Effect of Coupled Mechanical-Chemical Activation on Hydration Activity of Copper Slag Powder
Abstract
:1. Introduction
2. Raw Materials and Experimental Methods
2.1. Raw Materials
2.2. Particle Size Distribution of Copper Slag
2.3. Effect of Mechanical Activation on the Activity of Copper Slag Powder
2.4. Effect of Coupled Mechanical–Chemical Activation on Hydration Activity of Copper Slag Powder
2.5. Hydration Heat-Release Characteristics
2.6. Characterization
3. Results and Discussion
3.1. Grinding Characteristics
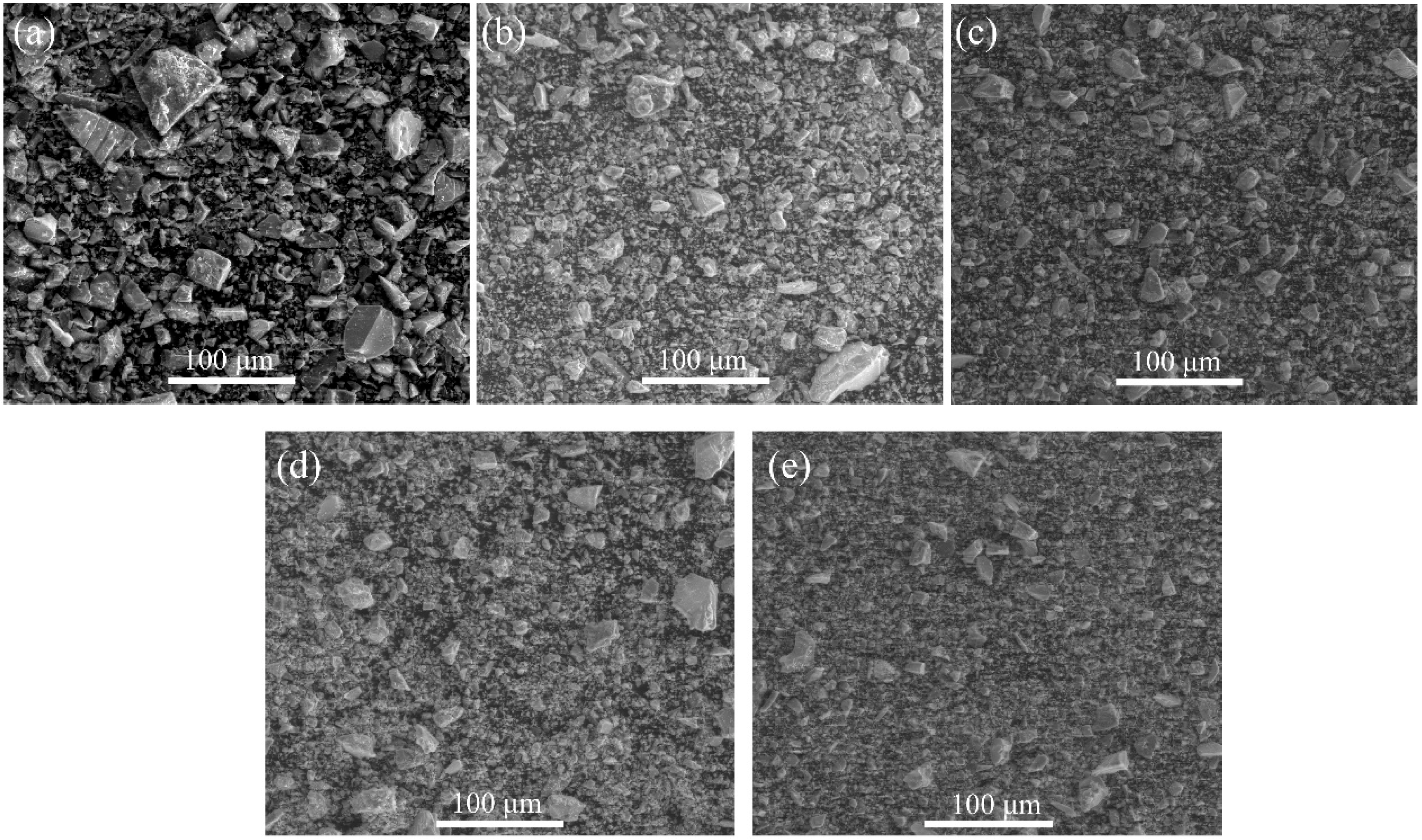
3.1.1. Micromorphology
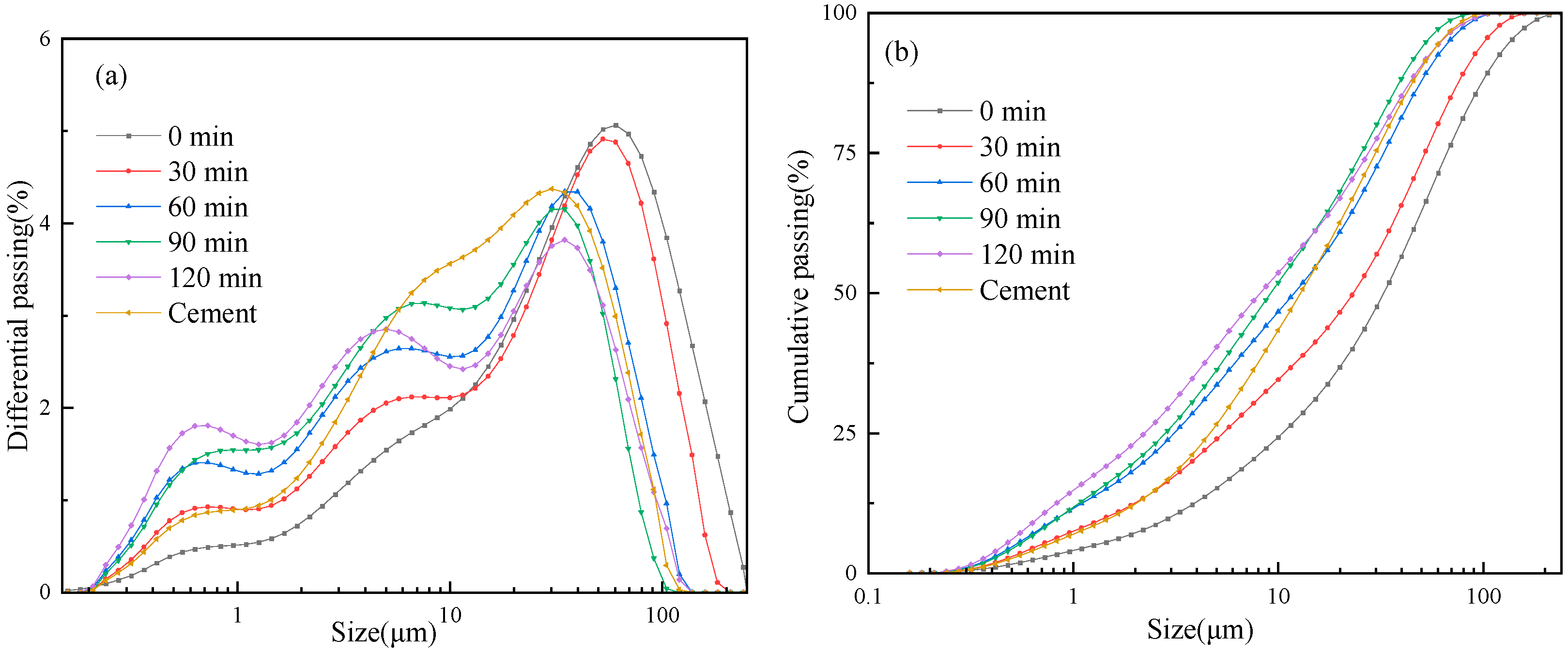
3.1.2. Particle Size Distribution
