Solvents for Membrane-Based Post-Combustion CO2 Capture for Potential Application in the Marine Environment
Abstract
:1. Introduction
- Alternative fuels (the vast majority (>95%) of sea-going vessels is operated with either heavy fuel oil (HFO) or marine diesel oil (MDO). Liquefied natural gas (LNG) is mainly used as a fuel in LNG carriers. Potential alternative fuels being considered by the maritime industry include ammonia, methanol, liquefied petroleum gas (LPG), and biofuels (including bio-oils and hydrotreated vegetable oils (HVOs));
- Propulsion and power system (engine efficiency improvement, propulsion efficiency devices, propeller optimization, waste heat recovery, and wind and solar assistance technologies);
- Electrification (on-board electricity production, fuel cells, battery storage, hybrid systems, and cold ironing);
- Ship design (hull hydrodynamics, hull coatings, and air lubrication);
- Operational measures (slow steaming, weather routing, route optimization, and ship energy management system integration).
2. Integration of Carbon Capture Technologies in Ships: Status and Requirements
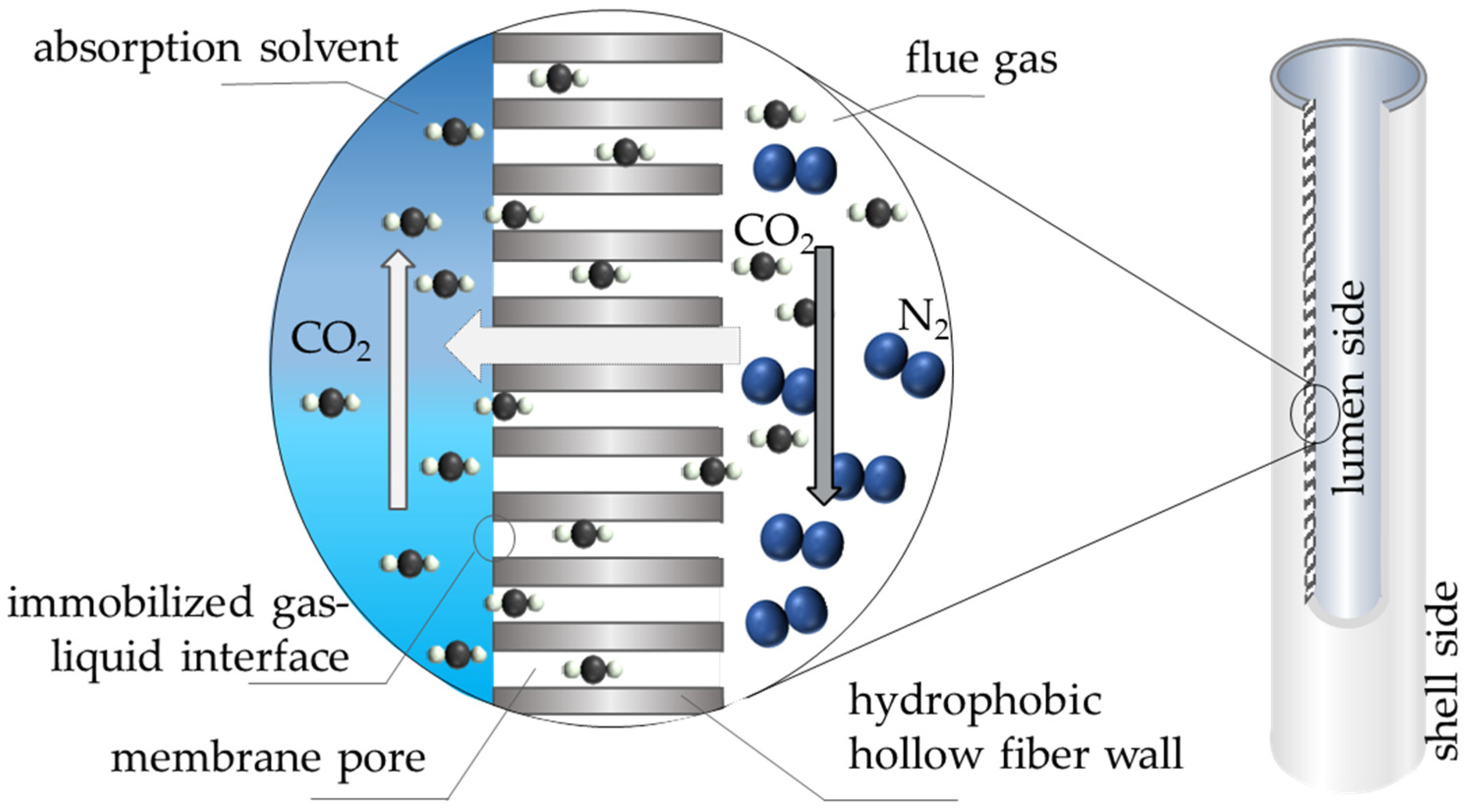
3. Membrane Contactors for Maritime CO2 Capture
- Inorganic solvents such as H2O, NaOH, K2CO3, etc. have high surface tension and do not easily wet the common hydrophobic polymeric membranes. However, they are typically less efficient than conventional amines.
- Amines have high CO2 capture efficiency but they have typically lower surface tension compared with inorganic solvents and they tend to more easily wet the common hydrophobic polymeric membranes. Among the different types of amines that are typically used, MEA has the highest wetting potential and typically leads to significant flux declines in all commercial hydrophobic membranes. DEA and MDEA tend to have milder effects on membrane performance.
- Novel solvents, such as the amino acid salts (e.g., potassium glycinate) or composite solutions with an amino acid salt, typically combine the high performance with a very low wetting potential.
- Ionic liquids typically require high operating temperatures, which can be tolerated only by PTFE or ceramic membranes.
