; Sharma, G.D. Ferrocene-Diketopyrrolopyrrole Based Small Molecule Donors for Bulk Heterojunction Solar Cells. Phys. Chem. Chem. Phys. 2017, 19, 7262–7269. [Google Scholar] [CrossRef]
- Manfredi, N.; Decavoli, C.; Boldrini, C.L.; Coluccini, C.; Abbotto, A. Ferrocene Derivatives Functionalized with Donor/Acceptor (Hetero)Aromatic Substituents: Tuning of Redox Properties. Energies 2020, 13, 3937. [Google Scholar] [CrossRef]
- Jia, J.; Duan, L.; Chen, Y.; Zong, X.; Sun, Z.; Wu, Q.; Xue, S. New Ferrocenyl-Containing Organic Hole-Transporting Materials for Perovskite Solar Cells in Regular (n-i-p) and Inverted (p-i-n) Architectures. RSC Adv. 2019, 9, 216–223. [Google Scholar] [CrossRef]
- Daeneke, T.; Mozer, A.J.; Kwon, T.H.; Duffy, N.W.; Holmes, A.B.; Bach, U.; Spiccia, L. Dye Regeneration and Charge Recombination in Dye-Sensitized Solar Cells with Ferrocene Derivatives as Redox Mediators. Energy Environ. Sci. 2012, 5, 7090–7099. [Google Scholar] [CrossRef]
- Saygili, Y.; Stojanovic, M.; Flores-Díaz, N.; Zakeeruddin, S.M.; Vlachopoulos, N.; Grätzel, M.; Hagfeldt, A. Metal Coordination Complexes as Redox Mediators in Regenerative Dye-Sensitized Solar Cells. Inorganics 2019, 7, 30. [Google Scholar] [CrossRef]
- Chauhan, R.; Auvinen, S.; Aditya, A.S.; Trivedi, M.; Prasad, R.; Alatalo, M.; Amalnerkar, D.P.; Kumar, A. Light Harvesting Properties of Ferrocenyl Based Sensitizer with Sulfur Rich Dithiocarabamates and Xanthate as Anchoring Group. Sol. Energy 2014, 108, 560–569. [Google Scholar] [CrossRef]
- Chauhan, R.; Shahid, M.; Trivedi, M.; Amalnerkar, D.P.; Kumar, A. Dye-Sensitized Solar Cells with Biferrocenyl Antennae Having Quinoxaline Spacers. Eur. J. Inorg. Chem. 2015, 2015, 3700–3707. [Google Scholar] [CrossRef]
- Singh, A.; Kociok-Köhn, G.; Chauhan, R.; Muddassir, M.; Gosavi, S.W.; Kumar, A. Ferrocene Appended Asymmetric Sensitizers with Azine Spacers with Phenolic/Nitro Anchors for Dye-Sensitized Solar Cells. J. Mol. Struct. 2022, 1249, 131630. [Google Scholar] [CrossRef]
- Chauhan, R.; Yadav, R.; Singh, A.K.; Trivedi, M.; Kociok-Köhn, G.; Kumar, A.; Gosavi, S.; Rane, S. Ferrocenyl Chalcones with Phenolic and Pyridyl Anchors as Potential Sensitizers in Dye-Sensitized Solar Cells. RSC Adv. 2016, 6, 97664–97675. [Google Scholar] [CrossRef]
- Maragani, R.; Misra, R.; Roy, M.S.; Singh, M.K.; Sharma, G.D. (D-π-A)2-π-D-A Type Ferrocenyl Bisthiazole Linked Triphenylamine Based Molecular Systems for DSSC: Synthesis, Experimental and Theoretical Performance Studies. Phys. Chem. Chem. Phys. 2017, 19, 8925–8933. [Google Scholar] [CrossRef]
- Yadav, R.; Singh, A.; Kociok-Köhn, G.; Chauhan, R.; Kumar, A.; Gosavi, S. Ferrocenyl Benzimidazole with Carboxylic and Nitro Anchors as Potential Sensitizers in Dye-Sensitized Solar Cells. New J. Chem. 2017, 41, 7312–7321. [Google Scholar] [CrossRef]
- Singh, A.; Singh, P.; Kociok-Köhn, G.; Trivedi, M.; Kumar, A.; Chauhan, R.; Rane, S.B.; Terashima, C.; Gosavi, S.W.; Fujishima, A. 1,1′-Bis(Diphenylphosphino)Ferrocene-Appended Nickel(Ii) Dithiolates as Sensitizers in Dye-Sensitized Solar Cells. New J. Chem. 2018, 42, 9306–9316. [Google Scholar] [CrossRef]
