Co-Culture of THP-1 Cells and Normal Human Epidermal Keratinocytes (NHEK) for Modified Human Cell Line Activation Test (h-CLAT)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagent
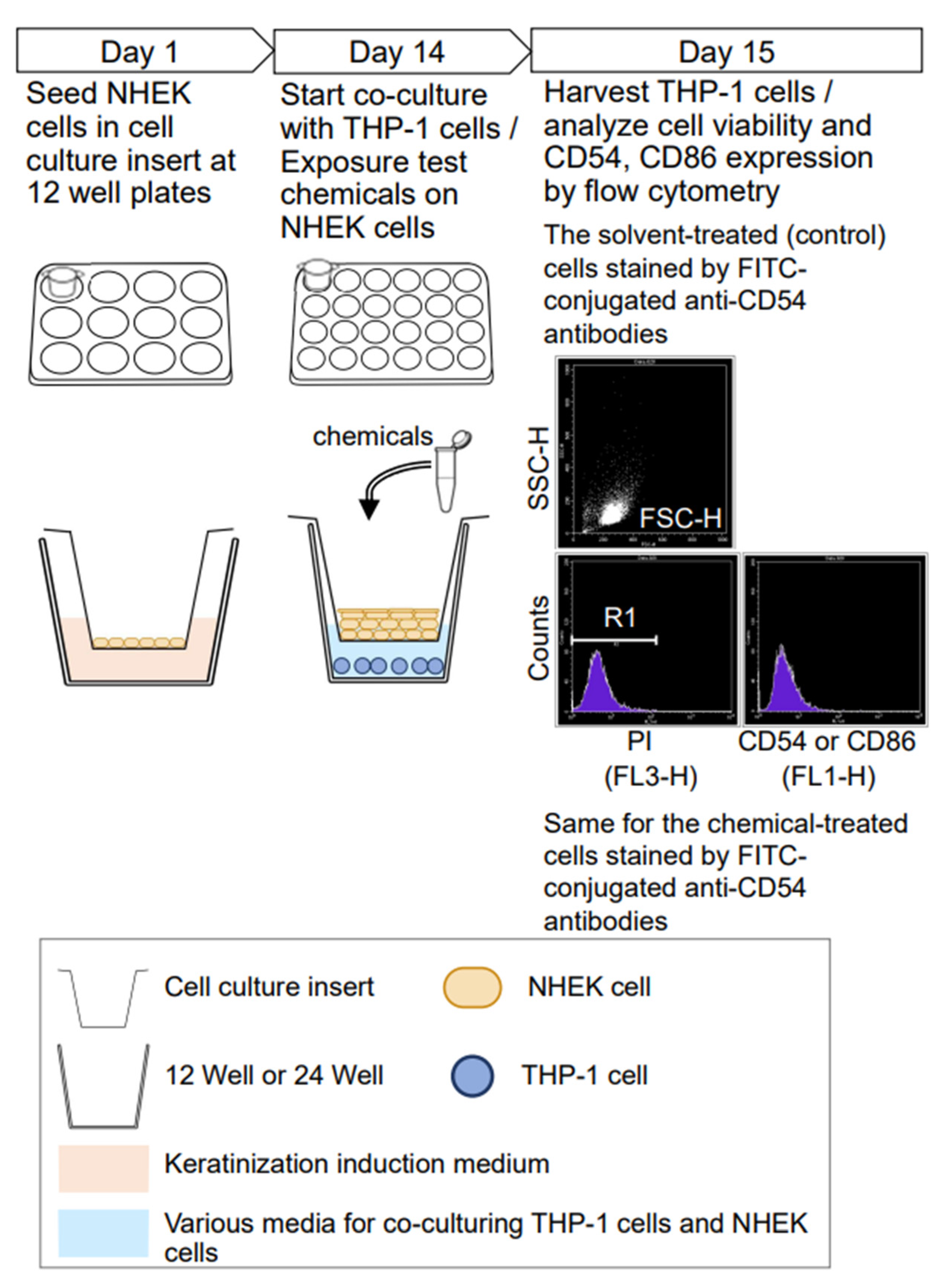
2.2. Cell Culture and Maintenance
2.3. Preparation of Human Epidermis Model
2.4. Preparation of Conditioned Medium from Keratinized NHEK Cells
2.5. Treatment of Medium and Chemical in Monoculture and Co-Culture
2.6. Analysis of CD54, CD86 Expression Level and Cell Viability
2.7. Data Analysis and Criteria
2.8. Statistical Analysis
3. Results
3.1. Comparison of Normal h-CLAT and Co-Culture System h-CLAT
3.2. Effects of Medium and Co-Culturing with NHEK Cells on CD54 and CD86 Expression Levels of THP-1 Cells
3.3. Effects of KG2 Medium and Co-Culturing with Undifferentiated NHEK Cells
3.4. Effects of Conditioned Medium from Keratinized NHEK Cells on the Expression of CD54 and CD86
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- United Nations. Globally Harmonized System of Classification and Labelling of Chemicals (GHS), 7th ed.; UN: New York, NY, USA; Geneva, Switzerland, 2017.