4. Solvents for CO2 Capture
4.1. Physical Solvents
4.2. Chemical Solvents
4.2.1. Single Amine Solvents
4.2.2. Solvent Blends
4.2.3. Phase Change Solvents
4.2.4. Ionic Liquids
4.2.5. Other Solvents
5. Solvent Evaluation
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ritchie, H.; Roser, M. CO2 and Greenhouse Gas Emissions. 2020. Available online: https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions (accessed on 16 March 2022).
- IEA. Greenhouse Gas Emissions from Energy: Overview, International Energy Agency; IEA: Paris, France, 2021; Available online: https://www.iea.org/reports/greenhouse-gas-emissions-from-energy-overview (accessed on 16 March 2022).
- IMO. Fourth IMO Greenhouse Gas Study; International Maritime Organization: London, UK, 2021.
- DNV. Maritime Forecast to 2050, Energy Transition Outlook 2021; DNV: Bærum, Norway, 2021. [Google Scholar]
- IRENA. A Pathway to Decarbonise the Shipping Sector by 2050; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2021. [Google Scholar]
- Mallouppas, G.; Yfantis, E.A. Decarbonization in shipping industry: A review of research, technology development, and innovation proposals. J. Mar. Sci. Eng. 2021, 9, 415. [Google Scholar] [CrossRef]
- Foretich, A.; Zaimes, G.G.; Hawkins, T.R.; Newes, E. Challenges and opportunities for alternative fuels in the maritime sector. Marit. Transp. Res. 2021, 2, 100033. [Google Scholar] [CrossRef]
- Bouman, E.A.; Lindstad, E.; Rialland, A.I.; Stromman, A.H. State-of-the-art technologies, measures, and potential for reducing GHG emissions from shipping—A review. Transport. Res. Part D Transp. Environ. 2017, 52, 408–421. [Google Scholar] [CrossRef]
- Balcombe, P.; Brierley, J.; Lewis, C.; Skatvedt, L.; Speirs, J.; Hawkes, A.; Staffell, I. How to decarbonise international shipping: Options for fuels, technologies and policies. Energy Convers. Manag. 2019, 182, 72–88. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fenell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef] [Green Version]
- IEA. Energy Technology Perspectives 2017; International Energy Agency: Paris, France, 2017. [Google Scholar]
- IEA. Energy Technology Perspectives 2020—Special Report on Carbon Capture Utilisation and Storage; International Energy Agency: Paris, France, 2020. [Google Scholar]
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kus, T. Methods and techniques for CO2 capture: Review of potential solutions and applications in modern energy technologies. Energies 2022, 15, 887. [Google Scholar] [CrossRef]
- Chao, C.; Deng, Y.; Dewil, R.; Baeyens, J.; Fan, X. Post-combustion CO2 capture. Renew. Sustain. Energy Rev. 2021, 138, 110490. [Google Scholar] [CrossRef]
- Raganati, F.; Miccio, F.; Ammendola, P. Adsorption of carbon dioxide for post-combustion capture: A review. Energy Fuels 2021, 35, 12845–12868. [Google Scholar] [CrossRef]
- Vaz, S., Jr.; de Souza, A.P.R.; Baeta, B.E.L. Technologies for carbon dioxide capture: A review applied to energy sectors. Clean Eng Technol. 2022, 8, 100456. [Google Scholar] [CrossRef]
- Wilcox, J. Carbon Capture, 1st ed.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Mc Dowell, N.; Florin, N.; Buchard, A.; Hallett, J.; Galindo, A.; Jackson, G.; Adjiman, C.S.; Williams, C.K.; Shah, N.; Fennell, P. An overview of CO2 capture technologies. Energy Environ. Sci. 2010, 3, 1645–1669. [Google Scholar] [CrossRef] [Green Version]
- DNV. Maritime CCS Project. 2011. Available online: https://www.era-learn.eu/network-information/networks/eurostars/eurostars-cut-off-2/novel-process-designs-forreduction-of-maritime-carbon-emissions (accessed on 1 May 2022).
- Van den Akker, J.T. Carbon Capture on-Board LNG-Fuelled Vessels. Master’s Thesis, TU Delft, Delft, The Netherlands, 2017. [Google Scholar]
- Luo, X.; Wang, M. Study of solvent-based carbon capture for cargo ships through process modelling and simulation. Appl. Energy 2017, 195, 402–413. [Google Scholar] [CrossRef] [Green Version]
- Feenstra, M.; Monteiro, J.; Gilling, E.; Goetheer, E.; van den Akker, J.; Abu-Zahra, M. Ship-based carbon capture on-board of diesel or LNG-fuelled ships. Int. J. Greenh. Gas Contr. 2019, 85, 1–10. [Google Scholar] [CrossRef]
- Ragland, K.W.; Bryden, K.M. Combustion Engineering 2011, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Purwasasmita, M.; Nabu, E.B.P.; Khoiruddin, K.; Wenten, I.G. Non dispersive chemical deacidification of crude palm oil in hollow fiber membrane contactor. J. Eng. Technol. Sci. 2015, 47, 426–446. [Google Scholar] [CrossRef] [Green Version]
- Pantoleontos, G.; Theodoridis, T.; Mavroudi, M.; Kikkinides, E.S.; Koutsonikolas, D.; Kaldis, S.P.; Pagana, A.E. Modelling, simulation, and membrane wetting estimation in gas-liquid contacting processes. Canadian J. Chem. Eng. 2017, 95, 1352–1363. [Google Scholar] [CrossRef]
- Siagian, U.W.R.; Raksajati, A.; Himma, N.F.; Khoiruddin, K.; Wenten, I.G. Membrane-based carbon capture technologies: Membrane gas separation vs. membrane contactor. J. Nat. Gas Sci. Eng. 2019, 67, 172–195. [Google Scholar] [CrossRef]