- Hussein, B.A.; Huynh, J.T.; Prieto, P.L.; Barran, C.P.; Arnold, A.E.; Sarycheva, O.V.; Lough, A.J.; Koivisto, B.D. Molecular Lemmings: Strategies to Avoid When Designing BODIPY Ferrocene Dyads for Dye-Sensitized Solar Cell Applications. Dalt. Trans. 2018, 47, 4916–4920. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Y.; Wu, F.Y.; Liu, J.C. New Type of Ferrocene Group Substituted Porphyrin Axial Coordinate Self-Assembly for Dye-Sensitized Solar Cells. Org. Electron. 2019, 71, 290–295. [Google Scholar] [CrossRef]
- Ji, J.M.; Zhou, H.; Eom, Y.K.; Kim, C.H.; Kim, H.K. 14.2% Efficiency Dye-Sensitized Solar Cells by Co-Sensitizing Novel Thieno[3,2-b]Indole-Based Organic Dyes with a Promising Porphyrin Sensitizer. Adv. Energy Mater. 2020, 10, 2000124. [Google Scholar] [CrossRef]
- Noirbent, G.; Brunel, D.; Bui, T.T.; Péralta, S.; Aubert, P.H.; Gigmes, D.; Dumur, F. D-A Dyads and A-D-A Triads Based on Ferrocene: Push-Pull Dyes with Unusual Behaviours in Solution. New J. Chem. 2021, 45, 13475–13498. [Google Scholar] [CrossRef]
- Hagberg, D.P.; Marinado, T.; Karlsson, K.M.; Nonomura, K.; Qin, P.; Boschloo, G.; Brinck, T.; Hagfeldt, A.; Sun, L. Tuning the HOMO and LUMO Energy Levels of Organic Chromophores for Dye Sensitized Solar Cells. J. Org. Chem. 2007, 72, 9550–9556. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Chen, J. Arylamine Organic Dyes for Dye-Sensitized Solar Cells. Chem. Soc. Rev. 2013, 42, 3453–3488. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, K.; Ma, L.; Zhan, X. Triarylamine: Versatile Platform for Organic, Dye-Sensitized, and Perovskite Solar Cells. Chem. Rev. 2016, 116, 14675–14725. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A. Triphenylamine Based Dyes for Dye Sensitized Solar Cells: A Review. Sol. Energy 2016, 123, 127–144. [Google Scholar] [CrossRef]
- Rybakiewicz, R.; Zagorska, M.; Pron, A. Triphenylamine-Based Electroactive Compounds: Synthesis, Properties and Application to Organic Electronics. Chem. Pap. 2017, 71, 243–268. [Google Scholar] [CrossRef]
- Misra, R.; Maragani, R.; Patel, K.R.; Sharma, G.D. Synthesis, Optical and Electrochemical Properties of New Ferrocenyl Substituted Triphenylamine Based Donor-Acceptor Dyes for Dye Sensitized Solar Cells. RSC Adv. 2014, 4, 34904–34911. [Google Scholar] [CrossRef]
- Maragani, R.; Misra, R. Ferrocenyl Substituted Triphenylamine Based Donor-Acceptor Molecular Systems; Synthesis, Photophysical, Electrochemical, and Theoretical Studies. Tetrahedron 2014, 70, 3390–3399. [Google Scholar] [CrossRef]
- El-Zohry, A.M.; Karlsson, M. Gigantic Relevance of Twisted Intramolecular Charge Transfer for Organic Dyes Used in Solar Cells. J. Phys. Chem. C 2018, 122, 23998–24003. [Google Scholar] [CrossRef]
- El-Zohry, A.M.; Cong, J.; Karlsson, M.; Kloo, L.; Zietz, B. Ferrocene as a Rapid Charge Regenerator in Dye-Sensitized Solar Cells. Dyes Pigments 2016, 132, 360–368. [Google Scholar] [CrossRef]
