Sustainable Treatment Techniques for Emerging Pollutants—The Case of Personal Hygiene Products
Abstract
:1. Introduction
2. Materials and Methods
Modified Saaty Method
3. Results
3.1. Technique 1: Co-Composting
3.2. Technique 2: Combined Anaerobic–Aerobic Method
3.3. Technique 3: Adsorption Method
3.4. Modified Saaty Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Index of Abbreviations
| ABR | Anaerobic baffle reactor |
| AHR | Hybrid anaerobic reactor |
| AHP | Analysis hierarchy process |
| AnCSTR | Anaerobic fully stirred treatment reactor |
| AS | Aerobic activated sludge |
| CBZ | Carbamazepine |
| CC | Construction cost |
| CNT | Carbon nanotubes |
| COD | Chemical oxygen demand |
| EC | Emerging pollutants |
| ED | Stage of development |
| Ef | Efficiency |
| HUASB | Hybrid upflow anaerobic sludge blanket |
| MC | Maintenance cost |
| MWCNT | Multi-walled carbon nanotubes |
| PAHs | Polycyclic aromatic hydrocarbons |
| PCP | Personal care products |
| PPCP | Pharmaceuticals and personal care products |
| SWCNT | Single-walled carbon nanotubes |
| TCS | Triclosan |
| UBAF | Upflow aerated biological filter |
| UK | United kingdom |
| US | United States |
| μA (Vo) | Behavior of the variable or criterion |
| Vo | Original value |
| Vmin | Minimum value |
| Vmáx | Maximum value |
| WWTPs | Wastewater treatment plants |
| Wi | Weight of each criterion |
References
- Ávila, C.; García, J. Pharmaceuticals and Personal Care Products (PPCPs) in the Environment and Their Removal from Wastewater through Constructed Wetlands. Compr. Anal. Chem. 2015, 67, 195–244. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.F.; Mofijur, M.; Nuzhat, S.; Chowdhury, A.T.; Rafa, N.; Uddin, M.A.; Inayat, A.; Mahlia, T.M.I.; Ong, H.C.; Chia, W.Y.; et al. Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J. Hazard. Mater. 2021, 416, 125912. [Google Scholar] [CrossRef] [PubMed]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef] [PubMed]
- Ebele, A.J.; Abdallah, M.A.E.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Akhbarizadeh, R.; Dobaradaran, S.; Schmidt, T.C.; Nabipour, I.; Spitz, J. Worldwide bottled water occurrence of emerging contaminants: A review of the recent scientific literature. J. Hazard. Mater. 2020, 392, 122171. [Google Scholar] [CrossRef]
- Ji, J.; Kakade, A.; Yu, Z.; Khan, A.; Liu, P.; Li, X. Anaerobic membrane bioreactors for treatment of emerging contaminants: A review. J. Environ. Manag. 2020, 270, 110913. [Google Scholar] [CrossRef]
- Wilkinson, J.; Hooda, P.S.; Barker, J.; Barton, S.; Swinden, J. Occurrence, fate and transformation of emerging contaminants in water: An overarching review of the field. Environ. Pollut. 2017, 231, 954–970. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, J.L.; Hooda, P.S.; Barker, J.; Barton, S.; Swinden, J. Ecotoxic pharmaceuticals, personal care products, and other emerging contaminants: A review of environmental, receptor-mediated, developmental, and epigenetic toxicity with discussion of proposed toxicity to humans. Crit. Rev. Environ. Sci. Technol. 2016, 46, 336–381. [Google Scholar] [CrossRef] [Green Version]
- Blair, B.D.; Crago, J.P.; Hedman, C.J.; Klaper, R.D. Pharmaceuticals and personal care products found in the Great Lakes above concentrations of environmental concern. Chemosphere 2013, 93, 2116–2123. [Google Scholar] [CrossRef] [Green Version]
- Richardson, S.D.; Ternes, T.A. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 2018, 90, 398–428. [Google Scholar] [CrossRef]
- Fang, C.; Yang, X.; Ding, S.; Luan, X.; Xiao, R.; Du, Z.; Wang, P.; An, W.; Chu, W. Characterization of Dissolved Organic Matter and Its Derived Disinfection Byproduct Formation along the Yangtze River. Environ. Sci. Technol. 2021, 55, 12326–12336. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Carrillo, M.; Abrell, L.; Ramírez-Hernández, J.; Reyes-López, J.A.; Carreón-Diazconti, C. Pharmaceuticals as emerging contaminants in the aquatic environment of Latin America: A review. Environ. Sci. Pollut. Res. 2020, 27, 44863–44891. [Google Scholar] [CrossRef] [PubMed]