3.2. Mechanical Properties
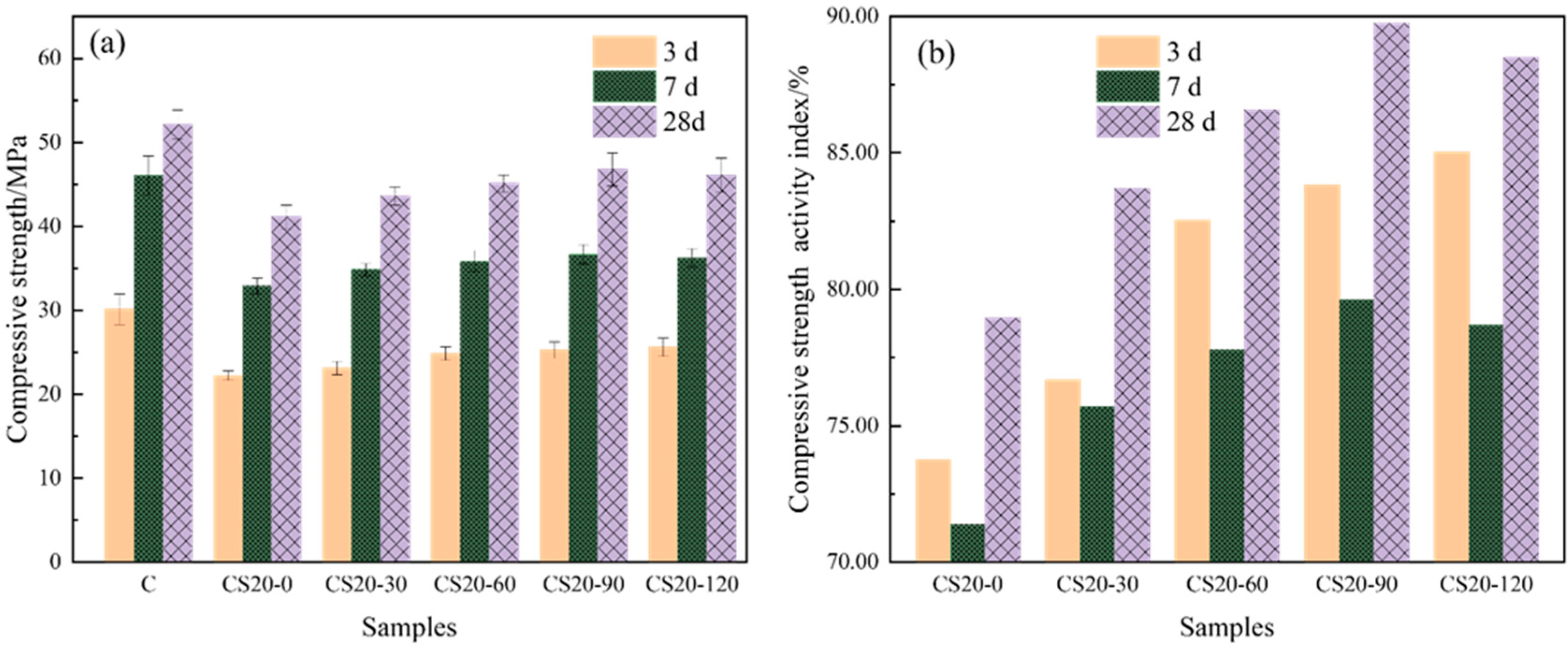
3.2.1. The Effect of Mechanical Grinding on the Hydration Activity of Copper Slag Powder
3.2.2. Effect of Coupled Mechanical–Chemical Activation on Hydration Activity of Copper Slag Powder
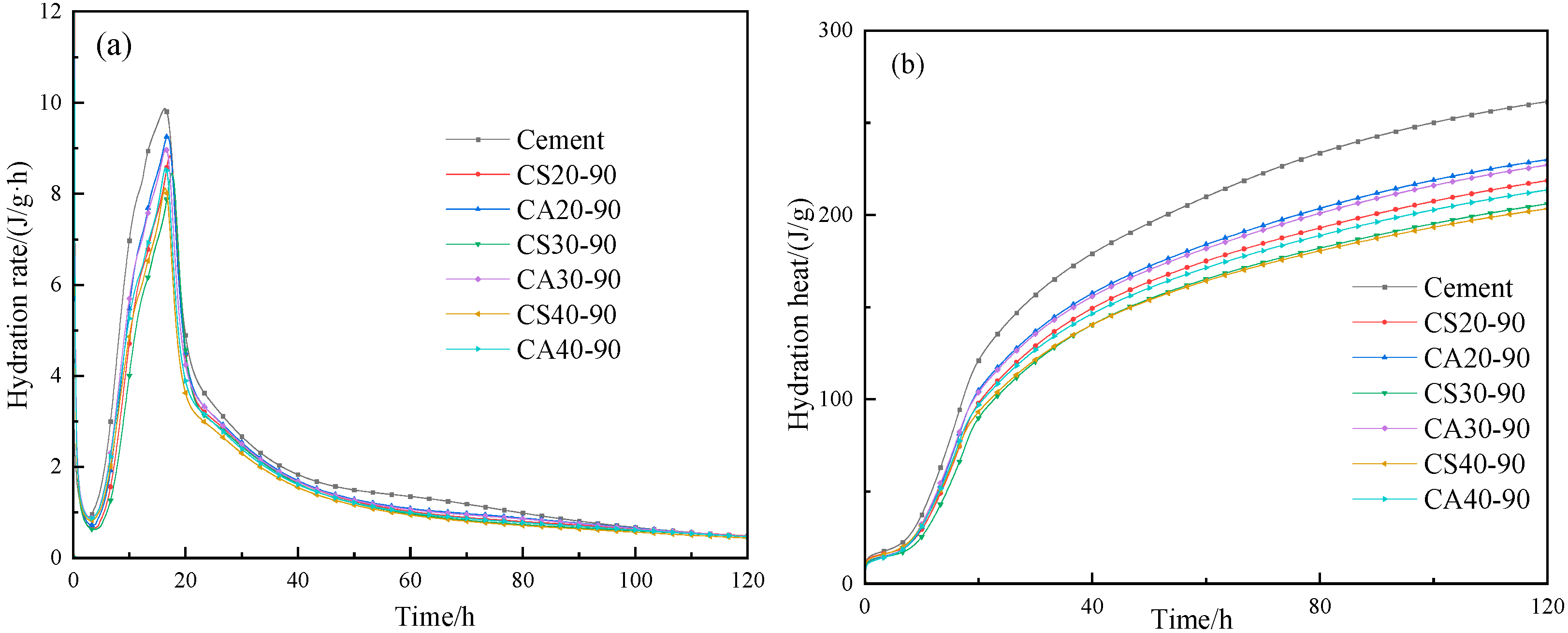
3.3. Hydration Heat-Release Characteristics
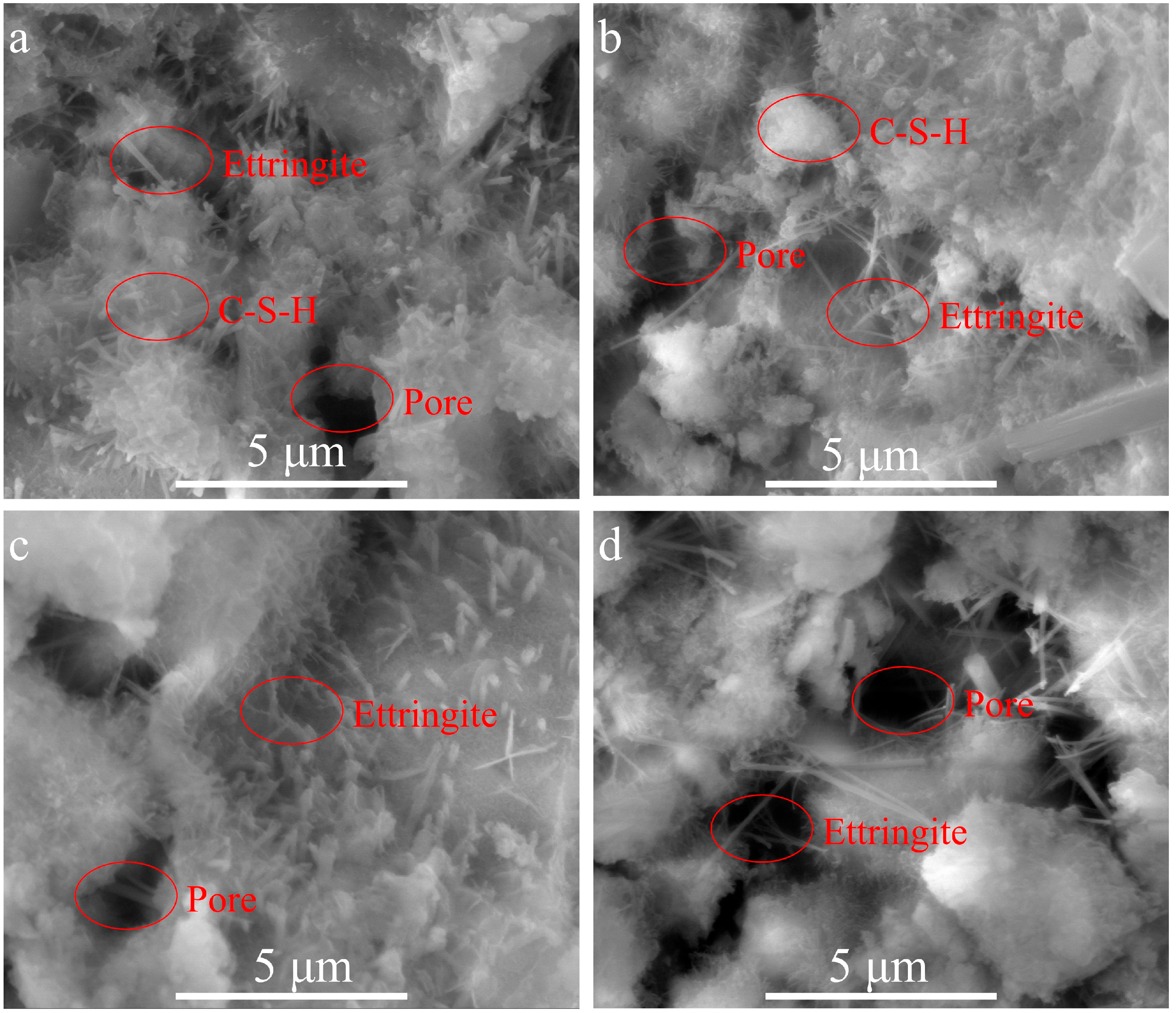
3.4. Hydration Products
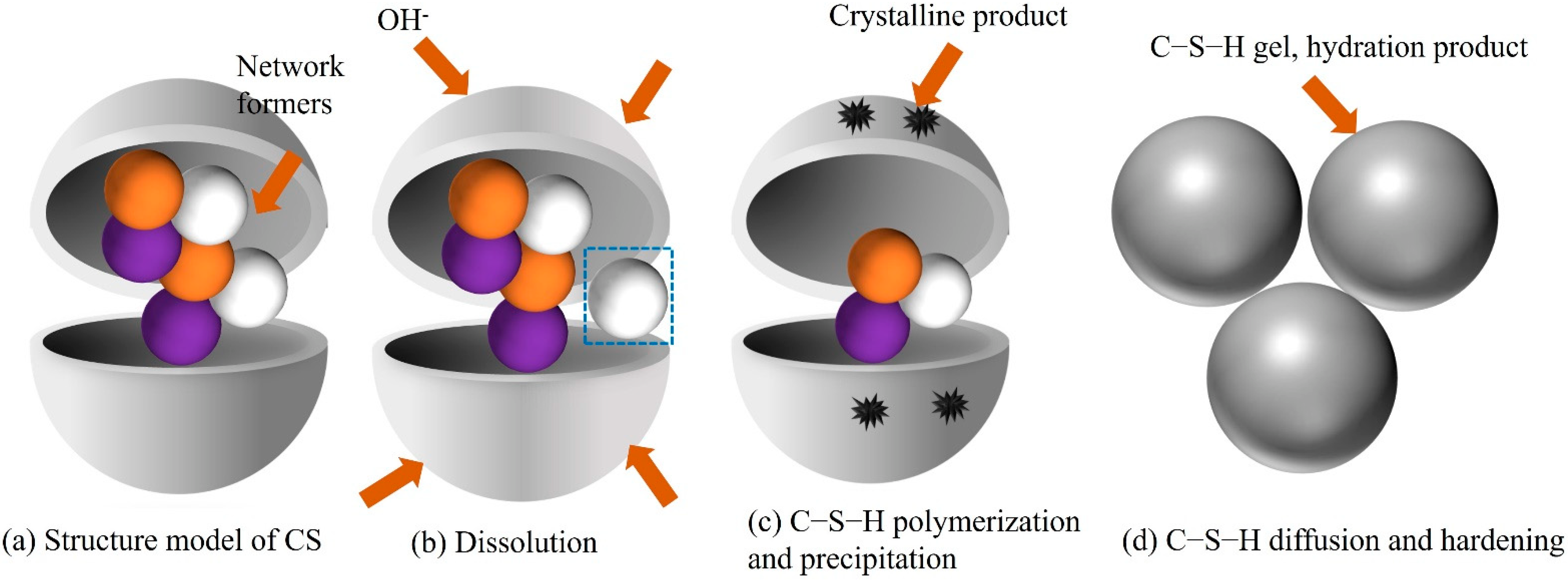
3.5. Coupled Mechanical–Chemical Activation Mechanism
4. Conclusions
- (1)
- The specific surface area of the copper slag powder increased by 27.84%, 20.14%, and 10.92% when the grinding time increased from 30 min to 120 min. The particle