Methane Production Potential from Apple Pomace, Cabbage Leaves, Pumpkin Residue and Walnut Husks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates and Inoculum
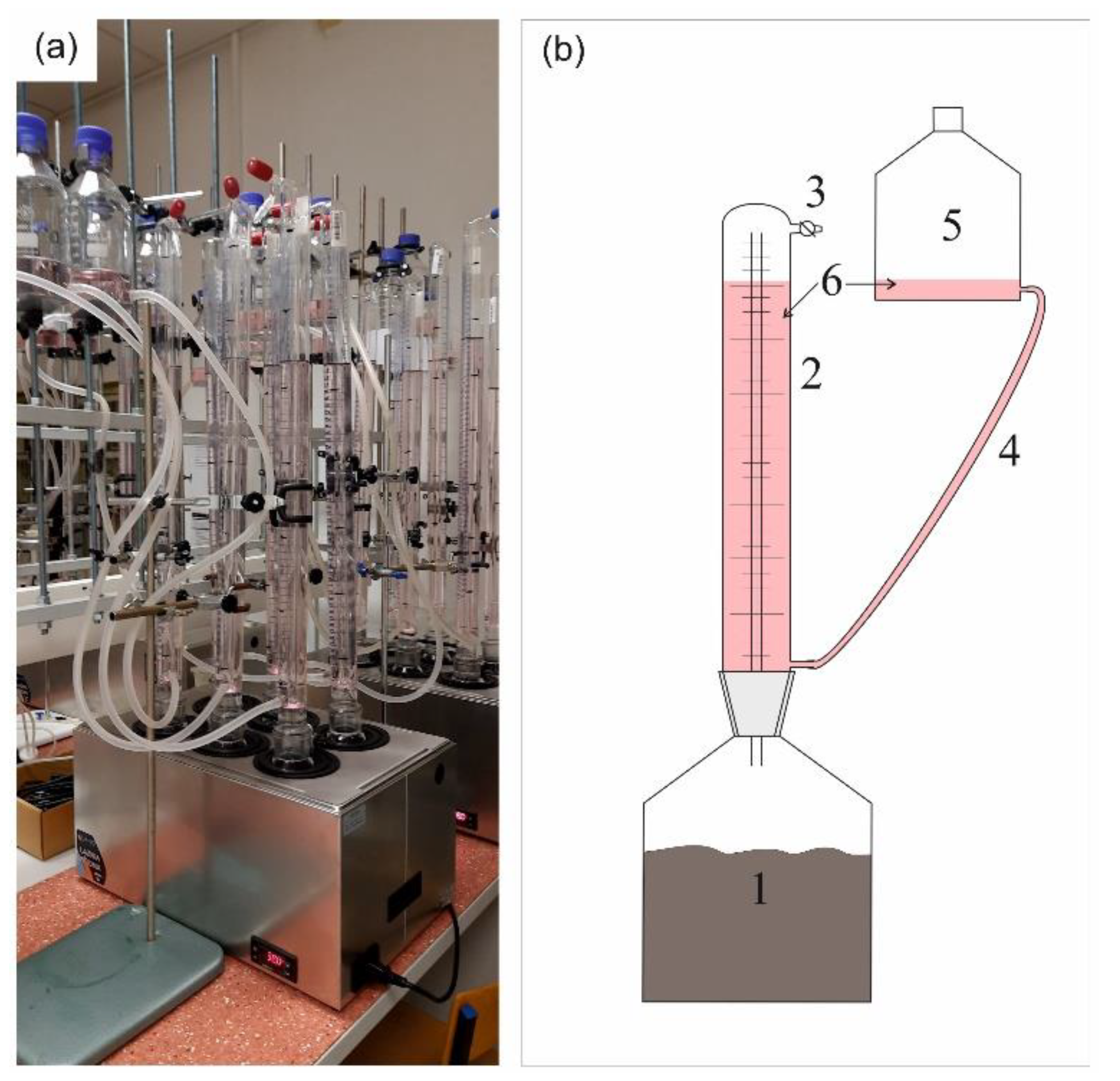
2.2. The Experimental Set-Up and Biogas Calculations
- G(t)—cumulative methane production at specific time t (mL)
- G0—methane production potential (mL)
- Rmax—maximum methane production rate (mL day−1)
- λ—duration of lag phase (minimum time to produce methane) (days)
- t—cumulative time for methane production (days)
- e—mathematical constant (2.71828)
2.3. Analytical Methods
2.4. Statistical Analyses
2.5. Calculations of Energy and GHG Emissions
3. Results
3.1. Properties of Analysed FVR and Inoculum
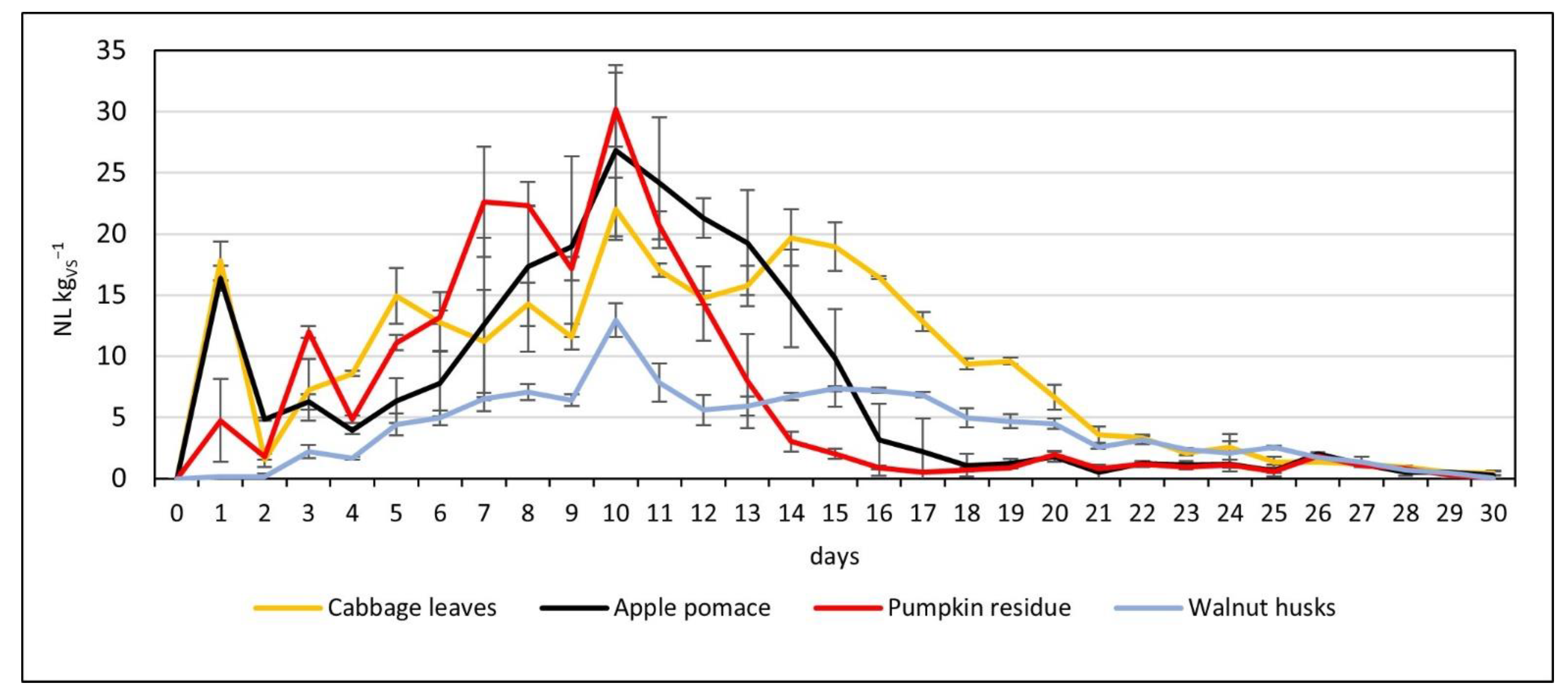
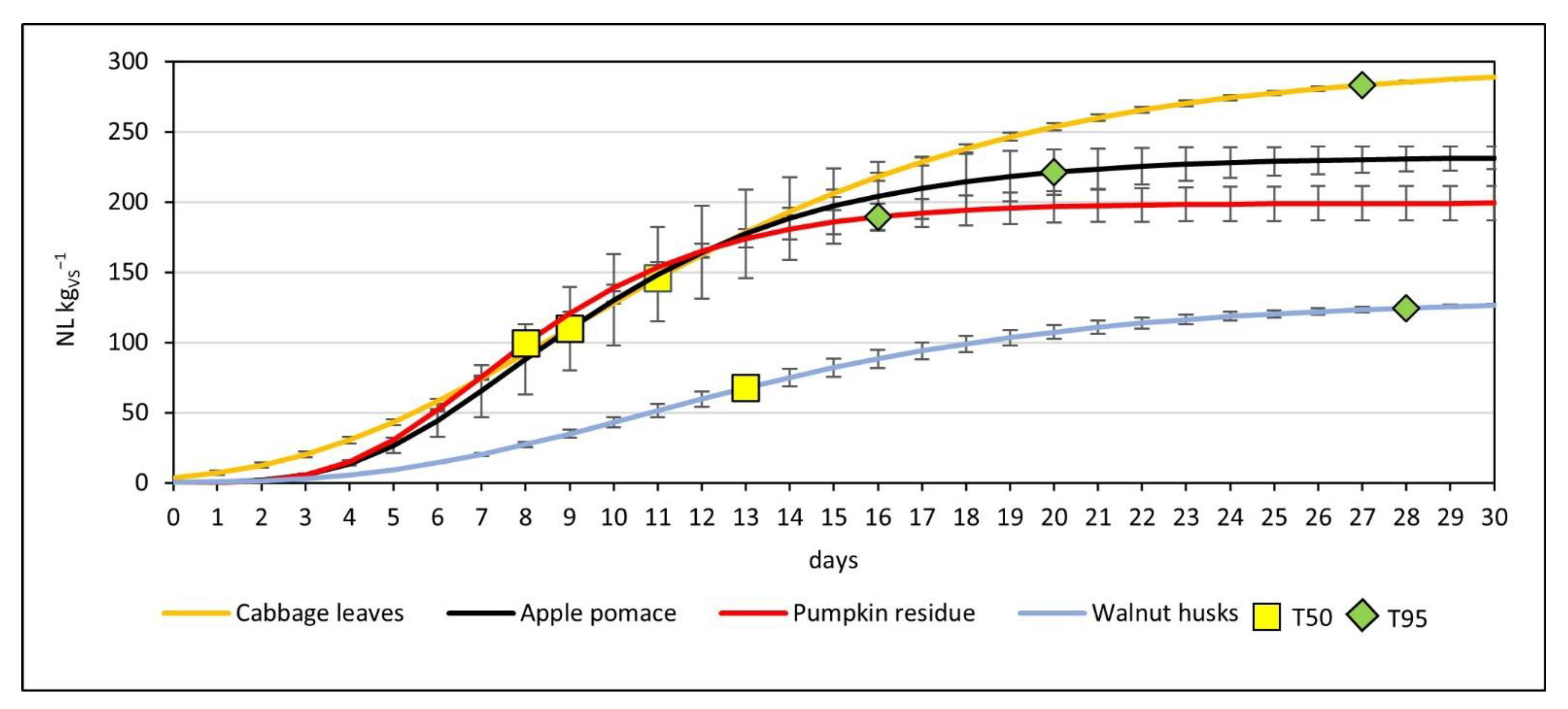
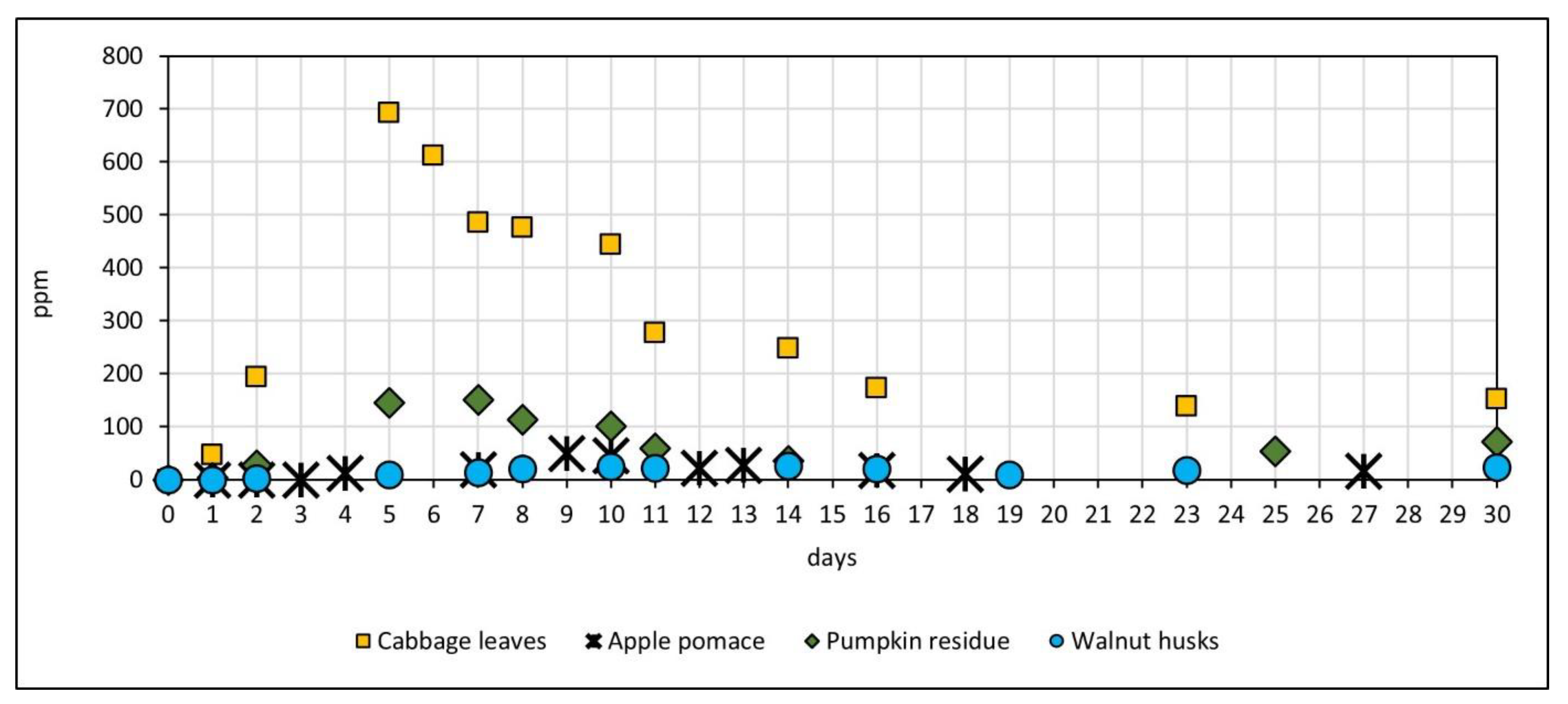
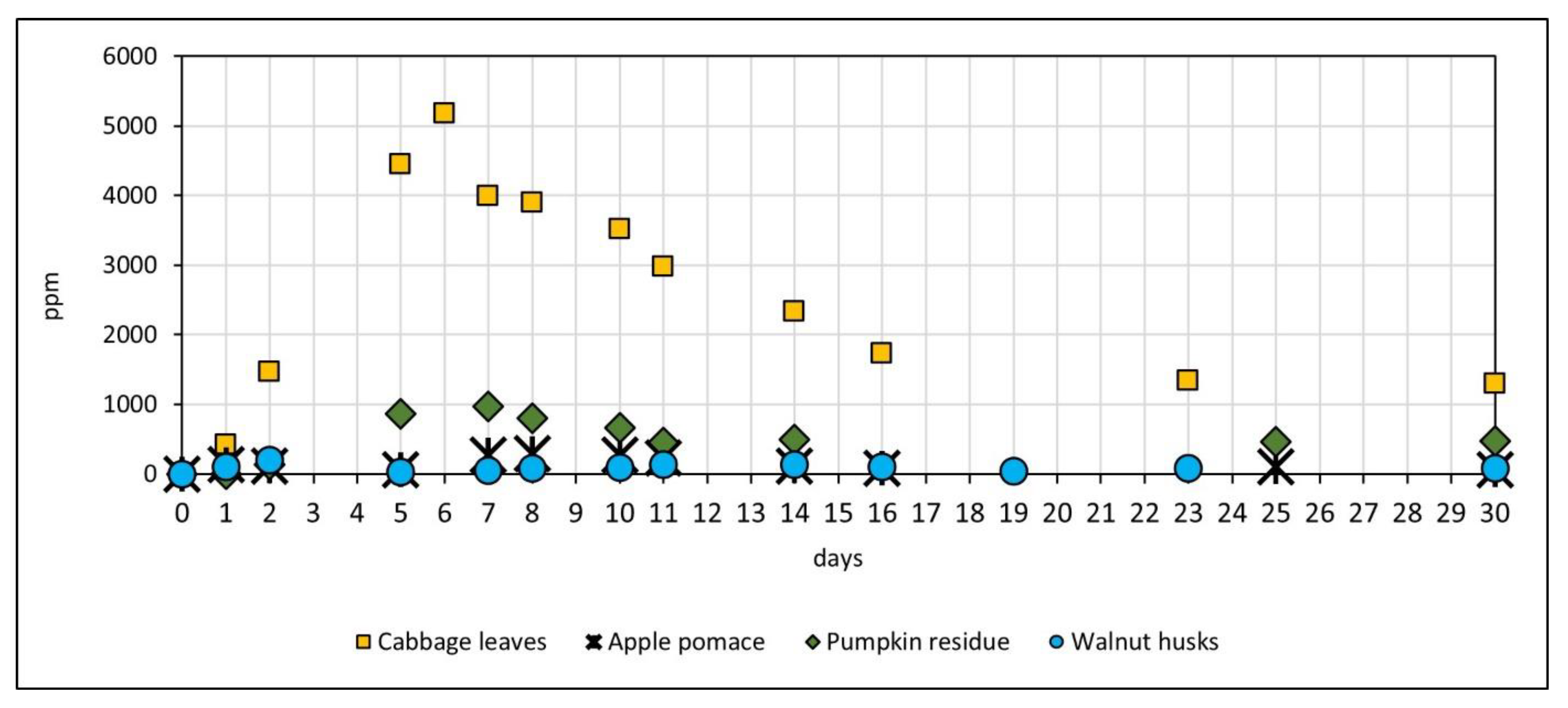
3.2. Production of Methane