- Salmon, I.R.; Cambier, N.; Luis, P. CO2 capture by alkaline solution for carbonate production: A comparison between a packed column and a membrane contactor. Appl. Sci. 2018, 8, 996. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Kim, M.K.; Park, J.H.; Magnone, E. Temperature and pressure dependence of the CO2 absorption through a ceramic hollow fiber membrane contactor module. Chem. Eng. Process. 2020, 150, 107871. [Google Scholar] [CrossRef]
- Sohaib, Q.; Vadillo, J.M.; Gómez-Coma, L.; Albo, J.; Druon-Bocquet, S.; Irabien, A.; Sanchez-Marcano, J. CO2 capture with room temperature ionic liquids; coupled absorption/desorption and single module absorption in membrane contactor. Chem. Eng. Sci. 2020, 223, 115719. [Google Scholar] [CrossRef]
- Usman, M.; Hillestad, M.; Deng, L. Assessment of a membrane contactor process for pre-combustion CO2 capture by modelling and integrated process simulation. Int. J. Greenh. Gas Control 2018, 71, 95–103. [Google Scholar] [CrossRef]
- Khaisri, S.; Montigny, D.; Tontiwachwuthikul, P.; Jiraratananon, R. Comparing membrane resistance and absorption performance of three different membranes in a gas absorption membrane contactor. Sep. Purif. Technol. 2009, 65, 290–297. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Kim, K.; Lee, J.; Koh, D.-Y.; Bae, T.-H. CO2 absorption using membrane contactors: Recent progress and future perspective. Ind. Eng. Chem. Res. 2020, 59, 6773–6794. [Google Scholar] [CrossRef]
- An, L.; Yu, X.; Yang, J.; Tu, S.-T.; Yan, J. CO2 capture using a superhydrophobic ceramic membrane contactor. Energ. Procedia 2015, 75, 2287–2292. [Google Scholar] [CrossRef] [Green Version]
- Nogalska, A.; Trojanowska, A.; Garcia-Valls, R. Membrane contactors for CO2 capture processes—Critical review. Phys. Sci. Rev. 2017, 2, 1–7. [Google Scholar] [CrossRef]
- Xu, Y.; Malde, C.; Wang, R. Correlating Physicochemical Properties of Commercial Membranes with CO2 Absorption Performance in Gas-Liquid Membrane Contactor. J. Membrane Sci. 2020, 6, 630–639. [Google Scholar]
- Mavroudi, M.; Kaldis, S.P.; Sakellaropoulos, G.P. A study of mass transfer resistance in membrane gas–liquid contacting processes. J. Membrane Sci. 2006, 272, 103–115. [Google Scholar] [CrossRef]
- Cui, Z.; deMontigny, D. Part 7: A review of CO2 capture using hollow fiber membrane contactors. Carbon Manag. 2013, 4, 69–89. [Google Scholar] [CrossRef]
- Mosadegh-Sedghi, S.; Rodrigue, D.; Brisson, J.; Iliuta, M.C. Wetting phenomenon in membrane contactors—Causes and prevention. J. Membrane Sci. 2014, 452, 332–353. [Google Scholar] [CrossRef]
- Dai, Z.; Ansaloni, L.; Deng, L. Precombustion CO2 Capture in Polymeric Hollow Fiber Membrane Contactors Using Ionic Liquids: Porous Membrane versus Nonporous Composite Membrane. Ind. Eng. Chem. Res. 2016, 55, 5983–5992. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; El-Naas, M.H.; Zhang, Z.; Van der Bruggen, B. CO2 Capture using Hollow Fiber Membranes: A review of membrane wetting. Energ. Fuel. 2018, 32, 963–978. [Google Scholar] [CrossRef]
- Yan, X.; Anguille, S.; Bendahan, M.; Moulin, P. Ionic liquids combined with membrane separation processes: A review. Sep. Purif. Technol. 2019, 222, 230–253. [Google Scholar] [CrossRef]
- Olajire, A.A. CO2 capture and separation technologies for end-of pipe applications—A review. Energy 2010, 35, 2610–2628. [Google Scholar] [CrossRef]
- Owens, W.; Buchanan, T.; DeLallo, M.; Schoff, R.; White, J.; Wolk, R. Evaluation of Innovative Fossil Fuel Power Plants with CO2 Removal; EPRI: Palo Alto, CA, USA, 2000. [Google Scholar]
- Mumford, K.A.; Wu, Y.; Smith, K.H.; Stevens, G.W. Review of solvent based carbon-dioxide capture technologies. Front. Chem. Sci. Eng. 2015, 9, 125–141. [Google Scholar] [CrossRef]
- Gainar, I.; Anitescu, G. The solubility of CO2, N2 and H2 in a mixture of dimethylether polyethylene glycols at high pressures. Fluid Phase Equilibria 1995, 109, 281–289. [Google Scholar] [CrossRef]
- Kohl, A.L.; Nielsen, R.B. Alkanolamines for Hydrogen Sulfide and Carbon Dioxide Removal. In Gas Purification, 5th ed.; Gulf Publication Company: Houston, TX, USA, 1997; pp. 40–186. [Google Scholar]
- Vega, F.; Cano, M.; Camino, S.; Gallego Fernandez, L.M.; Portillo, E.; Navarrete, B. Solvents for CO2 capture. In Carbon Dioxide Chemistry, Capture and Oil Recovery; Karamé, I., Shaya, J., Srour, H., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Dashti, S.S.; Shariati, A.; Nikou, M.R.K. Sensitivity analysis for selection of an optimum amine gas sweetening process with minimum cost requirement. Asia-Pacific J. Chem. Eng. 2015, 10, 709–715. [Google Scholar] [CrossRef]
- Damartzis, T.; Papadopoulos, A.I.; Seferlis, P. Process flowsheet design optimization for various amine-based solvents in post-combustion CO2 capture plants. J. Clean. Prod. 2016, 111, 204–216. [Google Scholar] [CrossRef]