- OECD (Ed.) OECD TG 442C in Chemico Skin Sensitization Assays Addressing the Adverse Outcome Pathway Key Event on Covalent Binding to Proteins; OECD: Pairs, France, 2019. [Google Scholar]
- OECD (Ed.) OECD TG 442D In Vitro Skin Sensitization Assays Addressing the Key Event on Activation of Dendritic Cells on the Adverse Outcome Pathway for Skin Sensitization; OECD: Pairs, France, 2019. [Google Scholar]
- OECD (Ed.) G 442E In Vitro Skin Sensitization Assays Addressing the Key Event on Activation of Dendritic Cells on the Adverse Outcome Pathway for Skin Sensitization; OECD: Pairs, France, 2017. [Google Scholar]
- Rovida, C.; Alépée, N.; Api, A.M.; Basketter, D.A.; Bois, F.Y.; Caloni, F.; Corsini, E.; Daneshian, M.; Eskes, C.; Ezendam, J. Integrated Testing Strategies (ITS) for safety assessment. ALTEX 2015, 32, 25–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbisch, D.; Mehling, A.; Guth, K.; Ramirez, T.; Honarvar, N.; Kolle, S.; Landsiedel, R.; Jaworska, J.; Kern, P.S.; Gerberick, F. Assessing skin sensitization hazard in mice and men using non-animal test methods. Regul. Toxicol. Pharmacol. 2015, 71, 337–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OECD (Ed.) OECD Guidance Document on the Reporting of Defined Approaches and Individual Information Sources to Be Used within Integrated Approaches to Testing and Assessment (IATA) for Skin Sensitization; OECD: Pairs, France, 2016. [Google Scholar]
- Kleinstreuer, N.C.; Hoffmann, S.; Alépée, N.; Allen, D.; Ashikaga, T.; Casey, W.; Clouet, E.; Cluzel, M.; Desprez, B.; Gellatly, N. Non-animal methods to predict skin sensitization (II): An assessment of defined approaches. Crit. Rev. Toxicol. 2018, 48, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Thélu, A.; Catoire, S.; Kerdine-Römer, S. Immune-competent in vitro co-culture models as an approach for skin sensitization assessment. Toxicol. Vitr. 2020, 62, 104691. [Google Scholar] [CrossRef]
- Galbiati, V.; Maddalon, A.; Iulini, M.; Marinovich, M.; Corsini, E. Human keratinocytes and monocytes co-culture cell system: An important contribution for the study of moderate and weak sensitizers. Toxicol. Vitr. 2020, 68, 104929. [Google Scholar] [CrossRef] [PubMed]
- Hennen, J.; Aeby, P.; Goebel, C.; Schettgen, T.; Oberli, A.; Kalmes, M.; Blömeke, B. Cross talk between keratinocytes and dendritic cells: Impact on the prediction of sensitization. Toxicol. Sci. 2011, 123, 501–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eskes, C.; Hennen, J.; Schellenberger, M.T.; Hoffmann, S.; Frey, S.; Goldinger-Oggier, D.; Peter, N.; Van Vliet, E.; Blömeke, B. The HaCaT/THP-1 cocultured activation test (COCAT) for skin sensitization: A study of intra-laboratory reproducibility and predictivity. ALTEX 2019, 36, 613–622. [Google Scholar] [CrossRef]