3.3. Energy Production and Potential GHG Reduction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, S.N.; Sharma, M.; Lata, K.; Singh, U.; Kumar, V.; Sangwan, R.S.; Singh, S.P. Improved Operational Stability of d -psicose 3-epimerase by a Novel Protein Engineering Strategy, and d -psicose Production from Fruit and Vegetable Residues. Bioresour. Technol. 2016, 216, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.D.; Reay, D.S.; Higgins, P.; Bomberg, E. A Half-Century of Production-Phase Greenhouse Gas Emissions from Food Loss & Waste in the Global Food Supply Chain. Sci. Total Environ. 2016, 571, 721–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. The State of Food and Agriculture 2019. In Moving Forward on Food Loss and Waste Reduction; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Gustavsson, J. (Ed.) Global Food Losses and Food Waste: Extent, Causes and Prevention; Study Conducted for the International Congress Save Food! At Interpack 2011, [16–17 May], Düsseldorf, Germany; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011; ISBN 978-92-5-107205-9. [Google Scholar]
- Šelo, G.; Planinić, M.; Tišma, M.; Tomas, S.; Koceva Komlenić, D.; Bucić-Kojić, A. A Comprehensive Review on Valorization of Agro-Food Industrial Residues by Solid-State Fermentation. Foods 2021, 10, 927. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, N.; Arendt, E.; Gallagher, E. Enhancing an Extruded Puffed Snack by Optimising Die Head Temperature, Screw Speed and Apple Pomace Inclusion. Food Bioprocess Technol. 2014, 7, 1767–1782. [Google Scholar] [CrossRef]
- KOWR. Poland Tastes Good. A Brief Guide to Polish Agri-Food Offer; National Support Centre for Agriculture: Warsaw, Poland, 2021. [Google Scholar]
- Statistics Poland. Production of Agricultural and Horticultural Crops in 2021, 1st ed.; Zakład Wydawnictw Statystycznych: Warsaw, Poland, 2022. [Google Scholar]
- Eurostat. Crop Production in EU Standard Humidity. Available online: https://ec.europa.eu/eurostat/databrowser/view/APRO_CPSH1__custom_2724766/default/table?lang=en (accessed on 28 April 2022).