- Chen, E.; Zhang, Y.; Lin, Y.; Nielsen, P.; Rochelle, G. Review of recent pilot plant activities with concentrated piperazine. Energy Procedia 2017, 114, 1110–1127. [Google Scholar] [CrossRef]
- Chen, X.; Rochelle, G.T. Aqueous piperazine derivatives for CO2 capture: Accurate screening by a wetted wall column. Chem. Eng. Res. Des. 2011, 89, 1693–1710. [Google Scholar] [CrossRef]
- Vaidya, P.D.; Kenig, E.Y. CO2-alkanolamine reaction kinetics: A review of recent studies. Chem. Eng. Technol. 2007, 30, 1467–1474. [Google Scholar] [CrossRef]
- Hüser, N.; Schmitz, O.; Kenig, E.Y. A comparative study of different amine-based solvents for CO2-capture using the rate-based approach. Chem. Eng. Sci. 2017, 157, 221–231. [Google Scholar] [CrossRef]
- Buvik, V.; Vevelstad, S.J.; Brakstad, O.G.; Knuutila, H.K. Stability of structurally varied aqueous amines for CO2 capture. Ind. Eng. Chem. Res. 2021, 60, 5627–5638. [Google Scholar] [CrossRef]
- Bruder, P.; Svendsen, H.F. Capacity and kinetics of solvents for post-combustion CO2 capture. Energy Procedia 2012, 23, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Cavaignac, R.S.; Ferreira, N.L.; Guardani, R. Techno-economic and environmental process evaluation of biogas upgrading via amine scrubbing. Renew. Energy 2021, 171, 868–880. [Google Scholar] [CrossRef]
- Mathias, P.M.; Reddy, S.; Smith, A.; Afshar, K. Thermodynamic analysis of CO2 capture solvents. Int. J. Greenh. Gas Control 2013, 19, 262–270. [Google Scholar] [CrossRef]
- Kim, Y.E.; Kim, J.A.; Jeong, S.K.; Yoon, Y.I.; Bae, S.T.; Nam, S.C. Comparison of carbon dioxide absorption in aqueous MEA, DEA, TEA and AMP solutions. Bull. Korean Chem. Soc. 2013, 34, 783–787. [Google Scholar] [CrossRef] [Green Version]
- Dubuis, L.; Thomas, D. Optimization of the post-combustion CO2 capture applied to cement plant flue gases: Parametric study with different solvents and configurations combined with intercooling. In Proceedings of the 14th International Conference on Greenhouse Gas Control Technologies, GHGT-14, Melbourne, Australia, 21–25 October 2018. [Google Scholar]
- Damartzis, T.; Papadopoulos, A.I.; Seferlis, P. Solvent effects on design with operability considerations in post-combustion CO2 capture plants. Chem. Eng. Res. Des. 2018, 131, 414–429. [Google Scholar] [CrossRef]
- Nwaoha, C.; Supap, T.; Idem, R.; Saiwan, C.; Tontiwachwuthikul, P.; Al-Marri, M.J.; Benamor, A. Advancement and new perspectives of using formulated reactive amine blends for post-combustion carbon dioxide (CO2) capture technologies. Petroleum 2017, 3, 10–36. [Google Scholar] [CrossRef]
- Idem, R.; Wilson, M.; Tontiwachwuthikul, P.; Chakma, A.; Veawab, A.; Aroonwilas, A.; Gelowitz, D. Pilot plant studies of the CO2 capture performance of aqueous MEA and mixed MEA/MDEA solvents at the University of Regina CO2 capture technology development plant and the boundary dam CO2 capture demonstration plant. Ind. Eng. Chem. Res. 2006, 45, 2414–2420. [Google Scholar] [CrossRef]
- Mangalapally, H.P.; Hasse, H. Pilot plant experiments for post combustion carbon dioxide capture by reactive absorption with novel solvents. Energy Procedia 2011, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Mudhasakul, S.; Ku, H.; Douglas, P.L. A simulation model of a CO2 absorption process with methyldiethanolamine solvent and piperazine as an activator. Int. J. Greenh. Gas Control 2013, 15, 134–141. [Google Scholar] [CrossRef]
- Adeosun, A.; Abu-Zahra, M.R.M. Evaluation of amine-blend solvent systems for CO2 post-combustion capture applications. Energy Procedia 2013, 37, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Dash, S.K.; Samanta, A.N.; Bandyopadhyay, S.S. Simulation and parametric study of post combustion CO2 capture process using (AMP + PZ) blended solvent. Int. J. Greenh. Gas Contr. 2014, 21, 130–139. [Google Scholar] [CrossRef]
- Choi, W.-J.; Seo, J.-B.; Jang, S.-Y.; Jung, J.-H.; Oh, K.-J. Removal characteristics of CO2 using aqueous MEA/AMP solutions in the absorption and regeneration process. J. Environ. Sci. 2009, 21, 907–913. [Google Scholar] [CrossRef]
- Bruder, P.; Grimstvedt, A.; Mejdell, T.; Svendsen, H.F. CO2 capture into aqueous solutions of piperazine activated 2-amino-2-methyl-1-propanol. Chem. Eng. Sci. 2011, 66, 6193–6198. [Google Scholar] [CrossRef]
- Haghtalab, A.; Eghbali, H.; Shojaeian, A. Experiment and modeling solubility of CO2 in aqueous solutions of diisopropanolamine + 2-amino-2-methyl-1-propanol + piperazine at high pressures. J. Chem. Thermodyn. 2014, 71, 71–83. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, W.; Wang, K.; Wang, J. Studies of CO2 absorption/regeneration performances of novel aqueous monoethanolamine (MEA)—based solution. J. Clean. Prod. 2016, 112, 4012–4021. [Google Scholar] [CrossRef]
- Nwaoha, C. CO2 Absorption: Solubility of CO2 in 2-amino-2-methyl-1-Propanol Solvent Promoted by Piperazine and Monoethanolamine Blends. Master’s Thesis, The Petroleum and Petrochemical College, Chulalongkorn University, Bangkok, Thailand, 2015. [Google Scholar]