size distribution of the copper slag powder after grinding for 90 min and 120 min was superior to that of cement.
- (2)
- The strengthening effect of the copper slag powder on the composite cement-hardened paste at 3 d of age mainly had a “filling effect,” and the strengthening effect of the copper slag powder on the composite cement-hardened paste at 28 d of age showed a “pozzolanic effect.” The chemical activator CaO further stimulated the hydration activation of the copper slag and promoted the development of the compressive strength of the copper slag powder-based composite cement at various ages.
- (3)
- The incorporation of the copper slag powder prolonged the induction period and the occurrence time of the second exothermic peak and reduced the total heat release of the composite cementitious system. The reduction ratio of the hydration heat was lower than the mass ratio of the copper slag in the hydration reaction system. With the same content of copper slag powder, the addition of alkaline activator CaO improved the hydration heat and the hydration heat of the copper slag powder-based composite cement.
- (4)
- Micro measurements demonstrated that the hydration products of the copper slag powder-based composite cementitious system mainly included calcium hydroxide, ettringite, hydrated calcium silicate gel, and unhydrated cement particles. The incorporation of copper slag powder reduced the compactness of the composite cementitious system. The new hydration product of calcium iron oxide was detected in the hardened paste of the copper slag powder-based composite cement.
- (5)
- Mechanical grinding increased the fineness and specific surface area of the copper slag powder and exposed the active mineral components to the alkaline environment of cement-based materials. The copper slag underwent hydration reactions such as dissociation of glass body structure, precipitation of internal ions, C-S-H gel formation, and hardening in the alkaline environment of cement-based materials.