- FAOSTAT. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 30 April 2022).
- Płocharski, W.; Mieszczakowska-Frąc, M.; Rutkowski, K.; Konopacka, D. Traditional and Innovative Directions of Apple Management in Poland; Instytut Ogrodnictwa: Skierniewice, Poland, 2019. (In Polish) [Google Scholar]
- Vendruscolo, F.; Albuquerque, P.M.; Streit, F.; Esposito, E.; Ninow, J.L. Apple Pomace: A Versatile Substrate for Biotechnological Applications. Crit. Rev. Biotechnol. 2008, 28, 1–12. [Google Scholar] [CrossRef] [PubMed]
- De Laurentiis, V.; Corrado, S.; Sala, S. Quantifying Household Waste of Fresh Fruit and Vegetables in the EU. Waste Manag. 2018, 77, 238–251. [Google Scholar] [CrossRef]
- Seabra, I.J.; Braga, M.E.M.; Oliveira, R.A.; de Sousa, H.C. Two-Step High Pressure Solvent Extraction of Walnut (Juglans regia L.) Husks: ScCO2 + CO2/Ethanol/H2O. J. CO2 Util. 2019, 34, 375–385. [Google Scholar] [CrossRef]
- Directive (EU) 2018/851 of the European Parliament and of the Council of 30 May 2018 Amending Directive 2008/98/EC on Waste (Text with EEA Relevance). O. J. Eur. Union L. 2018, 328, 82–209.
- Neto, J.G.; Ozorio, L.V.; Campos de Abreu, T.C.; Ferreira dos Santos, B.; Pradelle, F. Modeling of Biogas Production from Food, Fruits and Vegetables Wastes Using Artificial Neural Network (ANN). Fuel 2021, 285, 119081. [Google Scholar] [CrossRef]
- Md Noh, N.A.N.; Karim, L.; Omar, S.R. Value-Added Products from Pumpkin Wastes: A Review. Malays. J. Sci. Health Technol. 2022, 8, 77–84. [Google Scholar] [CrossRef]
- Bijmans, M.F.M.; Buisman, C.J.N.; Meulepas, R.J.W.; Lens, P.N.L. Sulfate Reduction for Inorganic Waste and Process Water Treatment. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Burlington, NJ, USA, 2011; pp. 435–446. ISBN 978-0-08-088504-9. [Google Scholar]
- Mu, H.; Zhao, C.; Zhao, Y.; Li, Y.; Hua, D.; Zhang, X.; Xu, H. Enhanced Methane Production by Semi-Continuous Mesophilic Co-Digestion of Potato Waste and Cabbage Waste: Performance and Microbial Characteristics Analysis. Bioresour. Technol. 2017, 236, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Tamelová, B.; Malaťák, J.; Velebil, J. Hydrothermal Carbonization and Torrefaction of Cabbage Waste. Agron. Res. 2019, 17, 862–871. [Google Scholar] [CrossRef]
- Dahunsi, S.O.; Olayanju, A.; Izebere, J.O.; Oluyori, A.P. Data on Energy and Economic Evaluation and Microbial Assessment of Anaerobic Co-Digestion of Fruit Rind of Telfairia occidentalis (Fluted Pumpkin) and Poultry Manure. Data Brief 2018, 21, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Dahunsi, S.O.; Oranusi, S.; Owolabi, J.B.; Efeovbokhan, V.E. Comparative Biogas Generation from Fruit Peels of Fluted Pumpkin (Telfairia occidentalis) and Its Optimization. Bioresour. Technol. 2016, 221, 517–525. [Google Scholar] [CrossRef]
- Dahunsi, S.O.; Olayanju, T.M.A.; Adesulu-Dahunsi, A.T. Data on Optimization of Bioconversion of Fruit Rind of Telfairia occidentalis (Fluted Pumpkin) and Poultry Manure for Biogas Generation. Chem. Data Collect. 2019, 20, 100192. [Google Scholar] [CrossRef] [Green Version]
- Scarlat, N.; Dallemand, J.-F.; Fahl, F. Biogas: Developments and Perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Obaideen, K.; Abdelkareem, M.A.; Wilberforce, T.; Elsaid, K.; Sayed, E.T.; Maghrabie, H.M.; Olabi, A.G. Biogas Role in Achievement of the Sustainable Development Goals: Evaluation, Challenges, and Guidelines. J. Taiwan Inst. Chem. Eng. 2022, 131, 104207. [Google Scholar] [CrossRef]
- UN. Transforming Our World: The 2030 Agenda for Sustainable Development. Available online: https://sdgs.un.org/2030agenda (accessed on 4 May 2022).