- Papadopoulos, A.I.; Perdomo, F.A.; Tzirakis, F.; Shavalieva, G.; Tsivintzelis, I.; Kazepidis, P.; Nessi, E.; Papadokonstantakis, S.; Seferlis, P.; Galindo, A.; et al. Molecular engineering of sustainable phase-change solvents: From digital design to scaling-up for CO2 capture. Chem. Eng. J. 2021, 420, 127624. [Google Scholar] [CrossRef]
- Papadopoulos, A.I.; Tzirakis, F.; Tsivintzelis, I.; Seferlis, P. Phase-change solvents and processes for post-combustion CO2 capture: A detailed review. Ind. Eng. Chem. Res. 2019, 58, 5088–5111. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Shen, Y.; Wang, L.; Chen, J.; Lu, Y. Phase change solvents for post-combustion CO2 capture: Principle, advances and challenges. Applied Energy 2019, 239, 876–897. [Google Scholar] [CrossRef]
- Zhang, X. Studies on Multiphase CO2 Capture Systems. Ph.D. Thesis, TU-Dortmund, Dortmund, Germany, 2007. [Google Scholar]
- Pinto, D.D.D.; Zaidy, S.A.H.; Hartono, A.; Svendsen, H.F. Evaluation of a phase change solvent for CO2 capture: Absorption and desorption tests. Int. J. Greenh. Gas Control 2014, 28, 318–327. [Google Scholar] [CrossRef] [Green Version]
- Arshad, M.W.; Von Solms, N.; Thomsen, K.; Svendsen, H.F. Heat of absorption of CO2 in aqueous solutions of DEEA, MAPA and their mixture. Energy Procedia 2013, 37, 1532–1542. [Google Scholar] [CrossRef] [Green Version]
- Shavalieva, G.; Kazepidis, P.; Papadopoulos, A.I.; Seferlis, P.; Papadokonstantakis, S. Environmental, health and safety assessment of post-combustion CO2 capture processes with phase-change solvents. Sustain. Prod. Consum. 2021, 25, 60–76. [Google Scholar] [CrossRef]
- Shavalieva, G.; Papadokonstantakis, S.; Kazepidis, P.; Papadopoulos, A.I.; Seferlis, P. Sustainability analysis of phase-change solvents for post-combustion CO2 capture. Chem Eng Transact 2019, 76, 1045–1050. [Google Scholar]
- Tzirakis, F.; Tsivintzelis, I.; Papadopoulos, A.I.; Seferlis, P. Experimental measurement and assessment of equilibrium behaviour for phase change solvents used in CO2 capture. Chem. Eng. Sci. 2019, 18, 20–27. [Google Scholar] [CrossRef]
- Kazepidis, P.; Papadopoulos, A.I.; Tzirakis, F.; Seferlis, P. Optimum design of industrial post-combustion CO2 capture processes using phase-change solvents. Chem. Eng. Res. Des. 2021, 175, 209–222. [Google Scholar] [CrossRef]
- Friess, K.; Izák, P.; Kárásova, M.; Pasichnyk, M.; Lanc, M.; Nikolaeva, D.; Luis, P.; Jansen, J.C. A review on ionic liquid gas separation membranes. Membranes 2021, 11, 97. [Google Scholar] [CrossRef]
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. A systematic review on CO2 capture with ionic liquids: Current status and future prospects. Renew. Sustain. Energy Rev. 2018, 96, 502–525. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Dong, H.; Zhao, Z.; Zhang, S.; Huang, Y. Carbon capture with ionic liquids: Overview and progress. Energy Environ. Sci. 2012, 5, 6668–6681. [Google Scholar] [CrossRef]
- Sarmad, S.; Mikkola, J.-P.; Ji, X. Carbon dioxide capture with ionic liquids and deep eutectic solvents: A new generation of sorbents. ChemSusChem 2017, 10, 324–352. [Google Scholar] [CrossRef]
- Stevanovic, S.; Podgorsek, A.; Moura, L.; Santini, C.C.; Padua, A.A.H.; Costa Gomes, M.F. Absorption of carbon dioxide by ionic liquids with carboxylate anions. Int. J. Greenh. Gas Control 2013, 17, 78–88. [Google Scholar] [CrossRef]
- Tian, Q.; Li, R.; Sun, H.; Xue, Z.; Mu, T. Theoretical and experimental study on the interaction between 1-butyl-3-methylimidazolium acetate and CO2. J. Mol. Liq. 2015, 208, 259–268. [Google Scholar] [CrossRef]
- Cabaço, M.I.; Besnard, M.; Danten, Y.; Coutinho, J.A.P. Carbon dioxide in 1-Butyl-3-methylimidazolium acetate. I. Unusual solubility investigated by Raman Spectroscopy and DFT calculations. J. Phys. Chem. A 2012, 116, 1605–1620. [Google Scholar] [CrossRef] [PubMed]
- Mota-Martinez, M.T.; Hallett, J.P.; Mc Dowell, N. Solvent selection and design for CO2 capture—how we might have been missing the point. Sustain. Energy Fuels 2017, 1, 2078–2090. [Google Scholar] [CrossRef] [Green Version]
- Almeida, H.F.D.; Passos, H.; Lopes-da-Silva, J.A.; Fernandes, A.M.; Freire, M.G.; Coutinho, J.A.P. Thermophysical properties of five acetate-based ionic liquids. J. Chem. Eng. Data 2012, 57, 3005–3013. [Google Scholar] [CrossRef]
- Williams, M.L.; Holahan, S.P.; McCorkill, M.E.; Dickmann, J.S.; Kiran, E. Thermal and spectral characterization and stability of mixtures of ionic liquids [EMIM]Ac and [BMIM]Ac with ethanol, methanol, and water at ambient conditions and at elevated temperatures and pressures. Thermochim. Acta 2018, 669, 126–139. [Google Scholar] [CrossRef]
- Chen, F.F.; Huang, K.; Zhou, Y.; Tian, Z.Q.; Zhu, X.; Tao, D.J.; Jiang, D.E.; Dai, S. Multi-molar absorption of CO2 by the activation of carboxylate groups in amino acid ionic liquids. Angew. Chem. 2016, 55, 7166–7170. [Google Scholar] [CrossRef]
