Molecularly Imprinted Polymers Exhibit Low Cytotoxic and Inflammatory Properties in Macrophages In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of SA-MIPs
2.2. Reference Particles
2.3. Cell Culturing
2.4. Size Distribution of SA-MIPs and Alhydrogel
2.5. Protein Adsorption
2.6. Flow Cytometry Analysis
THP-1 Cells
2.7. RAW 264.7 Cells
2.8. Fluorescence Microscopy
2.9. Stimulation of THP-1 Cells
2.10. Stimulation of RAW Cells
2.11. Detection of IL-1β, IL-6 and TNF-α by Enzyme-Linked Immunosorbent Assay (ELISA)
2.12. Lactate Dehydrogenase (LDH) Activity
2.13. Digital Holographic Cytometry (DHC) and Cell Tracking
2.14. Statistical Methods
3. Results
3.1. Particle Characteristics
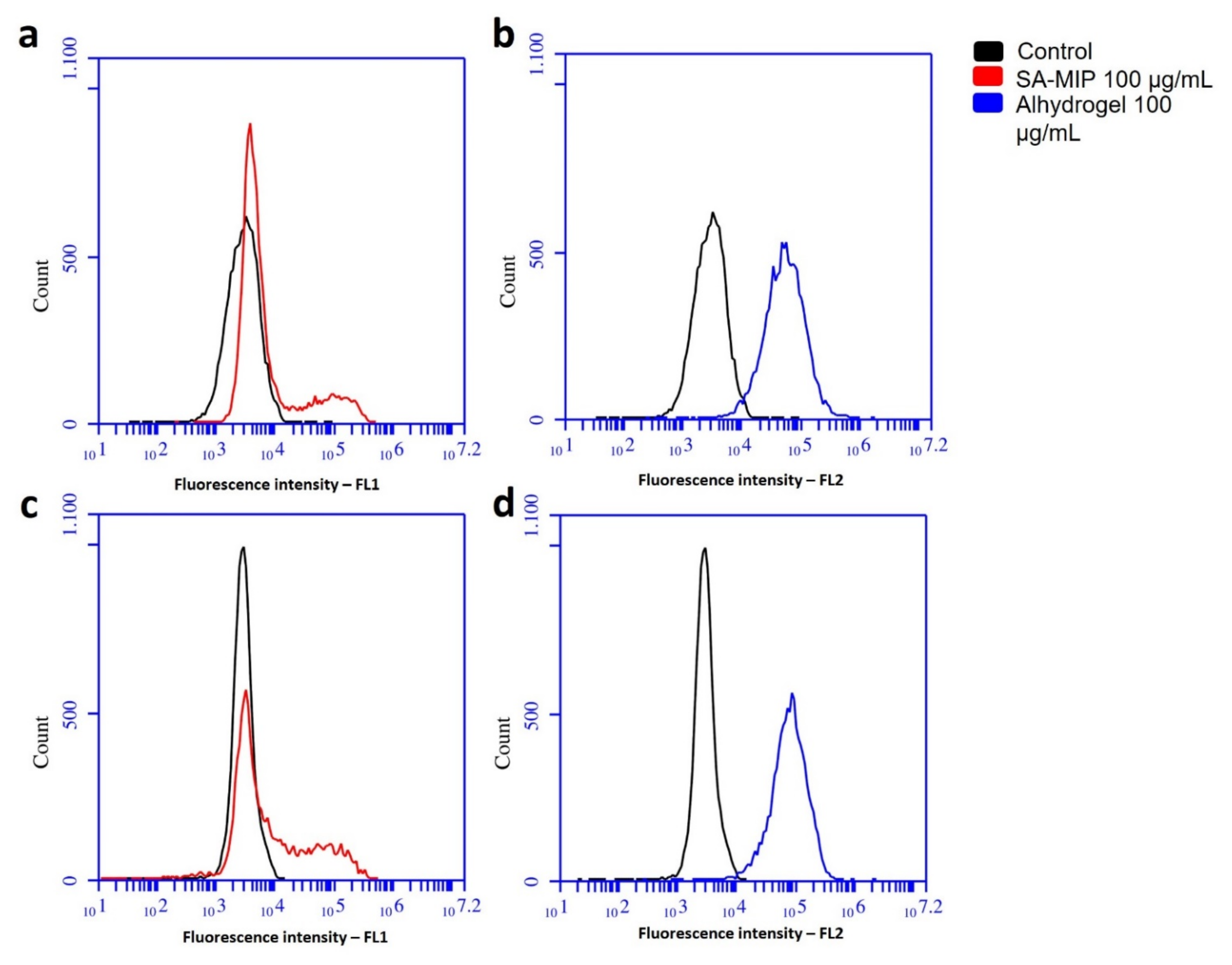
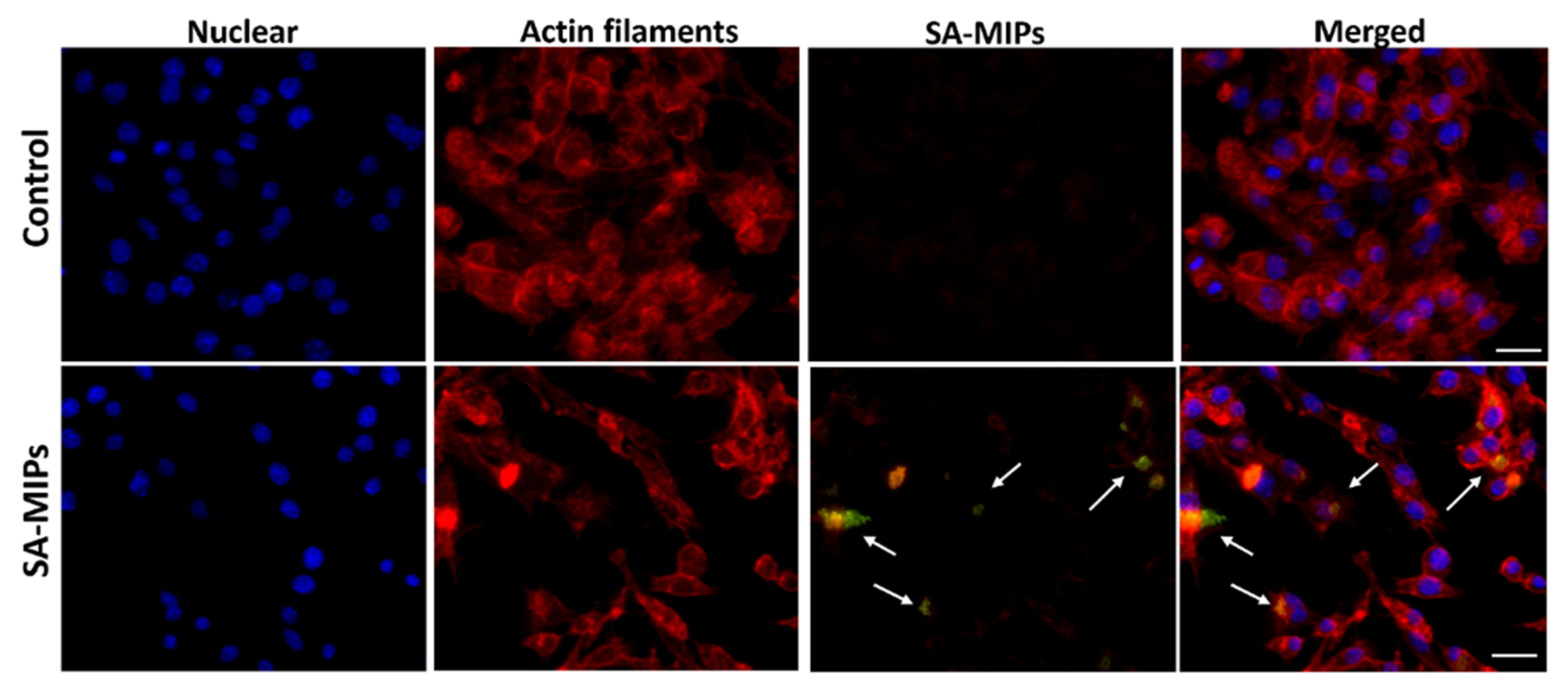
3.2. Association of SA-MIPs and Phagocytosing Cells
3.3. Incubation with SA-MIPs Did Not Result in Increased Cytokine Secretion
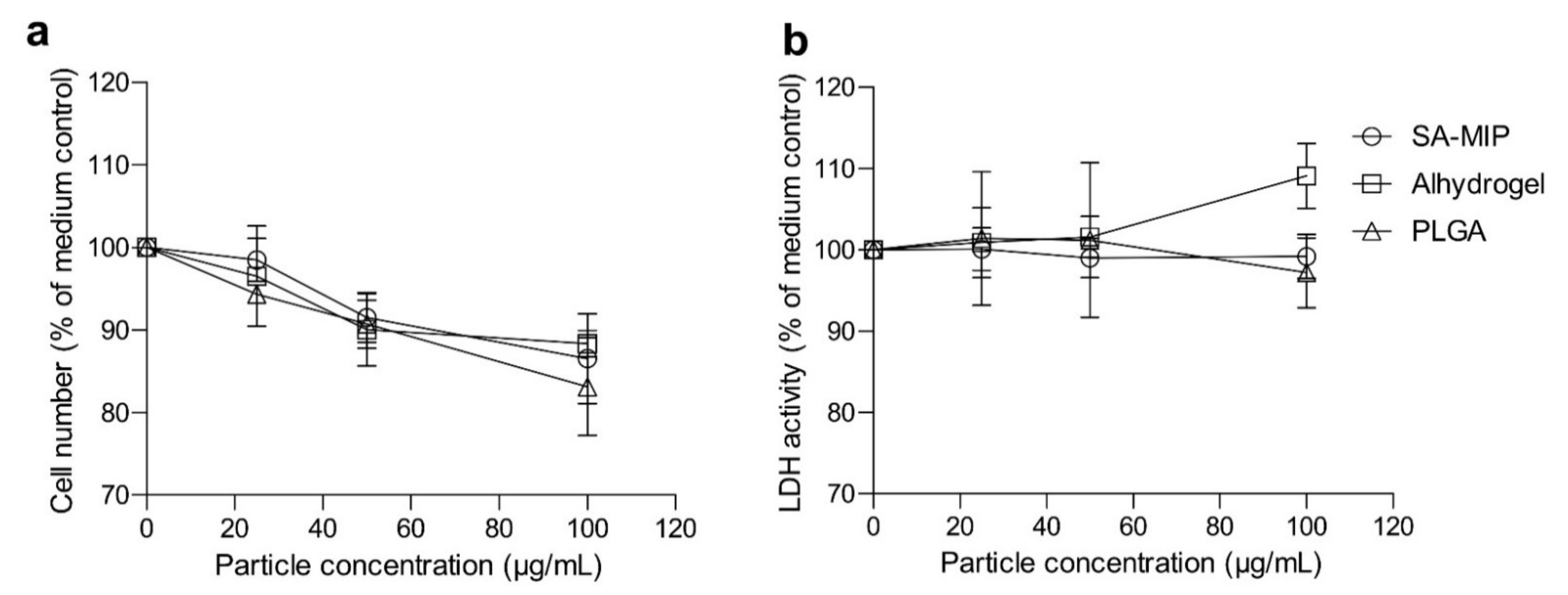
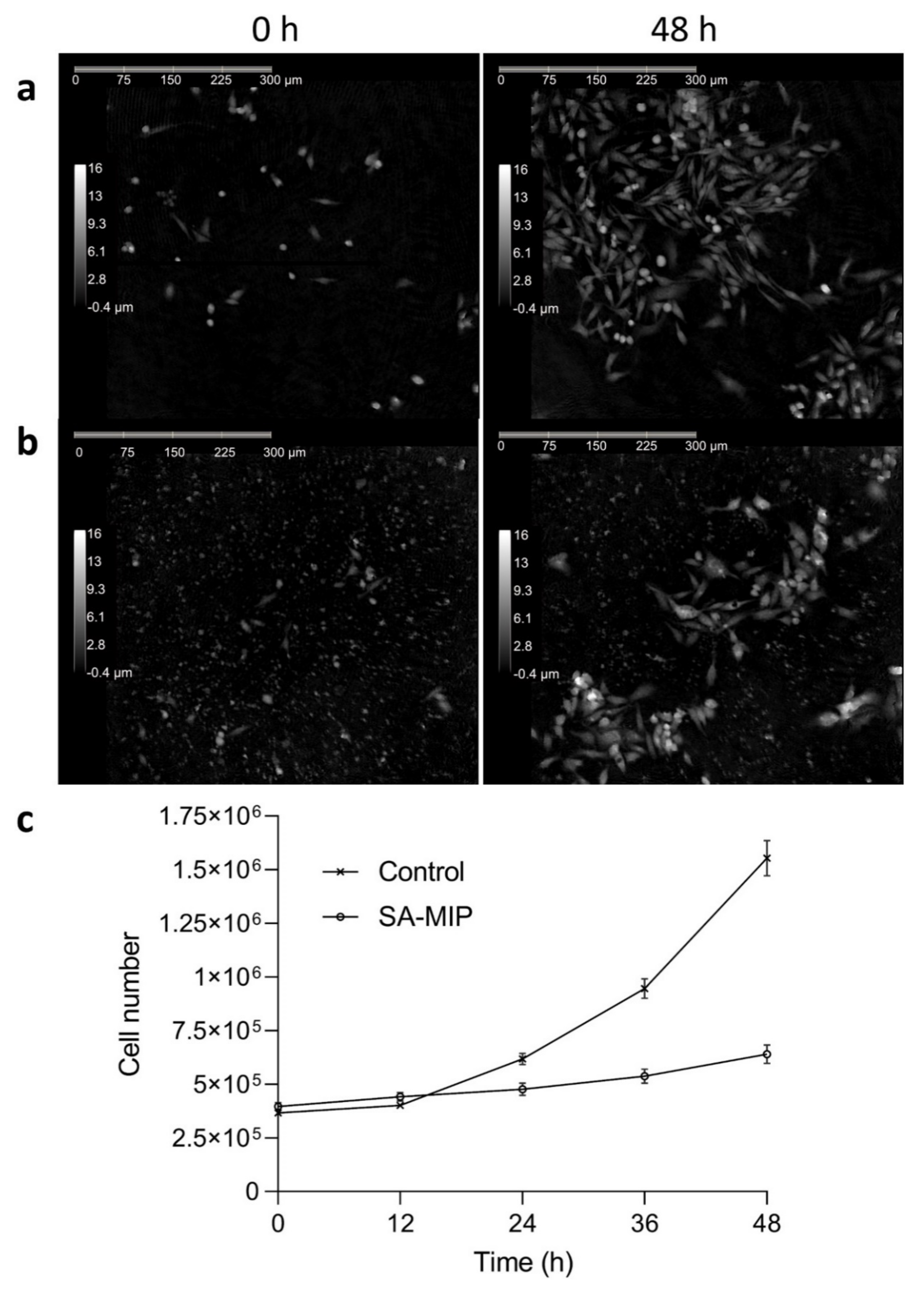