- Feiz, R.; Larsson, M.; Ekstrand, E.-M.; Hagman, L.; Ometto, F.; Tonderski, K. The Role of Biogas Solutions for Enhanced Nutrient Recovery in Biobased Industries—Three Case Studies from Different Industrial Sectors. Resour. Conserv. Recycl. 2021, 175, 105897. [Google Scholar] [CrossRef]
- Esposito, E.; Dellamuzia, L.; Moretti, U.; Fuoco, A.; Giorno, L.; Jansen, J.C. Simultaneous Production of Biomethane and Food Grade CO2 from Biogas: An Industrial Case Study. Energy Environ. Sci. 2019, 12, 281–289. [Google Scholar] [CrossRef]
- Golmakani, A.; Nabavi, S.A.; Manović, V. Production of Negative-Emission Biomethane by Twin Double-Bed Pressure Swing Adsorption with Tail Gas Sequestration. Chem. Eng. J. 2021, 408, 127312. [Google Scholar] [CrossRef]
- Fagerström, A.; Al Seadi, T.; Rasi, S.; Briseid, T.; Murphy, J.D.; IEA Bioenergy Task 37; IEA Bioenergy Programme. The Role of Anaerobic Digestion and Biogas in the Circular Economy; IEA Bioenergy: Cork, Ireland, 2018; ISBN 978-1-910154-45-8. [Google Scholar]
- Banaszuk, P.; Wysocka-Czubaszek, A.; Czubaszek, R.; Roj-Rojewski, S. Implications of Biomass Use for Energy Production. Wieś I Rol. 2015, 169, 139–152. (In Polish) [Google Scholar]
- Prochnow, A.; Heiermann, M.; Plöchl, M.; Linke, B.; Idler, C.; Amon, T.; Hobbs, P.J. Bioenergy from Permanent Grassland—A Review: 1. Biogas. Bioresour. Technol. 2009, 100, 4931–4944. [Google Scholar] [CrossRef]
- Feiz, R.; Metson, G.S.; Wretman, J.; Ammenberg, J. Key Factors for Site-Selection of Biogas Plants in Sweden. J. Clean. Prod. 2022, 354, 131671. [Google Scholar] [CrossRef]
- Golmakani, A.; Ali Nabavi, S.; Wadi, B.; Manovic, V. Advances, Challenges, and Perspectives of Biogas Cleaning, Upgrading, and Utilisation. Fuel 2022, 317, 123085. [Google Scholar] [CrossRef]
- Gupta, A.; Behl, T.; Panichayupakaranan, P. A Review of Phytochemistry and Pharmacology Profile of Juglans regia. Obes. Med. 2019, 16, 100142. [Google Scholar] [CrossRef]
- Kafle, G.K.; Kim, S.H. Effects of Chemical Compositions and Ensiling on the Biogas Productivity and Degradation Rates of Agricultural and Food Processing By-Products. Bioresour. Technol. 2013, 142, 553–561. [Google Scholar] [CrossRef]
- Scano, E.A.; Grosso, M.; Pistis, A.; Carboni, G.; Cocco, D. An In-Depth Analysis of Biogas Production from Locally Agro-Industrial by-Products and Residues. An Italian Case. Renew. Energy 2021, 179, 308–318. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Kobayashi, T.; Kumar, G.; Xu, K. Anaerobic Co-Digestion on Improving Methane Production from Mixed Microalgae (Scenedesmus sp., Chlorella sp.) and Food Waste: Kinetic Modeling and Synergistic Impact Evaluation. Chem. Eng. J. 2016, 299, 332–341. [Google Scholar] [CrossRef]
- Yan, H.; Zhao, C.; Zhang, J.; Zhang, R.; Xue, C.; Liu, G.; Chen, C. Study on Biomethane Production and Biodegradability of Different Leafy Vegetables in Anaerobic Digestion. AMB Express 2017, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Czubaszek, R.; Wysocka-Czubaszek, A.; Banaszuk, P. GHG Emissions and Efficiency of Energy Generation through Anaerobic Fermentation of Wetland Biomass. Energies 2020, 13, 6497. [Google Scholar] [CrossRef]
- Sagagi, B.; Garba, B.; Usman, N. Studies on Biogas Production from Fruits and Vegetable Waste. Bayero J. Pure Appl. Sci. 2010, 2, 115–118. [Google Scholar] [CrossRef]
- Chakravarty, I.; Mandavgane, S.A. Valorization of Fruit and Vegetable Waste for Biofertilizer and Biogas. J. Food Process. Eng. 2021, 44, e13512. [Google Scholar] [CrossRef]
- Miramontes-Martínez, L.R.; Rivas-García, P.; Albalate-Ramírez, A.; Botello-Álvarez, J.E.; Escamilla-Alvarado, C.; Gomez-Gonzalez, R.; Alcalá-Rodríguez, M.M.; Valencia-Vázquez, R.; Santos-López, I.A. Anaerobic Co-Digestion of Fruit and Vegetable Waste: Synergy and Process Stability Analysis. J. Air Waste Manag. Ass. 2021, 71, 620–632. [Google Scholar] [CrossRef]
- Sitorus, B.; Sukandar; Panjaitan, S.D. Biogas Recovery from Anaerobic Digestion Process of Mixed Fruit -Vegetable Wastes. Energy Procedia 2013, 32, 176–182. [Google Scholar] [CrossRef] [Green Version]
- Pilarska, A.A.; Pilarski, K.; Ryniecki, A.; Tomaszyk, K.; Dach, J.; Wolna-Maruwka, A. Utilization of Vegetable Dumplings Waste from Industrial Production by Anaerobic Digestion. Int. Agrophys. 2017, 31, 93–102. [Google Scholar] [CrossRef]
- Morales-Polo, C.; Cledera-Castro, M.M.; Soria, B.Y.M. Biogas Production from Vegetable and Fruit Markets Waste—Compositional and Batch Characterizations. Sustainability 2019, 11, 6790. [Google Scholar] [CrossRef] [Green Version]
- Tura, A.M.; Lemma, T.S. Production and Evaluation of Biogas from Mixed Fruits and Vegetable Wastes Collected from Arba Minch Market. Am. J. Appl. Chem. 2019, 7, 185. [Google Scholar] [CrossRef]
- Pavi, S.; Kramer, L.E.; Gomes, L.P.; Miranda, L.A.S. Biogas Production from Co-Digestion of Organic Fraction of Municipal Solid Waste and Fruit and Vegetable Waste. Bioresour. Technol. 2017, 228, 362–367. [Google Scholar] [CrossRef]
- Phetyim, N.; Wanthong, T.; Kannika, P.; Supngam, A. Biogas Production from Vegetable Waste by Using Dog and Cattle Manure. Energy Procedia 2015, 79, 436–441. [Google Scholar] [CrossRef] [Green Version]
- Shen, F.; Yuan, H.; Pang, Y.; Chen, S.; Zhu, B.; Zou, D.; Liu, Y.; Ma, J.; Yu, L.; Li, X. Performances of Anaerobic Co-Digestion of Fruit & Vegetable Waste (FVW) and Food Waste (FW): Single-Phase vs. Two-Phase. Bioresour. Technol. 2013, 144, 80–85. [Google Scholar] [CrossRef]
- Deressa, L.; Libsu, S.; Chavan, R.B.; Manaye, D.; Dabassa, A. Production of Biogas from Fruit and Vegetable Wastes Mixed with Different Wastes. Environ. Ecol. Res. 2015, 3, 65–71. [Google Scholar] [CrossRef]