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Khalid, M.K.; Hamuyuni, J.; Agarwal, V.; Pihlasalo, J.; Haapalainen, M.; Lundström, M. Sulfuric acid leaching for capturing value from copper rich converter slag. J. Clean. Prod. 2019, 215, 1005–1013. [Google Scholar] [CrossRef]
- Li, S.; Pan, J.; Zhu, D.; Guo, Z.; Xu, J.; Chou, J. A novel process to upgrade the copper slag by direct reduction -magnetic separation with the addition of Na2CO3 and CaO. Powder Technol. 2019, 347, 159–169. [Google Scholar] [CrossRef]
- Gupta, N.; Siddique, R. Sulfate resistance and drying shrinkage of self-compacting concrete incorporating copper slag. J. Mater. Civ. Eng. 2020, 32, 04020389. [Google Scholar] [CrossRef]
- Gong, W.; Chen, Q.; Miao, J.J. Bond behaviors between copper slag concrete and corroded steel bar after exposure to high temperature. J. Build. Eng. 2021, 44, 103312. [Google Scholar] [CrossRef]
- Murari, K.; Siddique, R.; Jain, K.K. Use of waste copper slag, a sustainable material. J. Mater. Cycles Waste Manag. 2015, 17, 13–26. [Google Scholar] [CrossRef]
- Sharma, R.; Khan, R.A. Sustainable use of copper slag in self compacting concrete containing supplementary cementitious materials. J. Clean. Prod. 2017, 151, 179–192. [Google Scholar] [CrossRef]
- Singh, J.; Singh, S.P. Development of Alkali-activated cementitious material using copper slag. Constr. Build. Mater. 2019, 211, 73–79. [Google Scholar] [CrossRef]
- Chithra, S.; Kumar, S.R.R.S.; Chinnaraju, K.; Ashmita, F.A. A comparative study on the compressive strength prediction models for high performance concrete containing nano-silica and copper slag using regression analysis and artificial neural networks. Constr. Build. Mater. 2016, 114, 528–535. [Google Scholar] [CrossRef]
- Ambily, P.S.; Umarani, C.; Ravisankar, K.; Prem, P.R.; Bharatkumar, B.H.; Iyer, N.R. Studies on ultra-high performance concrete incorporating copper slag as fine aggregate. Constr. Build. Mater. 2015, 77, 233–240. [Google Scholar] [CrossRef]
- Feng, Y.; Kero, J.; Yang, Q.; Chen, Q.; Engström, F.; Samuelsson, C.; Qi, C. Mechanical Activation of Granulated Copper Slag and Its Influence on Hydration Heat and Compressive Strength of Blended Cement. Materials 2019, 12, 772. [Google Scholar] [CrossRef] [Green Version]
- He, R.; Zhang, S.; Zhang, X.; Zhang, Z.; Zhao, Y.; Ding, H. Copper slag: The leaching behavior of heavy metals and its applicability as a supplementary cementitious material. J. Environ. Chem. Eng. 2021, 9, 105132. [Google Scholar] [CrossRef]
- Chen, Q.; Tao, Y.; Feng, Y.; Zhang, Q.; Liu, Y. Utilization of modified copper slag activated by Na2SO4 and CaO for unclassified lead/zinc mine tailings based cemented paste backfill. J. Environ. Manag. 2021, 290, 112608. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Q.; Wang, Q.; Huang, Z.X. Reuse of copper slag as a supplementary cementitious material: Reactivity and safety. Resour. Conserv. Recycl. 2020, 162, 105037. [Google Scholar] [CrossRef]
- Ahmari, S.; Parameswaran, K.; Zhang, L. Alkali Activation of Copper Mine Tailings and Low-Calcium Flash-Furnace Copper Smelter Slag. J. Mater. Civ. Eng. 2015, 27, 04014193. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Bandopadhyay, A.; Alex, T.; Kumar, B.R.; Das, S.; Mehrotra, S. Mechanical activation of granulated blast furnace slag and its effect on the properties and structure of portland slag cement. Cem. Concr. Compos. 2008, 30, 679–685. [Google Scholar] [CrossRef]
- Bouaziz, A.; Hamzaoui, R.; Guessasma, S.; Lakhal, R.; Achoura, D.; Leklou, N. Efficiency of high energy over conventional milling of granulated blast furnace slag powder to improve mechanical performance of slag cement paste. Powder Technol. 2017, 308, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Al-Jabri, K.; Shoukry, H. Use of nano-structured waste materials for improving mechanical, physical and structural properties of cement mortar. Constr. Build. Mater. 2014, 73, 636–644. [Google Scholar] [CrossRef]