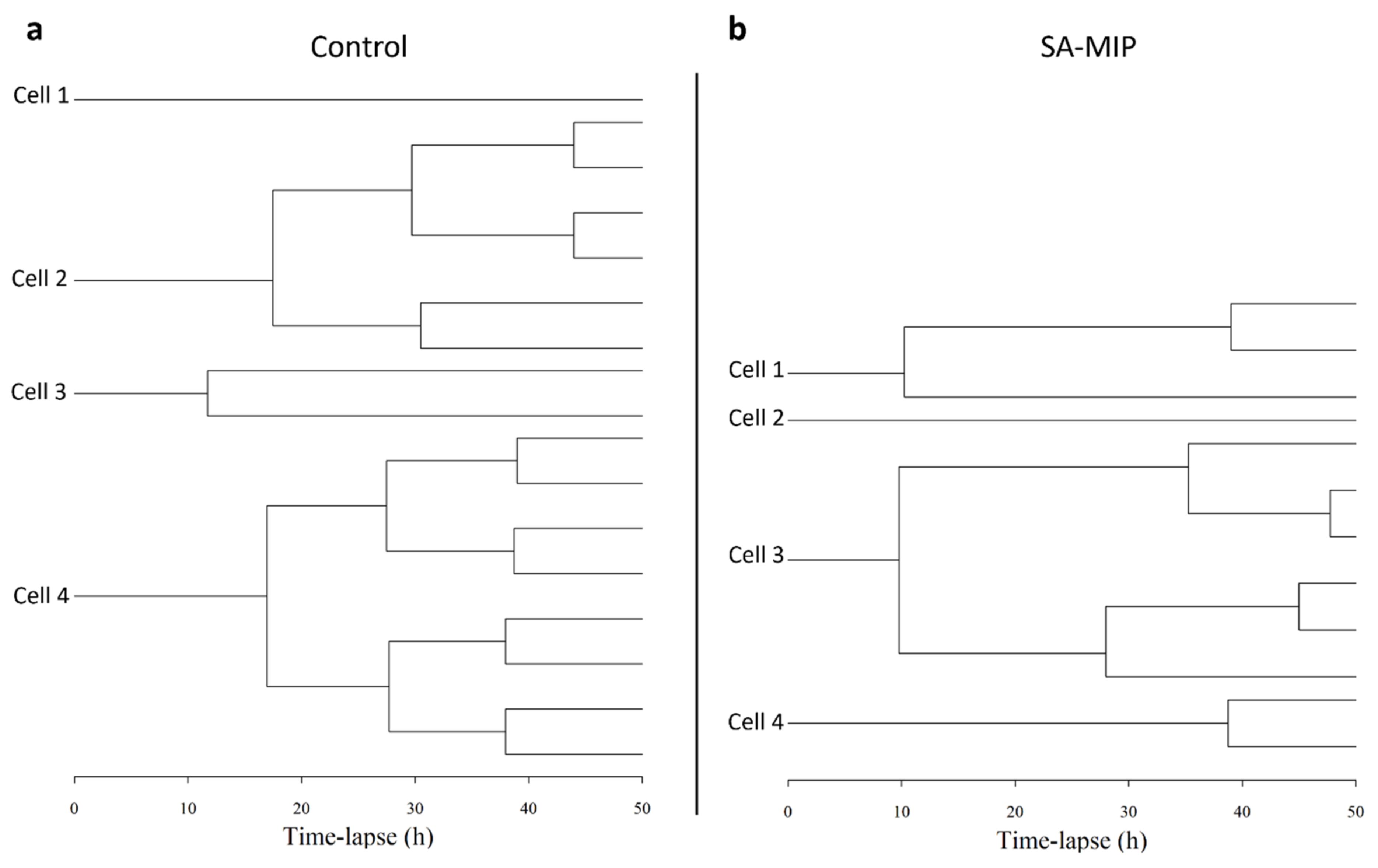
3.4. SA-MIPs Cause Decreased Cell Proliferation, but Are Not Cytotoxic
3.5. SA-MIPs Affect Cell Cycle Progression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaneckova, T.; Vanickova, L.; Tvrdonova, M.; Pomorski, A.; Krezel, A.; Vaculovic, T.; Kanicky, V.; Vaculovicova, M.; Adam, V. Molecularly imprinted polymers coupled to mass spectrometric detection for metallothionein sensing. Talanta 2019, 198, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Haupt, K.; Medina Rangel, P.X.; Bui, B.T.S. Molecularly Imprinted Polymers: Antibody Mimics for Bioimaging and Therapy. Chem. Rev. 2020, 120, 9554–9582. [Google Scholar] [CrossRef] [PubMed]
- Piletsky, S.; Canfarotta, F.; Poma, A.; Bossi, A.M.; Piletsky, S. Molecularly Imprinted Polymers for Cell Recognition. Trends Biotechnol. 2020, 38, 368–387. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.; El-Schich, Z.; Malakpour, A.; Wan, W.; Dizeyi, N.; Mohammadi, R.; Rurack, K.; Gjorloff Wingren, A.; Sellergren, B. Sialic Acid-Imprinted Fluorescent Core-Shell Particles for Selective Labeling of Cell Surface Glycans. J. Am. Chem. Soc. 2015, 137, 13908–13912. [Google Scholar] [CrossRef] [PubMed]
- Kimani, M.; Beyer, S.; El-Schich, Z.; Gawlitza, K.; Gjörloff-Wingren, A.; Rurack, K. Imprinted Particles for Direct Fluorescence Detection of Sialic Acid in Polar Media and on Cancer Cells with Enhanced Control of Nonspecific Binding. ACS Appl. Polym. Mater. 2021, 3, 2363–2373. [Google Scholar] [CrossRef]
- El-Schich, Z.; Zhang, Y.; Feith, M.; Beyer, S.; Sternbaek, L.; Ohlsson, L.; Stollenwerk, M.; Wingren, A.G. Molecularly imprinted polymers in biological applications. Biotechniques 2020, 69, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Sternbæk, L.; Kimani Wamaitha, M.; Gawlitza, K.; Janicke, B.; Alm, K.; Wingren Gjörloff, A. Digital holographic microscopy: Macrophage uptake of nanoprobes. Imaging Microsc. 2019, 1, 21–23. [Google Scholar]