- Sistla, Y.S.; Khanna, A. CO2 absorption studies in amino acid-anion based ionic liquids. Chem. Eng. J. 2015, 273, 268–276. [Google Scholar] [CrossRef]
- Santiago, R.; Lemus, J.; Moya, C.; Moreno, D.; Alonso-Morales, N.; Palomar, J. Encapsulated ionic liquids to enable the practical application of amino acid-based ionic liquids in CO2 capture. ACS Sustain. Chem. Eng. 2018, 6, 14178–14187. [Google Scholar] [CrossRef]
- Wu, J.; Lv, B.; Wu, X.; Zhou, Z.; Jing, G. Aprotic heterocyclic anion-based dual-functionalized ionic liquid solutions for efficient CO2 uptake: Quantum chemistry calculation and experimental research. ACS Sustain. Chem. Eng. 2019, 7, 7312–7323. [Google Scholar] [CrossRef]
- Hong, B.; Simoni, L.D.; Bennett, J.E.; Brennecke, J.F.; Stadtherr, M.A. Simultaneous process and material design for aprotic N-heterocyclic anion ionic liquids in post-combustion CO2 capture. Ind. Eng. Chem. Res. 2016, 55, 8432–8449. [Google Scholar] [CrossRef] [Green Version]
- Seo, S.; Quiroz-Guzman, M.; DeSilva, M.A.; Lee, T.B.; Huang, Y.; Goodrich, B.F.; Schneider, W.F.; Brennecke, J.F. Chemically tunable ionic liquids with aprotic heterocyclic anion (AHA) for CO2 capture. J. Phys. Chem. B 2014, 118, 5740–5751. [Google Scholar] [CrossRef]
- Fillion, J.J.; Bennett, J.E.; Brennecke, J.F. The viscosity and density of ionic liquid + tetraglyme mixtures and the effect of tetraglyme on CO2 colubility. J Chem. Eng. Data 2017, 62, 608–622. [Google Scholar] [CrossRef]
- Oko, E.; Zacchello, B.; Wang, M.; Fethi, A. Process analysis and economic evaluation of mixed aqueous ionic liquid and monoethanolamine (MEA) solvent for CO2 capture from a coke oven plant. Greenhouse Gas. Sci. Technol. 2018, 8, 686–700. [Google Scholar] [CrossRef] [Green Version]
- Zhai, H.; Rubin, E.S. Systems analysis of ionic liquids for post-combustion CO2 capture at coal-fired power plants. Energy Procedia 2014, 63, 1321–1328. [Google Scholar] [CrossRef] [Green Version]
- Shiflett, M.B.; Drew, D.W.; Cantini, R.A.; Yokozeki, A. Carbon dioxide capture using ionic liquid 1-Butyl-3-methylimidazolium acetate. Energy Fuels 2010, 24, 5781–5789. [Google Scholar] [CrossRef]
- De Riva, J.; Ferro, V.; Moya, C.; Stadtherr, M.A.; Brennecke, J.F.; Palomar, J. Aspen Plus supported analysis of the post-combustion CO2 capture by chemical absorption using the P-2228 CNPyr and P-66614 CNPyr AHA Ionic Liquids. Int. J. Greenh. Gas Control 2018, 78, 94–102. [Google Scholar] [CrossRef]
- Borhani, T.N.G.; Wang, M. Role of solvents in CO2 capture processes: The review of selection and design methods. Renew. Sust. Energ. Rev. 2019, 114, 109299. [Google Scholar] [CrossRef]
- Sartori, G.; Savage, D.W. Sterically hindered amines for carbon dioxide removal from gases. Ind. Eng. Chem. Fundam. 1983, 22, 239–249. [Google Scholar] [CrossRef]
- Asif, M.; Suleman, M.; Haq, I.; Jamal, S.A. Post-combustion CO2 capture with chemical absorption and hybrid system: Current status and challenges. Greenh. Gases Sci. Technol 2018, 8, 998–1031. [Google Scholar]
- Borhani, T.N.G.; Azarpour, A.; Akbari, V.; Wan Alwi, S.R.; Manan, Z.A. CO2 capture with potassium carbonate solutions: A state-of-the-art review. Int. J. Greenh. Gas Contr. 2015, 41, 142–162. [Google Scholar] [CrossRef]
- Song, H.-J.; Park, S.; Kim, H.; Gaur, A.; Park, J.-W.; Lee, S.-J. Carbon dioxide absorption characteristics of aqueous amino acid salt solutions. Int. J. Greenh. Gas Control 2012, 11, 64–72. [Google Scholar] [CrossRef]
- Yang, N.; Yu, H.; Xu, D.Y.; Conway, W.; Maeder, M.; Feron, P. Amino acids/NH3 mixtures for CO2 capture: Effect of neutralization methods on CO2 mass transfer and NH3 vapour loss. Energy Procedia 2014, 63, 773–780. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.S.; Hogendoorn, J.A.; Versteeg, G.F.; Feron, P.H.M. Kinetics of the reaction of CO2 with aqueous potassium salt of taurine and glycine. AIChE J 2003, 49, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Aronu, U.E.; Hartono, A.; Hoff, K.A.; Svendsen, H.F. Kinetics of carbon dioxide absorption into aqueous amino acid salt: Potassium salt of sarcosine solution. Ind. Eng. Chem. Res. 2011, 50, 10465–10475. [Google Scholar] [CrossRef]
- Nakjiri, A.T.; Heydarinasab, A.; Bakhtiari, O.; Mohammadi, T. Modeling and simulation of CO2 separation from CO2/CH4 gaseous mixture using potassium glycinate, potassium argininate and sodium hydroxide liquid absorbents in the hollow fiber membrane contactor. J. Environ. Chem. Eng. 2018, 6, 1500–1511. [Google Scholar] [CrossRef]
- Dave, N.; Do, T.; Puxty, G.; Rowland, R.; Feron, P.H.M.; Attalla, M.I. CO2 capture by aqueous amines and aqueous ammonia—A comparison. Energy Procedia 2009, 1, 949–954. [Google Scholar] [CrossRef] [Green Version]
- Resnik, K.P.; Pennline, H.W. Study of an ammonia-based wet scrubbing process in a continuous flow system. Fuel 2013, 105, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Guo, Y. Process simulations of NH3 abatement system for large-scale CO2 capture using aqueous ammonia solution. Int. J. Greenh. Gas Control 2013, 18, 114–127. [Google Scholar] [CrossRef]