- Gopalakrishnan, R.; Nithiyanantham, S. Microstructural, mechanical, and electrical properties of copper slag admixtured cement mortar. J. Build. Eng. 2020, 31, 101375. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, Q.; Chen, Q.; Kero, J.; Andersson, A.; Ahmed, H.; Engström, F.; Samuelsson, C. Characterization and evaluation of the pozzolanic activity of granulated copper slag modified with CaO. J. Clean. Prod. 2019, 232, 1112–1120. [Google Scholar] [CrossRef]
- Singh, J.; Singh, S.P. Utilization of alkali-activated copper slag as binder in concrete. Front. Struct. Civ. Eng. 2021, 15, 773–780. [Google Scholar] [CrossRef]
- Lan, W.T.; Wu, A.N.; Yu, P. Development of a new controlled low strength filling material from the activation of copper slag: Influencing factors and mechanism analysis. J. Clean. Prod. 2020, 246, 119060. [Google Scholar] [CrossRef]
- Tangpagasit, J.; Cheerarot, R.; Jaturapitakkul, C.; Kiattikomol, K. Packing effect and pozzolanic reaction of fly ash in mortar. Cem. Concr. Res. 2005, 35, 1145–1151. [Google Scholar] [CrossRef]
- Chang, Z.; Long, G.; Xie, Y.; Zhou, J.L. Pozzolanic reactivity of aluminum-rich sewage sludge ash: Influence of calcination process and effect of calcination products on cement hydration. Constr. Build. Mater. 2022, 318, 126096. [Google Scholar] [CrossRef]
- Tan, H.; Li, M.; He, X.; Su, Y.; Zhang, J.; Pan, H.; Yang, J.; Wang, Y. Preparation for micro-lithium slag via wet grinding and its application as accelerator in Portland cement. J. Clean. Prod. 2020, 250, 119528. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Mehringskoetter, M. Synthetic granulated blast furnace-like slag from bauxite residue smelting and its use in multi-component Portland composite cement. J. Clean. Prod. 2021, 329, 129667. [Google Scholar] [CrossRef]
- Sambangi, A.; Arunakanthi, E. Fresh and mechanical properties of SCC with fly ash and copper slag as mineral admixtures. Mater. Today-Proc. 2017, 45, 6687–6693. [Google Scholar] [CrossRef]
- Li, Q.; Li, B.; Li, X.; He, Z.; Zhang, P. Microstructure of pretreated steel slag and its influence on mechanical properties of cement stabilized mixture. Constr. Build. Mater. 2022, 317, 125799. [Google Scholar] [CrossRef]
- Han, F.; Zhang, H.; Pu, S.; Zhang, Z. Hydration heat and kinetics of composite binder containing blast furnace ferronickel slag at different temperatures. Thermochim. Acta 2021, 702, 178985. [Google Scholar] [CrossRef]
- Wang, X.Y. Analysis of hydration kinetics and strength progress in cement-slag binary composites. J. Build. Eng. 2021, 35, 101810. [Google Scholar] [CrossRef]
- Han, F.; He, X.; Zhang, Z.; Liu, J. Hydration heat of slag or fly ash in the composite binder at different temperatures. Thermochim. Acta 2017, 655, 202–210. [Google Scholar] [CrossRef]
- Huo, J.; Yu, B.; Peng, Z.; Wu, Z.; Zhang, L. Thermal control effects and mechanism of slag and fly ash on heat development of cement slurry used in hydrate formation. J. Nat. Gas Sci. Eng. 2021, 91, 103967. [Google Scholar] [CrossRef]
- Xu, G.; Du, S.; He, J.; Shi, X. The role of admixed graphene oxide in a cement hydration system. Carbon 2019, 148, 141–150. [Google Scholar] [CrossRef]
- Zhu, W.; Feng, Q.; Luo, Q.; Bai, X.; Lin, X.; Zhang, Z. Effects of PCE on the Dispersion of Cement Particles and Initial Hydration. Materials 2021, 14, 3195. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, N.; Yuan, J.; Wang, X.; Zhang, Y.; Chen, F.; Zhang, Y. Preparation and microstructural characterization of a novel 3D printable building material composed of copper tailings and iron tailings. Constr. Build. Mater. 2020, 249, 118779. [Google Scholar] [CrossRef]