- Wilinska-Lisowska, A.; Ossowska, M.; Czerwionka, K. The Influence of Co-Fermentation of Agri-Food Waste with Primary Sludge on Biogas Production and Composition of the Liquid Fraction of Digestate. Energies 2021, 14, 1907. [Google Scholar] [CrossRef]
- Wang, K.; Yun, S.; Xing, T.; Li, B.; Abbas, Y.; Liu, X. Binary and Ternary Trace Elements to Enhance Anaerobic Digestion of Cattle Manure: Focusing on Kinetic Models for Biogas Production and Digestate Utilization. Bioresour. Technol. 2021, 323, 124571. [Google Scholar] [CrossRef] [PubMed]
- Clesceri, L.S.; American Public Health Association; American Water Works Association; Water Pollution Control Federation (Eds.) Standard Methods: For the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998; ISBN 978-0-87553-235-6. [Google Scholar]
- Curkowski, A.; Mroczkowski, P.; Oniszk-Popławska, A.; Wiśniewski, G. Agricultural Biogas—Production and Usage; Mazowiecka Agencja Energetyczna: Warszawa, Poland, 2009. [Google Scholar]
- Selvam, A.; Ilamathi, P.M.K.; Udayakumar, M.; Murugesan, K.; Banu, J.R.; Khanna, Y.; Wong, J. Food Waste Properties. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2021; pp. 11–41. ISBN 978-0-12-819148-4. [Google Scholar]
- Al Seadi, T.; Rutz, D.; Prassl, H.; Köttner, M.; Finsterwalder, T.; Volk, S.; Janssen, R. Biogas Handbook; University of Southern Denmark Esbjerg: Esbjerg, Denmark, 2008. [Google Scholar]
- Jung, J.; Cavender, G.; Zhao, Y. Impingement Drying for Preparing Dried Apple Pomace Flour and Its Fortification in Bakery and Meat Products. J. Food Sci. Technol. 2015, 52, 5568–5578. [Google Scholar] [CrossRef]
- Fernandes, P.A.R.; Ferreira, S.S.; Bastos, R.; Ferreira, I.; Cruz, M.T.; Pinto, A.; Coelho, E.; Passos, C.P.; Coimbra, M.A.; Cardoso, S.M.; et al. Apple Pomace Extract as a Sustainable Food Ingredient. Antioxidants 2019, 8, 189. [Google Scholar] [CrossRef] [Green Version]
- Gajewski, M.; Radzanowska, J. Chemical Composition and Sensory Quality of White Cabbage Depending on the Cabbage Cultivar and Nitrogen Dose as Applied in Mineral Fertilization. Żywność. Nauka Technol. Jakość. 2004, 2, 108–120. [Google Scholar]
- de Rezende, A.V.; Rabelo, C.H.S.; da Silva, M.R.M.; Härter, C.J.; Veiga, R.M. Wasted Cabbage (Brassica oleracea) Silages Treated with Different Levels of Ground Corn Andsilage Inoculant. R. Bras. Zootec. 2015, 44, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.Y.; Kim, E.J.; Kim, Y.-N.; Choi, C.; Lee, B.-H. Comparison of the Chemical Compositions and Nutritive Values of Various Pumpkin (Cucurbitaceae) Species and Parts. Nutr. Res. Pract. 2012, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Valdez-Arjona, L.P.; Ramírez-Mella, M. Pumpkin Waste as Livestock Feed: Impact on Nutrition and Animal Health and on Quality of Meat, Milk, and Egg. Animals 2019, 9, 769. [Google Scholar] [CrossRef] [Green Version]
- Nansikombi, N.; Muyonga, J.H.; Byaruhanga, Y.B. Association between Fruit Characteristics and Postharvest Stability of Different Pumpkin (Cucurbita) Species. J. Food Res. 2019, 8, 131. [Google Scholar] [CrossRef]
- Calvete-Torre, I.; Muñoz-Almagro, N.; Pacheco, M.T.; Antón, M.J.; Dapena, E.; Ruiz, L.; Margolles, A.; Villamiel, M.; Moreno, F.J. Apple Pomaces Derived from Mono-Varietal Asturian Ciders Production Are Potential Source of Pectins with Appealing Functional Properties. Carbohydr. Polym. 2021, 264, 117980. [Google Scholar] [CrossRef] [PubMed]
- de Evan, T.; Vintimilla, A.; Marcos, C.N.; Ranilla, M.J.; Carro, M.D. Evaluation of Brassica Vegetables as Potential Feed for Ruminants. Animals 2019, 9, 588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shilev, S.; Naydenov, M.; Vancheva, V.; Aladjadjiyan, A. Composting of Food and Agricultural Wastes. In Utilization of By-Products and Treatment of Waste in the Food Industry; Oreopoulou, V., Russ, W., Eds.; Springer: New York, NY, USA, 2007; pp. 283–301. ISBN 978-0-387-33511-7. [Google Scholar]
- Cardenas, R.R.; Wang, L.K. Composting Process. In Solid Waste Processing and Resource Recovery; Wang, L.K., Pereira, N.C., Eds.; Humana Press: Totowa, NJ, USA, 1980; pp. 269–327. ISBN 978-1-4612-5994-7. [Google Scholar]
- Tanimu, M.I.; Ghazi, T.I.M.; Harun, R.M.; Idris, A. Effect of Carbon to Nitrogen Ratio of Food Waste on Biogas Methane Production in a Batch Mesophilic Anaerobic Digester. Int. J. Innov. 2014, 5, 116–119. [Google Scholar]
- Kwietniewska, E.; Tys, J. Process Characteristics, Inhibition Factors and Methane Yields of Anaerobic Digestion Process, with Particular Focus on Microalgal Biomass Fermentation. Renew. Sustain. Energy Rev. 2014, 34, 491–500. [Google Scholar] [CrossRef]
- European Commission. Joint Research Centre. Solid and Gaseous Bioenergy Pathways: Input Values and GHG Emissions: Calculated According to the Methodology Set in COM(2016) 767; Publications Office: Luxembourg, 2017. [Google Scholar]
- Gunaseelan, V.N. Biochemical Methane Potential of Fruits and Vegetable Solid Waste Feedstocks. Biomass Bioenergy 2004, 26, 389–399. [Google Scholar] [CrossRef]
- Sapkota, T.; Aryal, J.; Thapa, S.; Karki, A.B. Biogas Production from Anaerobic Digestion of Different Biodegradable Materials. Nepal J. Sci. Technol. 2012, 13, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Smurzyńska, A.; Czekała, W.; Lewicki, A.; Cieślik, M.; Kozłowski, K.; Janczak, D. The Biogas Output of Vegetables Utilized in the Polish Market Due to the Introduction of the Russian Embargo. Tech. Rol. Ogrod. Leśna 2016, 6, 24–27. [Google Scholar]
- Dubrovskis, V.; Plume, I. Biogas from Wastes of Pumpkin, Marrow and Apple. Agron. Res. 2017, 15, 069–078. [Google Scholar]