- Wang, S.; Yin, D.; Wang, W.; Shen, X.; Zhu, J.-J.; Chen, H.-Y.; Liu, Z. Targeting and Imaging of Cancer Cells via Monosaccharide-Imprinted Fluorescent Nanoparticles. Sci. Rep. 2016, 6, 22757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Wen, Y.; Wang, Y.; Ma, Y.; Liu, Z. Pattern Recognition of Cells via Multiplexed Imaging with Monosaccharide-Imprinted Quantum Dots. Anal. Chem. 2017, 89, 5646–5652. [Google Scholar] [CrossRef]
- Zhang, Y.; Llapashtica, K.; Shinde, S.; Sellergren, B.; El-Schich, Z.; Wingren, A.G. Determination of cytokine regulated glycan expression by using molecularly imprinted polymers targeting sialic acid. J. Cancer Metastasis Treat. 2019, 5, 56. [Google Scholar] [CrossRef]
- Medina Rangel, P.X.; Laclef, S.; Xu, J.; Panagiotopoulou, M.; Kovensky, J.; Tse Sum Bui, B.; Haupt, K. Solid-phase synthesis of molecularly imprinted polymer nanolabels: Affinity tools for cellular bioimaging of glycans. Sci. Rep. 2019, 9, 3923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Liu, R.; Huang, H.; Zhu, Q. Vinblastine-Loaded Nanoparticles with Enhanced Tumor-Targeting Efficiency and Decreasing Toxicity: Developed by One-Step Molecular Imprinting Process. Mol. Pharm. 2019, 16, 2675–2689. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Poma, A. Advances in Molecularly Imprinted Polymers as Drug Delivery Systems. Molecules 2021, 26, 3589. [Google Scholar] [CrossRef] [PubMed]
- Platel, A.; Carpentier, R.; Becart, E.; Mordacq, G.; Betbeder, D.; Nesslany, F. Influence of the surface charge of PLGA nanoparticles on their in vitro genotoxicity, cytotoxicity, ROS production and endocytosis. J. Appl. Toxicol. 2016, 36, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.A.; Walker, A.; Mitragotri, S. Role of Particle Size in Phagocytosis of Polymeric Microspheres. Pharm. Res. 2008, 25, 1815–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, D.E., 3rd; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Gref, R.; Minamitake, Y.; Peracchia, M.; Trubetskoy, V.; Torchilin, V.; Langer, R. Biodegradable long-circulating polymeric nanospheres. Science 1994, 263, 1600–1603. [Google Scholar] [CrossRef] [Green Version]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Taciak, B.; Białasek, M.; Braniewska, A.; Sas, Z.; Sawicka, P.; Kiraga, Ł.; Rygiel, T.; Król, M. Evaluation of phenotypic and functional stability of RAW 264.7 cell line through serial passages. PLoS ONE 2018, 13, e0198943. [Google Scholar] [CrossRef]
- Invivogen. Product Sheet—Alhydrogel Adjuvant 2%. Available online: https://www.invivogen.com/sites/default/files/invivogen/products/files/alhydrogel_tds.pdf (accessed on 24 January 2022).
- Sigma-Aldrich. Product Sheet of PLGA Microspheres—2 µm Average Diameter. Available online: https://www.sigmaaldrich.com/specification-sheets/634/404/805130-50MG-PW.pdf (accessed on 24 January 2022).