- Bonnier, C.; Machin, D.D.; Abdi, O.K.; Robson, K.C.D.; Koivisto, B.D. The Effect of Donor-Modification in Organic Light-Harvesting Motifs: Triphenylamine Donors Appended with Polymerisable Thienyl Subunits. Org. Biomol. Chem. 2013, 11, 7011–7015. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abdi, O.K.; Fischer, B.J.D.; Al-Faouri, T.; Buguis, F.L.; Devgan, H.S.; Schott, E.; Zarate, X.; Koivisto, B.D. Bipodal Dyes with Bichromic Triphenylamine Architectures for Use in Dye-Sensitized Solar Cell Applications. RSC Adv. 2018, 8, 42424–42428. [Google Scholar] [CrossRef]
- Al-Faouri, T.; Buguis, F.L.; Soldouz, S.A.; Sarycheva, O.V.; Hussein, B.A.; Mahmood, R.; Koivisto, B.D. Exploring Structure-Property Relationships in a Bio-Inspired Family of Bipodal and Electronically-Coupled Bistriphenylamine Dyes for Dye-Sensitized Solar Cell Applications. Molecules 2020, 25, 2260. [Google Scholar] [CrossRef]
- Dyadchenko, V.P.; Dyadchenko, M.A.; Okulov, V.N.; Lemenovskii, D.A. Alkynylation of Ferrocene by Terminal Alkynes. Part I. A Simple One-Step Synthesis of Ferrocenylacetylenes. J. Organomet. Chem. 2011, 696, 468–472. [Google Scholar] [CrossRef]
- Yin, Z.J.; Xu, S.Q.; Zhan, T.G.; Qi, Q.Y.; Wu, Z.Q.; Zhao, X. Ultrahigh Volatile Iodine Uptake by Hollow Microspheres Formed from a Heteropore Covalent Organic Framework. Chem. Commun. 2017, 53, 7266–7269. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Anari, E.H.B.; Romano, M.; Teh, W.X.; Black, J.J.; Jiang, E.; Chen, J.; To, T.Q.; Panchompoo, J.; Aldous, L. Substituted Ferrocenes and Iodine as Synergistic Thermoelectrochemical Heat Harvesting Redox Couples in Ionic Liquids. Chem. Commun. 2016, 52, 745–748. [Google Scholar] [CrossRef]
- Toma, Š.; Šebesta, R. Applications of Ferrocenium Salts in Organic Synthesis. Synthesis 2015, 47, 1683–1695. [Google Scholar] [CrossRef]
- Curiac, C.; Rodrigues, R.R.; Watson, J.; Hunt, L.A.; Devdass, A.; Jurss, J.W.; Hammer, N.I.; Fortenberry, R.C.; Delcamp, J.H. Iron Redox Shuttles with Wide Optical Gap Dyes for High-Voltage Dye-Sensitized Solar Cells. ChemSusChem 2021, 14, 3084–3096. [Google Scholar] [CrossRef]
- Sönmezolu, S.; Akyürek, C.; Akin, S. High-Efficiency Dye-Sensitized Solar Cells Using Ferrocene-Based Electrolytes and Natural Photosensitizers. J. Phys. D. Appl. Phys. 2012, 45, 425101. [Google Scholar] [CrossRef]
- Daeneke, T.; Kwon, T.H.; Holmes, A.B.; Duffy, N.W.; Bach, U.; Spiccia, L. High-Efficiency Dye-Sensitized Solar Cells with Ferrocene-Based Electrolytes. Nat. Chem. 2011, 3, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Hurvois, J.P.; Moinet, C. Reactivity of Ferrocenium Cations with Molecular Oxygen in Polar Organic Solvents: Decomposition, Redox Reactions and Stabilization. J. Organomet. Chem. 2005, 690, 1829–1839. [Google Scholar] [CrossRef]
- Boschloo, G.; Hagfeldt, A. Characteristics of the Iodide/Triiodide Redox Mediator in Dye-Sensitized Solar Cells. Acc. Chem. Res. 2009, 42, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- De Violet, P.F.; Logan, S.R. Mechanism of the Photochemical Reaction between Ferrocene and Iodine. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1980, 76, 578–582. [Google Scholar] [CrossRef]