- Nawirska, A.; Sokół-Łętowska, A.; Kucharska, A.Z.; Biesiada, A.; Bednarek, M. Comparing the contents of dietary fibre fractions in some varieties of Cucurbita maxima and Cucurbita pepo. Żywność. Nauka Technol. Jakość. 2008, 1, 65–73. (In Polish) [Google Scholar]
- Komolka, P.; Górecka, D.; Dziedzic, K. The Effect of Thermal Processing of Cruciferous Vegetables on Their Content of Dietary Fiber and Its Fractions. Acta Sci. Pol. Technol. Aliment. 2012, 11, 347–354. [Google Scholar]
- Ji, C.; Kong, C.-X.; Mei, Z.-L.; Li, J. A Review of the Anaerobic Digestion of Fruit and Vegetable Waste. Appl. Biochem. Biotechnol. 2017, 183, 906–922. [Google Scholar] [CrossRef] [PubMed]
- Czubaszek, R.; Wysocka-Czubaszek, A.; Wichtmann, W.; Banaszuk, P. Specific Methane Yield of Wetland Biomass in Dry and Wet Fermentation Technologies. Energies 2021, 14, 8373. [Google Scholar] [CrossRef]
- Dragoni, F.; Giannini, V.; Ragaglini, G.; Bonari, E.; Silvestri, N. Effect of Harvest Time and Frequency on Biomass Quality and Biomethane Potential of Common Reed (Phragmites australis) Under Paludiculture Conditions. Bioenerg. Res. 2017, 10, 1066–1078. [Google Scholar] [CrossRef]
- Anhuradha, S.; Arrrivukkarasan, S. Potentiality of Fruit and Vegetable Waste by Anaerobic Co-Digestion with Municipal Sewage Sludge and Biogas Yield; AIP Publishing: Puducherry, India, 2020; p. 070003. [Google Scholar]
- Kupryś-Caruk, M.; Kołodziejski, R. Evaluation of Ensilaged Apple Pomace Usefulness for Biogas Production. Post. Nauk. Technol. Przem. Roln. Spoż. 2014, 69, 5–13. [Google Scholar]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of Anaerobic Digestion Process: A Review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D.I.; Singh, G. A Critical Review on Inhibition of Anaerobic Digestion Process by Excess Ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef]
- Westerholm, M.; Moestedt, J.; Schnürer, A. Biogas Production through Syntrophic Acetate Oxidation and Deliberate Operating Strategies for Improved Digester Performance. Appl. Energy 2016, 179, 124–135. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Fotidis, I.A.; Kissas, K.; Angelidaki, I. Effect of Different Ammonia Sources on Aceticlastic and Hydrogenotrophic Methanogens. Bioresour. Technol. 2018, 250, 390–397. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Wang, W.; He, Y.; Zhang, R.; Liu, G. Effect of Ammonia on Methane Production, Methanogenesis Pathway, Microbial Community and Reactor Performance under Mesophilic and Thermophilic Conditions. Renew. Energy 2018, 125, 915–925. [Google Scholar] [CrossRef]
- Kalamaras, S.D.; Vitoulis, G.; Christou, M.L.; Sfetsas, T.; Tziakas, S.; Fragos, V.; Samaras, P.; Kotsopoulos, T.A. The Effect of Ammonia Toxicity on Methane Production of a Full-Scale Biogas Plant—An Estimation Method. Energies 2021, 14, 5031. [Google Scholar] [CrossRef]
- Theuerl, S.; Klang, J.; Prochnow, A. Process Disturbances in Agricultural Biogas Production—Causes, Mechanisms and Effects on the Biogas Microbiome: A Review. Energies 2019, 12, 365. [Google Scholar] [CrossRef] [Green Version]
- Krakat, N.; Demirel, B.; Anjum, R.; Dietz, D. Methods of Ammonia Removal in Anaerobic Digestion: A Review. Water Sci. Technol. 2017, 76, 1925–1938. [Google Scholar] [CrossRef] [PubMed]
- Mamun, M.R.A.; Torii, S. Removal of Hydrogen Sulfide (H2S) from Biogas Using Zero-Valent Iron. J. Clean Energy Technol. 2015, 3, 428–432. [Google Scholar] [CrossRef] [Green Version]
- Żarczyński, A.; Rosiak, K.; Anielak, P.; Wolf, W. Practical Methods of Cleaning Biogas from Hydrogen Sulphide. Part 1. Application of Solid Sorbents. Acta Innov. 2014, 12, 24–34. [Google Scholar]
- Czatzkowska, M.; Harnisz, M.; Korzeniewska, E.; Koniuszewska, I. Inhibitors of the Methane Fermentation Process with Particular Emphasis on the Microbiological Aspect: A Review. Energy Sci. Eng. 2020, 8, 1880–1897. [Google Scholar] [CrossRef] [Green Version]
- Tahir, M.S.; Shahzad, K.; Shahid, Z.; Sagir, M.; Rehan, M.; Nizami, A. Producing Methane Enriched Biogas Using Solvent Absorption Method. Chem. Eng. Trans. 2015, 45, 1309–1314. [Google Scholar] [CrossRef]
- Choudhury, A.; Shelford, T.; Felton, G.; Gooch, C.; Lansing, S. Evaluation of Hydrogen Sulfide Scrubbing Systems for Anaerobic Digesters on Two U.S. Dairy Farms. Energies 2019, 12, 4605. [Google Scholar] [CrossRef] [Green Version]
- Nkosi, B.; Meeske, R.; Ratsaka, M.; Langa, T.; Motiang, M.; Groenewald, I. Effects of Dietary Inclusion of Discarded Cabbage (Brassica Oleracea Var. Capitata) on the Growth Performance of South African Dorper Lambs. S. Afr. J. Anim. Sci. 2016, 46, 35. [Google Scholar] [CrossRef] [Green Version]
- Tanongkankit, Y.; Chiewchan, N.; Devahastin, S. Physicochemical Property Changes of Cabbage Outer Leaves upon Preparation into Functional Dietary Fiber Powder. Food Bioprod. Process. 2012, 90, 541–548. [Google Scholar] [CrossRef]
- Nilnakara, S.; Chiewchan, N.; Devahastin, S. Production of Antioxidant Dietary Fibre Powder from Cabbage Outer Leaves. Food Bioprod. Process. 2009, 87, 301–307. [Google Scholar] [CrossRef]
- Ramos, D.E. Walnut Production Manual; UCANR Publications: Oakland, CA, USA, 1997; ISBN 978-1-879906-27-3. [Google Scholar]
- Lyu, F.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ajlouni, S.; Ranadheera, C.S. Apple Pomace as a Functional and Healthy Ingredient in Food Products: A Review. Processes 2020, 8, 319. [Google Scholar] [CrossRef] [Green Version]
- Njokweni, S.G.; Weimer, P.J.; Botes, M.; van Zyl, W.H. Effects of Preservation of Rumen Inoculum on Volatile Fatty Acids Production and the Community Dynamics during Batch Fermentation of Fruit Pomace. Bioresour. Technol. 2021, 321, 124518. [Google Scholar] [CrossRef]