- Fritsch-Decker, S.; Marquardt, C.; Stoeger, T.; Diabaté, S.; Weiss, C. Revisiting the stress paradigm for silica nanoparticles: Decoupling of the anti-oxidative defense, pro-inflammatory response and cytotoxicity. Arch. Toxicol. 2018, 92, 2163–2174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alm, K.; El-Schich, Z.; Falck, M.; Gjrloff Wingren, A.; Janicke, B.; Oredsso, S. Cells and Holograms—Holograms and Digital Holographic Microscopy as a Tool to Study the Morphology of Living Cells. In Holography—Basic Principles and Contemporary Applications; BoD—Books on Demand: Norderstedt, Germany, 2013. [Google Scholar]
- Kamlund, S.; Strand, D.; Janicke, B.; Alm, K.; Oredsson, S. Influence of salinomycin treatment on division and movement of individual cancer cells cultured in normoxia or hypoxia evaluated with time-lapse digital holographic microscopy. Cell Cycle 2017, 16, 2128–2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallin, H.; Hunaiti, S.; Abrahamson, M. Externally added cystatin C reduces growth of A375 melanoma cells by increasing cell cycle time. FEBS Open Bio. 2021, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Mile, I.; Svensson, A.; Darabi, A.; Mold, M.; Siesjo, P.; Eriksson, H. Al adjuvants can be tracked in viable cells by lumogallion staining. J. Immunol. Methods 2015, 422, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Gao, Y.; Tao, Q.; Li, A.; Liu, Z.; Jiang, Y.; Liu, H.; Yang, R.; Liu, Y. Synthesizing a surface-imprinted polymer based on the nanoreactor SBA-15 for optimizing the adsorption of salicylic acid from aqueous solution by response surface methodology. New J. Chem. 2021, 45, 6192–6205. [Google Scholar] [CrossRef]
- An, S.S.A.; Kim, K.M.; Kim, H.M.; Lee, W.J.; Lee, C.W.; Kim, T.I.; Lee, J.-K.; Jeong, J.; Paek, S.M.; Shin, J.-H. Surface treatment of silica nanoparticles for stable and charge-controlled colloidal silica. Int. J. Nanomed. 2014, 29, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Danielsson, R.; Svensson, A.; Falkman, P.; Eriksson, H. Tracing Aluminium-based Adjuvants: Their Interactions with Immune Competent Cells and their Effect on Mitochondrial Activity. Open Immunol. J. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Harris, J.R.; Soliakov, A.; Lewis, R.J.; Depoix, F.; Watkinson, A.; Lakey, J.H. Alhydrogel® adjuvant, ultrasonic dispersion and protein binding: A TEM and analytical study. Micron 2012, 43, 192–200. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. Phagocytosis: Our Current Understanding of a Universal Biological Process. Front. Immunol. 2020, 11, 1066. [Google Scholar] [CrossRef]
- Ueno, T.; Yamamoto, Y.; Kawasaki, K. Phagocytosis of microparticles increases responsiveness of macrophage-like cell lines U937 and THP-1 to bacterial lipopolysaccharide and lipopeptide. Sci. Rep. 2021, 11, 6782. [Google Scholar] [CrossRef]
- El-Schich, Z.; Abdullah, M.; Shinde, S.; Dizeyi, N.; Rosen, A.; Sellergren, B.; Wingren, A.G. Different expression levels of glycans on leukemic cells—A novel screening method with molecularly imprinted polymers (MIP) targeting sialic acid. Tumour Biol. 2016, 37, 13763–13768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mold, M.; Eriksson, H.; Siesjo, P.; Darabi, A.; Shardlow, E.; Exley, C. Unequivocal identification of intracellular aluminium adjuvant in a monocytic THP-1 cell line. Sci. Rep. 2014, 4, 6287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mankan, A.K.; Kubarenko, A.; Hornung, V. Immunology in clinic review series; focus on autoinflammatory diseases: Inflammasomes: Mechanisms of activation. Clin. Exp. Immunol. 2012, 167, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Van de Veerdonk, F.L.; Netea, M.G.; Dinarello, C.A.; Joosten, L.A. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends Immunol. 2011, 32, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Mayor, A.; Tschopp, J. The inflammasomes: Guardians of the body. Annu. Rev. Immunol. 2009, 27, 229–265. [Google Scholar] [CrossRef] [Green Version]
- Sollberger, G.; Strittmatter, G.E.; Garstkiewicz, M.; Sand, J.; Beer, H.D. Caspase-1: The inflammasome and beyond. Innate Immun. 2014, 20, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Ohlsson, L.; Exley, C.; Darabi, A.; Sanden, E.; Siesjo, P.; Eriksson, H. Aluminium based adjuvants and their effects on mitochondria and lysosomes of phagocytosing cells. J. Inorg. Biochem. 2013, 128, 229–236. [Google Scholar] [CrossRef]