- Li, H.; Tang, Z.; Xing, X.; Guo, D.; Cui, L.; Mao, X.-Z. Study of CO2 capture by seawater and its reinforcement. Energy 2018, 104, 1135–1144. [Google Scholar] [CrossRef]
- Li, H.; Zhang, R.; Wang, T.; Wu, Y.; Xu, R.; Wang, Q.; Tang, Z. Performance evaluation and environment risk assessment of steel slag enhancement for seawater to capture CO2. Energy 2022, 238B, 121861. [Google Scholar] [CrossRef]
| KPI | Description |
|---|---|
| Maturity | Whilst land-based CCS is relatively mature, only limited demo cases are available for maritime CCS and at low capture rates. |
| Compactness | On-board space capacity is limited. The minimization of system dimensions and weight is important for on-board integration. |
| Operability range | The solvent needs to be effective within a range of variant operating conditions, including temperature, pressure, exhaust gas flow and CO2 content in the exhaust. When powered by LNG, the CO2 content in ship engine exhaust is about 4–6%, which is lower than that of land-based applications. Solvents need to be effective at such low CO2 content increasing the energy penalty for CO2 capture. |
| Energy penalty | Unlike land-based applications, the on-board ship environment has low availability of power, heat, and consumable resources. Therefore, the energy demand for regenerating the solvent must be kept as low as possible. |
| Impurity tolerance | Some solvents may be sensitive to impurities, e.g., sulfur, particulate matter, or methane traces, or their capture efficiency may be degraded in the presence of such compounds in the flue gas. Any requirement for pre-treatment equipment would add complexity, risk, volume, and weight. |
| CO2 product characteristics | The CO2 product form is important in accounting for on-board storage capacity and conditions (liquefied, compressed, etc.), thus affecting the on-board resource requirements. |
| CO2 loading | The molar ratio of CO2 over the pure solvent. It is often regarded as a measure of the solvent’s capacity for CO2 capture (i.e., higher loading leads to more CO2 captured per unit of solvent) and can be correlated with solvent needs, regeneration demands, and the capture efficiency within the process. |
| OPEX | Costs are associated with large uncertainties and impact the uptake of technologies in the industry. Costs include maintenance and consumables, as well as any additional fuel costs as a result of the energy penalty. Degradation of solvent performance through use also plays important role in OPEX costs. |
| Other consumables | Depending on the solvent, other consumables may be needed, for example, water. Such demands increase the on-board requirements for energy and storage capacity. |
| Health and safety | The solvent physicochemical properties, e.g., flammability and toxicity, may impose health and safety hazards that require assessment, monitoring, and prevention measures. In addition, operational features such as high pressure or temperature impose additional design considerations related to safety as the key properties that determine the flammability of a material (such as lower-upper flammability limits, flash point, autoignition temperature, minimum ignition energy, and laminar flame speed, e.g., [23]) are strongly dependent on the temperature, pressure, and geometry of the container or reactor. |
| Class | Type | Advantages | Disadvantages |
|---|---|---|---|
| Physical | Methanol | Solvent cost ↓ Toxicity ↓ | Vapor pressure ↑ Process complexity ↑ |
| Selexol DPEG | Vapor pressure ↓ Temperature range ↑ Selectivity for H2S ↑ | Viscosity ↑ | |
| Rectisol/NH3 | Temperature ↓ | Selectivity for CO2 ↓ | |
| Amines | Primary | Solvent cost ↓ Reaction rate ↑ | Vapor pressure ↑ Stability ↓ Corrosiveness ↑ Regeneration energy ↑ |
| Secondary | Vapor pressure ↓ Regeneration energy ↓ | Reaction rate ↓ Corrosiveness ↑ | |
| Tertiary | Vapor pressure ↓ Regeneration energy ↓ Stability ↑ CO2 loading ↑ | Reaction rate ↓ Corrosiveness ↑ | |
| Sterically hindered | Stability ↑ CO2 loading ↑ Corrosiveness ↓ | Reaction rate ↓ | |
| Bi-Blends | Reaction rate ↑ CO2 loading ↑ Regeneration energy ↓ | Process complexity ↑ | |
| Tri-Blends | Reaction rate ↑ CO2 loading ↑↑ Regeneration energy ↓ | Process complexity ↑↑ | |
| Phase change | CO2 loading ↑ Regeneration energy ↓ Operating Temperature ↓ | Solvent cost ↑ Process complexity ↑ | |
| Ionic Liquids | Imidazolium | Vapor pressure ↓↓ Regeneration energy ↓ | Viscosity ↑ Solvent cost ↑↑ |
| Amino acid (AA) | Reaction rate ↑ Vapor pressure ↓↓ Regeneration energy ↓↓ | Viscosity ↑↑ Solvent cost ↑↑ | |
| Aprotic-heterocyclic (AHA) | Reaction rate ↑ Stability ↑ Vapor pressure ↓↓ Regeneration energy ↓ | Viscosity ↑ Solvent cost ↑↑ | |
| Salts | Carbonate | Solvent cost ↓ Stability ↑ High temperature ↑ | Reaction rate ↓ Precipitation chance ↑ |