- Sotiriadis, K.; Mroz, R. Simulation of thaumasite Sulfate attack on portland cement mixtures using synthesized cement phases. J. Mater. Civ. Eng. 2019, 31, 04018393. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, G.; Zhang, L.; Zhou, C. Mineralogical and morphological factors affecting the separation of copper and arsenic in flash copper smelting slag flotation beneficiation process. J. Hazard. Mater. 2021, 401, 123293. [Google Scholar] [CrossRef]
- Muñoz-Cáceres, O.; Raposeiras, A.C.; Movilla-Quesada, D.; Castro-Fresno, D.; Lagos-Varas, M.; Andrés-Valeri, V.C.; Valdés-Vidal, G. Mechanical performance of sustainable asphalt mixtures manufactured with copper slag and high percentages of reclaimed asphalt pavement. Constr. Build. Mater. 2021, 304, 124653. [Google Scholar] [CrossRef]
- Yan, Z.; Sun, Z.; Yang, J.; Yang, H.; Ji, Y.; Hu, K. Mechanical performance and reaction mechanism of copper slag activated with sodium silicate or sodium hydroxide. Constr. Build. Mater. 2021, 266, 120900. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, B.; Feng, Y.; Qi, C.; Chen, Q.; Xiao, C. Hydration development of blended cement paste with granulated copper slag modified with CaO and Al2O3. J. Mater. Res. Technol. 2022, 18, 909–920. [Google Scholar] [CrossRef]



| Composition | CaO | MgO | Al2O3 | SiO2 | Fe2O3 | SO3 | P2O5 | Na2O | K2O | CuO | MnO | LOSS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Copper slag | 2.46 | 0.75 | 4.98 | 30.44 | 52.78 | 0.52 | 0.08 | 1.38 | 1.24 | 0.32 | 0.12 | 4.93 |
| OPC | 59.74 | 0.92 | 6.57 | 21.35 | 3.79 | 3.24 | 0.15 | 0.13 | 0.78 | 0.04 | 0.02 | 3.27 |
| Sample | OPC/% | Copper Slag | Water–Binder Ratio | |
|---|---|---|---|---|
| Ball-Milling Time/Min | Content/% | |||
| Cement | 100 | - | - | 0.35 |
| CS20-0 | 80 | 0 | 20 | 0.35 |
| CS20-30 | 80 | 30 | 20 | 0.35 |
| CS20-60 | 80 | 60 | 20 | 0.35 |
| CS20-90 | 80 | 90 | 20 | 0.35 |
| CS20-120 | 80 | 120 | 20 | 0.35 |
| Sample | OPC/% | Copper Slag | CaO/Extra-Mixing, % | Water–Binder Ratio | |
|---|---|---|---|---|---|
| Ball-Milling Time/Min | Content/% | ||||
| Cement | 100 | - | - | - | 0.35 |
| CS20-90 | 80 | 90 | 20 | - | 0.35 |
| CA20-90 | 80 | 90 | 20 | 2 | 0.35 |
| CS30-90 | 70 | 90 | 30 | - | 0.35 |
| CA30-90 | 70 | 90 | 30 | 3 | 0.35 |
| CS40-90 | 60 | 90 | 40 | - | 0.35 |
| CA40-90 | 60 | 90 | 40 | 4 | 0.35 |
| Ball-Milling Time/Min | Specific Surface Area/m2·kg−1 | d10/μm | d25/μm | d50/μm | d75/μm | d90/μm |
|---|---|---|---|---|---|---|
| 0 | 178 | 9.840 | 10.741 | 32.676 | 66.596 | 107.749 |
| 30 | 334 | 1.449 | 5.365 | 23.234 | 51.996 | 82.391 |
| 60 | 427 | 0.850 | 3.113 | 11.967 | 32.679 | 54.272 |
| 90 | 513 | 0.853 | 2.809 | 9.204 | 25.507 | 42.719 |
| 120 | 569 | 0.682 | 2.229 | 8.178 | 23.474 | 40.128 |
| Cement | 416 | 1.539 | 4.642 | 12.853 | 29.746 | 49.653 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Li, Q.; Li, X.; Zhou, Y.; Liu, F.; Song, J. Effect of Coupled Mechanical-Chemical Activation on Hydration Activity of Copper Slag Powder. Appl. Sci. 2022, 12, 6018. https://doi.org/10.3390/app12126018
Zhu J, Li Q, Li X, Zhou Y, Liu F, Song J. Effect of Coupled Mechanical-Chemical Activation on Hydration Activity of Copper Slag Powder. Applied Sciences. 2022; 12(12):6018. https://doi.org/10.3390/app12126018
Chicago/Turabian StyleZhu, Jielu, Qi Li, Xianglan Li, Yanhua Zhou, Fanghua Liu, and Junwei Song. 2022. "Effect of Coupled Mechanical-Chemical Activation on Hydration Activity of Copper Slag Powder" Applied Sciences 12, no. 12: 6018. https://doi.org/10.3390/app12126018
APA StyleZhu, J., Li, Q., Li, X., Zhou, Y., Liu, F., & Song, J. (2022). Effect of Coupled Mechanical-Chemical Activation on Hydration Activity of Copper Slag Powder. Applied Sciences, 12(12), 6018. https://doi.org/10.3390/app12126018