- Giribabu, L.; Bolligarla, R.; Panigrahi, M. Recent Advances of Cobalt(II/III) Redox Couples for Dye-Sensitized Solar Cell Applications. Chem. Rec. 2015, 15, 760–788. [Google Scholar] [CrossRef]



| Molecule | UV-Vis λmax nm (ɛ × 104 M−1 cm−1) | Fluorescence Emission λmax nm | E1/2 a (V vs. NHE) | ||||
|---|---|---|---|---|---|---|---|
| 1a | 285 (2.75) | 480 (2.43) | 640 | 1.22 | |||
| 1b | 337 (3.85) | 496 (3.77) | 635 | 0.66 | 1.34 | ||
| 1cb | 345 (4.22) | 505 (3.37) | 690 | 0.65 | 1.32 | ||
| 2a | 307 (2.34) | 400 (2.68) | 462 (3.34) | 571 | 1.14 | 1.36 | |
| 2b | 361 (0.97) | 460 (0.58) | 510 | ||||
| 2c | 342 (2.66) | 408 (sh, 2.50) | 465 (3.09) | 509 | |||
| 2d | 352 (1.23) | 450 (0.92) | 509 | ||||
| 2e | 301 (1.82) | 370 (3.32) | 441 (1.67) | 504 | |||
| 3a | 240 (2.65) | 365 (4.66) | 483 | 1.12 | 1.35 | ||
| 3b | 240 (3.50) | 370 (5.10) | 474 | 0.65 | 1.22 | 1.38 | |
| 3c | 245 (5.05) | 370 (6.15) | 486 | 0.66 | 1.23 | 1.39 | |
| 3d | 245 (5.50) | 370 (8.25) | 490 | 0.77 | 1.25 | 1.40 | |
| 3e | 245 (2.27) | 370 (3.32) | 476 | 0.77 | 1.26 | 1.40 | |
| 5d | 320 (3.81) | 440 (0.25) | 507 | 0.77 | 1.35 | ||
| 5e | 330 (3.54) | 450 (0.24) | 508 | 0.78 | 1.38 | ||
| 7c | 325 (2.31) | 415 (2.48) | 657 | 0.67 | 1.34 | ||
| Dye | VOC (V) | JSC (mA/cm2) | FF | η (%) | N a |
|---|---|---|---|---|---|
| 1a | 0.65 ± 0.004 | 3.97 ± 0.570 | 0.58 ± 0.026 | 2.71 ± 0.345 | 3 |
| 1ab | 0.69 ± 0.002 | 10.4 ± 0.1 | 0.76 ± 0.50 | 5.5 ± 0.1 | - |
| 1b | 0.33 ± 0.044 | 0.22 ± 0.118 | 0.21 ± 0.117 | 0.028 ± 0.004 | 3 |
| 1cb | 0.57 ± 0.003 | 2.40 ± 0.1 | 0.53 ± 0.0008 | 0.70 ± 0.1 | - |
| 2a | 0.70 ± 0.013 | 4.10 ± 0.097 | 0.71 ± 0.077 | 3.70 ± 0.483 | 6 |
| 2b | 0.49 ± 0.141 | 0.70 ± 0.254 | 0.49 ± 0.154 | 0.30 ± 0.096 | 7 |
| 2c | 0.26 ± 0.028 | 0.17 ± 0.025 | 0.21 ± 0.026 | 0.018 ± 0.002 | 6 |
| 2d | 0.49 ± 0.014 | 0.69 ± 0.087 | 0.70 ± 0.307 | 0.43 ± 0.008 | 6 |
| 2e | 0.30 ± 0.033 | 0.11 ± 0.037 | 0.37 ± 0.150 | 0.022 ± 0.004 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, T.K.; Grandy, R.A.; Dennis, E.S.; Schouten, A.S.B.; Koivisto, B.D. Exploring Structure-Property Relationships in a Family of Ferrocene-Containing, Triphenylamine-Based Hybrid Organic Dyes. Appl. Sci. 2022, 12, 6001. https://doi.org/10.3390/app12126001
Singh TK, Grandy RA, Dennis ES, Schouten ASB, Koivisto BD. Exploring Structure-Property Relationships in a Family of Ferrocene-Containing, Triphenylamine-Based Hybrid Organic Dyes. Applied Sciences. 2022; 12(12):6001. https://doi.org/10.3390/app12126001
Chicago/Turabian StyleSingh, Tavneet K., Reese A. Grandy, Emma S. Dennis, Anja S. B. Schouten, and Bryan D. Koivisto. 2022. "Exploring Structure-Property Relationships in a Family of Ferrocene-Containing, Triphenylamine-Based Hybrid Organic Dyes" Applied Sciences 12, no. 12: 6001. https://doi.org/10.3390/app12126001
APA StyleSingh, T. K., Grandy, R. A., Dennis, E. S., Schouten, A. S. B., & Koivisto, B. D. (2022). Exploring Structure-Property Relationships in a Family of Ferrocene-Containing, Triphenylamine-Based Hybrid Organic Dyes. Applied Sciences, 12(12), 6001. https://doi.org/10.3390/app12126001