- Erinle, T.J.; Adewole, D.I. Fruit Pomaces—Their Nutrient and Bioactive Components, Effects on Growth and Health of Poultry Species, and Possible Optimization Techniques. Anim. Nutr. 2022, 9, 357–377. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Ye, F.; Zhou, G.; Gao, R.; Qin, D.; Zhao, G. Micronized Apple Pomace as a Novel Emulsifier for Food O/W Pickering Emulsion. Food Chem. 2020, 330, 127325. [Google Scholar] [CrossRef] [PubMed]
- Madrera, R.R.; Bedriñana, R.P.; Valles, B.S. Enhancement of the Nutritional Properties of Apple Pomace by Fermentation with Autochthonous Yeasts. Food Sci. Technol. 2017, 79, 27–33. [Google Scholar] [CrossRef]
- Mazurkiewicz, J.; Marczuk, A.; Pochwatka, P.; Kujawa, S. Maize Straw as a Valuable Energetic Material for Biogas Plant Feeding. Materials 2019, 12, 3848. [Google Scholar] [CrossRef] [Green Version]
- Przerwa, M. Innovative Methods of Vegetable Storage; Centrum Doradztwa Rolniczego w Brwinowie, Oddział w Radomiu: Radom, Poland, 2015. [Google Scholar]
- Watkins, C.B.; Nock, J.F. Production Guide for Storage of Organic Fruits and Vegetables; Department of Horticulture, Cornell University: New York, NY, USA, 2012. [Google Scholar]
- Halik, G.D.; Łozicki, A.; Koziorzębska, A.; Dymnicka, M.; Arkuszewska, E. Effect of Ensiling Pumpkin Cucurbita Maxima with the Addition of Inoculant or without It on Chemical Composition and Quality of Silages. Ann. Wars. Univ. Life Sci. SGGW Anim. Sci. 2014, 53, 103–110. [Google Scholar]
- Ülger, İ.; Kaliber, M.; Büyükkiliҫ Beyzi, S.; Konca, Y. Possible Ensiling of Pumpkin (Cucurbita pepo) Residues. Turk. J. Vet. Anim. Sci. 2020, 44, 853–859. [Google Scholar] [CrossRef]
- Koppelmäki, K.; Helenius, J.; Schulte, R.P.O. Nested Circularity in Food Systems: A Nordic Case Study on Connecting Biomass, Nutrient and Energy Flows from Field Scale to Continent. Resour. Conserv. Recycl. 2021, 164, 105218. [Google Scholar] [CrossRef]
- Camargo, G.G.T.; Ryan, M.R.; Richard, T.L. Energy Use and Greenhouse Gas Emissions from Crop Production Using the Farm Energy Analysis Tool. BioScience 2013, 63, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Holka, M.; Bieńkowski, J. Carbon Footprint and Life-Cycle Costs of Maize Production in Conventional and Non-Inversion Tillage Systems. Agronomy 2020, 10, 1877. [Google Scholar] [CrossRef]
- Walkowska, K. Energy Consumption in Households in 2018; Statistics Poland, Enterprises Department, Zakład Wydawnictw Statystycznych: Warsaw, Poland, 2019. Available online: https://stat.gov.pl/en/topics/environment-energy/energy/energyconsumption-in-households-in-2018,2,5.html (accessed on 8 June 2022).
| Inoculum | Apple Pomace | Pumpkin Residue | Walnut Husks | Cabbage Leaves | |
|---|---|---|---|---|---|
| Total solids (TS),% | 4.52 ± 0.03 a* | 19.41 ± 0.01 b | 6.13 ± 1.33 ac | 14.54 ± 1.09 d | 7.45 ± 0.85 c |
| Volatile solids (VS), %TS | 74.53 ± 0.01 a | 98.75 ± 0.36 b | 85.74 ± 2.18 c | 89.11 ± 1.09 d | 90.31 ± 2.40 d |
| Total Kjeldahl nitrogen (TKN), g kgDM−1 | 29.45 ± 4.84 a | 9.16 ± 3.82 b | 31.00 ± 6.43 a | 8.26 ± 0.04 b | 26.05 ± 0.88 a |
| Total phosphorus (TP), g kgDM−1 | 12.37 ± 0.18 a | 0.92 ± 0.01 b | 4.76 ± 0.95 c | 0.62 ± 0.05 b | 4.12 ± 0.36 c |
| Total potassium (K), g kgDM−1 | 60.54 ± 188 a | 6.69 ± 0.07 b | 57.12 ± 4.27 a | 41.36 ± 5.03 c | 27.51 ± 0.26 d |
| Total organic carbon (TOC), g kgDM−1 | 410.78 ± 2.19 a | 432.77 ± 5.24 b | 425.26 ± 1.28 ba | 442.15 ± 0.97 b | 426.27 ± 21.44 ba |
| C:N | 14 | 47 | 14 | 54 | 16 |
| N:P | 2 | 10 | 7 | 13 | 6 |
| Residues | Methane Production | Maximum of Daily Methane Production | Lag Phase |
|---|---|---|---|
| Cabbage leaves | 297.81 ± 0.65 | 22.05 ± 2.56 | 4.31 ± 0.68 |
| Apple pomace | 232.20 ± 6.88 | 26.83 ± 7.00 | 4.18 ± 0.11 |
| Pumpkin residue | 199.18 ± 12.45 | 30.18 ± 3.04 | 4.01 ± 0.13 |
| Walnut husks | 131.07 ± 1.30 | 12.98 ± 1.38 | 3.87 ± 0.06 |
| Residues | Electricity | Heat | Electricity | Heat |
|---|---|---|---|---|
| kWh tVS−1 | GJ tVS−1 | kWh ha−1 | GJ ha−1 | |
| Cabbage leaves | 944.35 ± 2.06 | 2.96 ± 0.01 | 594.94 ± 1.30 | 1.86 ± 0.004 |
| Apple pomace | 736.32 ± 21.82 | 2.31 ± 0.07 | 1060.30 ± 31.42 | 3.32 ± 1.09 |
| Pumpkin residue | 631.58 ± 39.49 | 1.98 ± 0.12 | 284.21 ± 17.77 | 0.89 ± 0.06 |
| Walnut husks | 415.62 ± 4.13 | 1.30 ± 0.01 | 62.34 ± 0.62 | 0.20 ± 0.002 |
| Maize sillage | 1093.99 | 3.43 | 14,703.23 | 46.07 |
| Residues | Cultivated Area of Crops in Poland | Maize Field Area Equivalent to 1 ha of Crops | Maize Field Area Equivalent to Area of Crop Cultivation in Poland | Reduction in GHG Emissions Resulting from the Use of Waste for Biogas Production |
|---|---|---|---|---|
| [ha] | [ha] | [ha] | [t CO2] | |
| Cabbage leaves | 14,599 | 0.041 | 598 | 2106 |
| Apple pomace | 161,948 | 0.072 | 11,660 | 41,066 |
| Pumpkin residue | 6700 | 0.020 | 134 | 472 |
| Walnut husks | 2700 | 0.004 | 11 | 38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czubaszek, R.; Wysocka-Czubaszek, A.; Tyborowski, R. Methane Production Potential from Apple Pomace, Cabbage Leaves, Pumpkin Residue and Walnut Husks. Appl. Sci. 2022, 12, 6128. https://doi.org/10.3390/app12126128
Czubaszek R, Wysocka-Czubaszek A, Tyborowski R. Methane Production Potential from Apple Pomace, Cabbage Leaves, Pumpkin Residue and Walnut Husks. Applied Sciences. 2022; 12(12):6128. https://doi.org/10.3390/app12126128
Chicago/Turabian StyleCzubaszek, Robert, Agnieszka Wysocka-Czubaszek, and Rafał Tyborowski. 2022. "Methane Production Potential from Apple Pomace, Cabbage Leaves, Pumpkin Residue and Walnut Husks" Applied Sciences 12, no. 12: 6128. https://doi.org/10.3390/app12126128