- Schellenberger, M.T.; Bock, U.; Hennen, J.; Groeber-Becker, F.; Walles, H.; Blömeke, B. A coculture system composed of THP-1 cells and 3D reconstructed human epidermis to assess activation of dendritic cells by sensitizing chemicals after topical exposure. Toxicol. Vitr. 2019, 57, 62–66. [Google Scholar] [CrossRef]
- Yoshida, Y.; Sakaguchi, H.; Ito, Y.; Okuda, M.; Suzuki, H. Evaluation of the skin sensitization potential of chemicals using expression of co-stimulatory molecules, CD54 and CD86, on the naive THP-1 cell line. Toxicol. Vitr. 2003, 17, 221–228. [Google Scholar] [CrossRef]
- Ashikaga, T.; Yoshida, Y.; Hirota, M.; Yoneyama, K.; Itagaki, H.; Sakaguchi, H.; Miyazawa, M.; Ito, Y.; Suzuki, H.; Toyoda, H. Development of an in vitro skin sensitization test using human cell lines: The human Cell Line Activation Test (h-CLAT): I. Optimization of the h-CLAT protocol. Toxicol. Vitr. 2006, 20, 767–773. [Google Scholar] [CrossRef]
- Sakaguchi, H.; Ashikaga, T.; Miyazawa, M.; Yoshida, Y.; Ito, Y.; Yoneyama, K.; Hirota, M.; Itagaki, H.; Toyoda, H.; Suzuki, H. Development of an in vitro skin sensitization test using human cell lines; human Cell Line Activation Test (h-CLAT) II. An inter-laboratory study of the h-CLAT. Toxicol. Vitr. 2006, 20, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, H.; Ashikaga, T.; Miyazawa, M.; Kosaka, N.; Ito, Y.; Yoneyama, K.; Sono, S.; Itagaki, H.; Toyoda, H.; Suzuki, H. The relationship between CD86/CD54 expression and THP-1 cell viability in an in vitro skin sensitization test–human cell line activation test (h-CLAT). Cell Biol. Toxicol. 2009, 25, 109–126. [Google Scholar] [CrossRef]
- Mitachi, T.; Mezaki, M.; Yamashita, K.; Itagaki, H. Acidic conditions induce the suppression of CD86 and CD54 expression in THP-1 cells. J. Toxicol. Sci. 2018, 43, 299–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukumo, H.; Matsunari, N.; Yamashita, K.; Kojima, H.; Itagaki, H. Lipopolysaccharide interferes with the use of the human Cell Line Activation Test to determine the allergic potential of proteins. J. Pharmacol. Toxicol. Methods 2018, 92, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi-Tsukumo, H.; Oiji, K.; Xie, D.; Sawada, Y.; Yamashita, K.; Ogata, S.; Kojima, H.; Itagaki, H. Eliminating the contribution of lipopolysaccharide to protein allergenicity in the human cell-line activation test (h-CLAT). J. Toxicol. Sci. 2019, 44, 283–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiGiovanni, J.; Gill, R.D.; Nettikumara, A.N.; Colby, A.B.; Reiners, J.J. Effect of extracellular calcium concentration on the metabolism of polycyclic aromatic hydrocarbons by cultured mouse keratinocytes. Cancer Res. 1989, 49, 5567–5574. Available online: https://cancerres.aacrjournals.org/content/49/20/5567 (accessed on 17 June 2022).