| Amino acids | Stability ↑ Vapor Pressure ↓ | Regeneration energy ↑ Precipitation chance ↑ | |
| Ammonia | Solvent cost ↓ Stability ↑ CO2 loading ↑ Regeneration energy ↓ | Vapor pressure ↑↑ Reaction rate ↓ Process complexity ↑ | |
| Seawater | Solvent cost ↓ Stability ↑ Potential for CCUS ↑ | CO2 loading ↓ Process complexity ↑↑ |
| Physical Solvents | Primary Amines | Secondary Amines | Tertiary Amines | Sterically Hindered Amines | Amine Blends | Phase Change Solvents | Ionic Liquids | Salts | Ammonia | Seawater | Degree of Criticality | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maturity | 5 | 5 | 5 | 4 | 4 | 3 | 1 | 1 | 4 | 4 | 1 | I |
| Compactness | 3 | 3 | 3 | 3 | 3 | 4 | 4 | 4 | 3 | 3 | 2 | I |
| Energy Penalty | 3 | 2 | 3 | 3 | 3 | 4 | 5 | 5 | 4 | 4 | 4 | I |
| CO2 Loading | 2 | 3 | 3 | 4 | 4 | 5 | 5 | 5 | 3 | 3 | 1 | I |
| Health and Safety | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 4 | 4 | 2 | 5 | I |
| Operability Range | 4 | 4 | 4 | 3 | 2 | 4 | 3 | 4 | 4 | 3 | 3 | II |
| Impurity Tolerance | 2 | 3 | 3 | 3 | 4 | 3 | 3 | 4 | 4 | 4 | 4 | II |
| OPEX | 4 | 2 | 2 | 3 | 3 | 2 | 2 | 2 | 4 | 4 | 5 | II |
| Other Consumables | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 2 | 4 | 2 | II |
| Physical Solvents | Primary Amines | Secondary Amines | Tertiary Amines | Sterically Hindered Amines | Amine Blends | Phase Change Solvents | Ionic Liquids | Salts | Ammonia | Seawater | Score | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Physical Solvents | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Primary Amines | 0 | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Secondary Amines | 1 | 1 | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Tertiary Amines | 0 | 0 | 0 | - | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Sterically Hindered Amines | 0 | 0 | 0 | 1 | - | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Amine Blends | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | 0 | 0 | 0 | 0 |
| Phase Change Solvents | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | 0 | 0 | 0 |
| Ionic Liquids | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | 0 | 0 | 0 | 1 |
| Salts | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | 1 | 0 | 1 |
| Ammonia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 |
| Seawater | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 |
| Physical Solvents | Primary Amines | Secondary Amines | Tertiary Amines | Sterically Hindered Amines | Amine Blends | Phase Change Solvents | Ionic Liquids | Salts | Ammonia | Seawater | Score | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Physical Solvents | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Primary Amines | 0 | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Secondary Amines | 1 | 1 | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Tertiary Amines | 1 | 1 | 1 | - | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Sterically Hindered Amines | 1 | 1 | 1 | 1 | - | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Amine Blends | 1 | 1 | 1 | 1 | 1 | - | 0 | 0 | 0 | 1 | 0 | 6 |
| Phase Change Solvents | 1 | 1 | 1 | 1 | 1 | 1 | - | 0 | 0 | 1 | 0 | 7 |
| Ionic Liquids | 1 | 1 | 1 | 1 | 1 | 1 | 1 | - | 1 | 1 | 0 | 9 |
| Salts | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | - | 1 | 0 | 4 |
| Ammonia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 |
| Seawater | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damartzis, T.; Asimakopoulou, A.; Koutsonikolas, D.; Skevis, G.; Georgopoulou, C.; Dimopoulos, G.; Nikolopoulos, L.; Bougiouris, K.; Richter, H.; Lubenau, U.; et al. Solvents for Membrane-Based Post-Combustion CO2 Capture for Potential Application in the Marine Environment. Appl. Sci. 2022, 12, 6100. https://doi.org/10.3390/app12126100
Damartzis T, Asimakopoulou A, Koutsonikolas D, Skevis G, Georgopoulou C, Dimopoulos G, Nikolopoulos L, Bougiouris K, Richter H, Lubenau U, et al. Solvents for Membrane-Based Post-Combustion CO2 Capture for Potential Application in the Marine Environment. Applied Sciences. 2022; 12(12):6100. https://doi.org/10.3390/app12126100
Chicago/Turabian StyleDamartzis, Theodoros, Akrivi Asimakopoulou, Dimitrios Koutsonikolas, George Skevis, Chara Georgopoulou, George Dimopoulos, Lampros Nikolopoulos, Konstantinos Bougiouris, Hannes Richter, Udo Lubenau, and et al. 2022. "Solvents for Membrane-Based Post-Combustion CO2 Capture for Potential Application in the Marine Environment" Applied Sciences 12, no. 12: 6100. https://doi.org/10.3390/app12126100
APA StyleDamartzis, T., Asimakopoulou, A., Koutsonikolas, D., Skevis, G., Georgopoulou, C., Dimopoulos, G., Nikolopoulos, L., Bougiouris, K., Richter, H., Lubenau, U., Economopoulos, S., Perinu, C., Hopkinson, D., & Panagakos, G. (2022). Solvents for Membrane-Based Post-Combustion CO2 Capture for Potential Application in the Marine Environment. Applied Sciences, 12(12), 6100. https://doi.org/10.3390/app12126100