- Hornung, V.; Bauernfeind, F.; Halle, A.; Samstad, E.O.; Kono, H.; Rock, K.L.; Fitzgerald, K.A.; Latz, E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008, 9, 847–856. [Google Scholar] [CrossRef]
- Li, H.; Nookala, S.; Re, F. Aluminum hydroxide adjuvants activate caspase-1 and induce IL-1beta and IL-18 release. J. Immunol. 2007, 178, 5271–5276. [Google Scholar] [CrossRef]
- Garapaty, A.; Champion, J.A. Shape of ligand immobilized particles dominates and amplifies the macrophage cytokine response to ligands. PLoS ONE 2019, 14, e0217022. [Google Scholar] [CrossRef]
- Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994, 12, 991–1045. [Google Scholar] [CrossRef] [PubMed]
- Rock, K.L.; Latz, E.; Ontiveros, F.; Kono, H. The sterile inflammatory response. Annu. Rev. Immunol. 2010, 28, 321–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rider, P.; Voronov, E.; Dinarello, C.A.; Apte, R.N.; Cohen, I. Alarmins: Feel the Stress. J. Immunol. 2017, 198, 1395–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.M.; Wang, X.; Marin-Muller, C.; Wang, H.; Lin, P.H.; Yao, Q.; Chen, C. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev. Mol. Diagn. 2009, 9, 325–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghitman, J.; Biru, E.I.; Stan, R.; Iovu, H. Review of hybrid PLGA nanoparticles: Future of smart drug delivery and theranostics medicine. Mater. Des. 2020, 193, 108805. [Google Scholar] [CrossRef]
- Legrand, C.; Bour, J.M.; Jacob, C.; Capiaumont, J.; Martial, A.; Marc, A.; Wudtke, M.; Kretzmer, G.; Demangel, C.; Duval, D.; et al. Lactate dehydrogenase (LDH) activity of the number of dead cells in the medium of cultured eukaryotic cells as marker. J. Biotechnol. 1992, 25, 231–243. [Google Scholar] [CrossRef]
- Hiebl, B.; Peters, S.; Gemeinhardt, O.; Niehues, S.M.; Jung, F. Impact of serum in cell culture media on in vitro lactate dehydrogenase (LDH) release determination. J. Cell. Biotechnol. 2017, 3, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Mölder, A.; Sebesta, M.; Gustafsson, M.; Gisselson, L.; Wingren, A.G.; Alm, K. Non-invasive, label-free cell counting and quantitative analysis of adherent cells using digital holography. J. Microsc. 2008, 232, 240–247. [Google Scholar] [CrossRef]
- Pauwels, A.M.; Trost, M.; Beyaert, R.; Hoffmann, E. Patterns, Receptors, and Signals: Regulation of Phagosome Maturation. Trends Immunol. 2017, 38, 407–422. [Google Scholar] [CrossRef] [Green Version]



| SA-MIP | Alhydrogel | PLGA | |
|---|---|---|---|
| Overall surface charge | Negative [28,29] | Positive [30] | Negative [14] |
| Single particle diameter | ~0.2 µm [4] | ~0.05 µm [31] | ~2 µm [22] |
| Particle size in medium (form aggregates) | 0.2–3 µm * | 0.5–4 µm * [30] | No aggregation ** |
| Protein adsorption in medium | ~50 kDa *** | ~ 50 kDa–198 kDa *** Several bands | Not investigated |
| Fluorescence dye | Nitrobenzoxadiazole [4] | Lumogallion [27] | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sternbæk, L.; Kimani, M.; Gawlitza, K.; Rurack, K.; Janicke, B.; Alm, K.; Wingren, A.G.; Eriksson, H. Molecularly Imprinted Polymers Exhibit Low Cytotoxic and Inflammatory Properties in Macrophages In Vitro. Appl. Sci. 2022, 12, 6091. https://doi.org/10.3390/app12126091
Sternbæk L, Kimani M, Gawlitza K, Rurack K, Janicke B, Alm K, Wingren AG, Eriksson H. Molecularly Imprinted Polymers Exhibit Low Cytotoxic and Inflammatory Properties in Macrophages In Vitro. Applied Sciences. 2022; 12(12):6091. https://doi.org/10.3390/app12126091
Chicago/Turabian StyleSternbæk, Louise, Martha Kimani, Kornelia Gawlitza, Knut Rurack, Birgit Janicke, Kersti Alm, Anette Gjörloff Wingren, and Håkan Eriksson. 2022. "Molecularly Imprinted Polymers Exhibit Low Cytotoxic and Inflammatory Properties in Macrophages In Vitro" Applied Sciences 12, no. 12: 6091. https://doi.org/10.3390/app12126091
APA StyleSternbæk, L., Kimani, M., Gawlitza, K., Rurack, K., Janicke, B., Alm, K., Wingren, A. G., & Eriksson, H. (2022). Molecularly Imprinted Polymers Exhibit Low Cytotoxic and Inflammatory Properties in Macrophages In Vitro. Applied Sciences, 12(12), 6091. https://doi.org/10.3390/app12126091