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011, 21, 745–754. [Google Scholar] [CrossRef] [Green Version]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [Green Version]
- OECD (Ed.) OECD TG 430 In Vitro Skin Corrosion: Transcutaneous Electrical Resistance Test Method (TER); OECD: Paris, France, 2013. [Google Scholar]
- Lepoittevin, J.P. Metabolism versus chemical transformation or pro-versus prehaptens? Contact Dermat. 2006, 54, 73–74. [Google Scholar] [CrossRef]
- Smith, C.M.; Hotchkiss, S.A.M. Enzymes and pathways of xenobiotic metabolism in skin. In Allergic Contact Dermatitis: Chemical and Metabolic Mechanisms; Taylor and Francis: London, UK, 2001; pp. 89–117. [Google Scholar] [CrossRef]
- Swanson, H.I. Cytochrome P450 expression in human keratinocytes: An aryl hydrocarbon receptor perspective. Chem. Biol. Interact. 2004, 149, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Chipinda, I.; Ruwona, T.B.; Templeton, S.P.; Siegel, P.D. Use of the human monocytic leukemia THP-1 cell line and co-incubation with microsomes to identify and differentiate hapten and prohapten sensitizers. Toxicology 2011, 280, 135–143. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, J.J.; Chou, C.Y. Protein kinase Cα but not p44/42 mitogen-activated protein kinase, p38, or c-Jun NH2-terminal kinase is required for intercellular adhesion molecule-1 expression mediated by interleukin-1β: Involvement of sequential activation of tyrosine kinase, nuclear factor-κB-inducing kinase, and IκB kinase 2. Mol. Pharmacol. 2000, 58, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Rossol, M.; Pierer, M.; Raulien, N.; Quandt, D.; Meusch, U.; Rothe, K.; Schubert, K.; Schöneberg, T.; Schaefer, M.; Krügel, U. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat. Commun. 2012, 3, 1329. [Google Scholar] [CrossRef] [Green Version]
- Mitachi, T.; Kouzui, M.; Maruyama, R.; Yamashita, K.; Ogata, S.; Kojima, H.; Itagaki, H. Some non-sensitizers upregulate CD54 expression by activation of the NLRP3 inflammasome in THP-1 cells. J. Toxicol. Sci. 2019, 44, 213–224. [Google Scholar] [CrossRef] [Green Version]
- Grodzki, A.C.G.; Giulivi, C.; Lein, P.J. Oxygen tension modulates differentiation and primary macrophage functions in the human monocytic THP-1 cell line. PLoS ONE 2013, 8, e54926. [Google Scholar] [CrossRef] [PubMed]
- Köck, A.; Schwarz, T.; Kirnbauer, R.; Urbanski, A.; Perry, P.; Ansel, J.C.; Luger, T.A. Human keratinocytes are a source for tumor necrosis factor alpha: Evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J. Exp. Med. 1990, 172, 1609–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enk, A.H.; Katz, S.I. Identification and induction of keratinocyte-derived IL-10. J. Immunol. 1992, 149, 92–95. Available online: https://www.jimmunol.org/content/149/1/92 (accessed on 17 June 2022).
- Ansel, J.; Perry, P.; Brown, J.; Damm, D.; Phan, T.; Hart, C.; Luger, T.; Hefeneider, S. Cytokine modulation of keratinocyte cytokines. J. Investig. Dermatol. 1990, 94, s101–s107. [Google Scholar] [CrossRef] [Green Version]
- Ioffreda, M.D.; Whitaker, D.; Murphy, G.F. Mast cell segranulation upregulates ∝6 integrins on epidermal langerhans cells. J. Investig. Dermatol. 1993, 101, 150–154. [Google Scholar] [CrossRef] [Green Version]
- Cumberbatch, M.; Kimber, I. Dermal tumour necrosis factor-alpha induces dendritic cell migration to draining lymph nodes, and possibly provides one stimulus for Langerhans’ cell migration. Immunology 1992, 75, 257–263. [Google Scholar]
- Enk, A.H.; Angeloni, V.L.; Udey, M.C.; Katz, S.I. Inhibition of Langerhans cell antigen-presenting function by IL-10. A role for IL-10 in induction of tolerance. J. Immunol. 1993, 151, 2390–2398. Available online: https://www.jimmunol.org/content/151/5/2390 (accessed on 17 June 2022).
- Witmer-Pack, M.D.; Olivier, W.; Valinsky, J.; Schuler, G.; Steinman, R.M. Granulocyte/macrophage colony-stimulating factor is essential for the viability and function of cultured murine epidermal langerhans cells. J. Exp. Med. 1987, 166, 1484–1498. [Google Scholar] [CrossRef] [Green Version]
- Heufler, C.; Koch, F.; Schuler, G. Granulocyte/macrophage colony-stimulating factor and interleukin 1 mediate the maturation of murine epidermal Langerhans cells into potent immunostimulatory dendritic cells. J. Exp. Med. 1988, 167, 700–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, E.L.; Hsu, Y.C.; Lee, J.A. Consideration of the cellular microenvironment: Physiologically relevant co-culture systems in drug discovery. Adv. Drug Deliv. Rev. 2014, 69, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Abbott, R.D.; Kaplan, D.L. Strategies for improving the physiological relevance of human engineered tissues. Trends Biotechnol. 2015, 33, 401–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peehl, D.M.; Ham, R.G. Growth and differentiation of human keratinocytes without a feeder layer or conditioned medium. Vitro 1980, 16, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.; Kusama, M.; Onda, M.; Nakahata, N. The effect of pantothenic acid deficiency on keratinocyte proliferation and the synthesis of keratinocyte growth factor and collagen in fibroblasts. J. Pharmacol. Sci. 2011, 115, 230–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gröne, A. Keratinocytes and cytokines. Vet. Immunol. Immunopathol. 2002, 88, 1–12. [Google Scholar] [CrossRef]
- Ghahary, A.; Marcoux, Y.; Karimi-Busheri, F.; Tredget, E.E. Keratinocyte differentiation inversely regulates the expression of involucrin and transforming growth factor β1. J. Cell. Biochem. 2001, 83, 239–248. [Google Scholar] [CrossRef]
- Ramsden, D.; Zhou, J.; Tweedie, D.J. Determination of a degradation constant for CYP3A4 by direct suppression of mRNA in a novel human hepatocyte model, HepatoPac. Drug Metab. Dispos. 2015, 43, 1307–1315. [Google Scholar] [CrossRef] [Green Version]
- OECD (Ed.) OECD TG 439 In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method; OECD: Paris, France, 2021. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawada, Y.; Tsukumo, H.; Fukuda, J.; Iijima, K.; Itagaki, H. Co-Culture of THP-1 Cells and Normal Human Epidermal Keratinocytes (NHEK) for Modified Human Cell Line Activation Test (h-CLAT). Appl. Sci. 2022, 12, 6207. https://doi.org/10.3390/app12126207
Sawada Y, Tsukumo H, Fukuda J, Iijima K, Itagaki H. Co-Culture of THP-1 Cells and Normal Human Epidermal Keratinocytes (NHEK) for Modified Human Cell Line Activation Test (h-CLAT). Applied Sciences. 2022; 12(12):6207. https://doi.org/10.3390/app12126207
Chicago/Turabian StyleSawada, Yuka, Hanae Tsukumo, Junji Fukuda, Kazutoshi Iijima, and Hiroshi Itagaki. 2022. "Co-Culture of THP-1 Cells and Normal Human Epidermal Keratinocytes (NHEK) for Modified Human Cell Line Activation Test (h-CLAT)" Applied Sciences 12, no. 12: 6207. https://doi.org/10.3390/app12126207
APA StyleSawada, Y., Tsukumo, H., Fukuda, J., Iijima, K., & Itagaki, H. (2022). Co-Culture of THP-1 Cells and Normal Human Epidermal Keratinocytes (NHEK) for Modified Human Cell Line Activation Test (h-CLAT). Applied Sciences, 12(12), 6207. https://doi.org/10.3390/app12126207