- Rego, R.M.; Kuriya, G.; Kurkuri, M.D.; Kigga, M. MOF based engineered materials in water remediation: Recent trends. J. Hazard. Mater. 2021, 403, 123605. [Google Scholar] [CrossRef] [PubMed]
- Dubey, M.; Mohapatra, S.; Tyagi, V.K.; Suthar, S.; Kazmi, A.A. Occurrence, fate, and persistence of emerging micropollutants in sewage sludge treatment. Environ. Pollut. 2021, 273, 116515. [Google Scholar] [CrossRef]
- Salazar-Vega, J.; Ortiz-Prado, E.; Solis, P.; Gomez, L. Thyroid Cancer in Ecuador, a 16 years population-based analysis (2001–2016). BMC Cancer 2019, 19, 294. [Google Scholar] [CrossRef]
- Witorsch, R.J.; Thomas, J.A. Personal care products and endocrine disruption: A critical review of the literature. Crit. Rev. Toxicol. 2010, 40 (Suppl. S3), 1–30. [Google Scholar] [CrossRef]
- Abidemi, B.L.; James, O.A.; Oluwatosin, A.T.; Akinropo, O.J.; Oraeloka, U.D.; Racheal, A.E. Treatment Technologies for Wastewater from Cosmetic Industry-A Review Experimental Determination of the Effect of Pretreatment on the Nutritional Quality of Cocoyam Chips View project Tensile Strength Assessment and Effluent Analysis of Multicoloured Textile Apparels Laundered in Anti-bleeding Solution View project Treatment Technologies for Wastewater from Cosmetic Industry-A Review. Int. J. Chem. Biomol. Sci. 2018, 4, 69–80. [Google Scholar]
- Ouda, M.; Kadadou, D.; Swaidan, B.; Al-Othman, A.; Al-Asheh, S.; Banat, F.; Hasan, S.W. Emerging contaminants in the water bodies of the Middle East and North Africa (MENA): A critical review. Sci. Total Environ. 2021, 754, 142177. [Google Scholar] [CrossRef]
- Kumar, M.; Ram, B.; Honda, R.; Poopipattana, C.; Canh, V.D.; Chaminda, T.; Furumai, H. Concurrence of antibiotic resistant bacteria (ARB), viruses, pharmaceuticals and personal care products (PPCPs) in ambient waters of Guwahati, India: Urban vulnerability and resilience perspective. Sci. Total Environ. 2019, 693, 133640. [Google Scholar] [CrossRef]
- Bi, R.; Zeng, X.; Mu, L.; Hou, L.; Liu, W.; Li, P.; Chen, H.; Li, D.; Bouchez, A.; Tang, J.; et al. Sensitivities of seven algal species to triclosan, fluoxetine and their mixtures. Sci. Rep. 2018, 8, 15361. [Google Scholar] [CrossRef] [Green Version]
- de García, S.O.; García-Encina, P.A.; Irusta-Mata, R. Dose–response behavior of the bacterium Vibrio fischeri exposed to pharmaceuticals and personal care products. Ecotoxicology 2016, 25, 141–162. [Google Scholar] [CrossRef] [PubMed]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Investigation of PPCPs in wastewater treatment plants in Greece: Occurrence, removal and environmental risk assessment. Sci. Total Environ. 2014, 466–467, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhou, Y.; Shi, B.; Meng, J.; He, B.; Yang, H.; Yoon, S.J.; Kim, T.; Kwon, B.O.; Khim, J.S.; et al. Anthropogenic impacts on the contamination of pharmaceuticals and personal care products (PPCPs) in the coastal environments of the Yellow and Bohai seas. Environ. Int. 2020, 135, 105306. [Google Scholar] [CrossRef] [PubMed]
- Cizmas, L.; Sharma, V.K.; Gray, C.M.; McDonald, T.J. Pharmaceuticals and personal care products in waters: Occurrence, toxicity, and risk. Environ. Chem. Lett. 2015, 13, 381–394. [Google Scholar] [CrossRef] [Green Version]
- Mohiuddin, A.K. Heavy Metals in Cosmetics: The Notorious Daredevils and Burning Health Issues. Am. J. Biomed. Sci. Res. 2019, 4, 332–337. [Google Scholar] [CrossRef]
- Nafisi, S.; Maibach, H.I. Nanotechnology in cosmetics. In Cosmetic Science and Technology: Theoretical Principles and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 337–361. [Google Scholar] [CrossRef]
- Ahammad, S.Z.; Bereslawski, J.L.; Dolfing, J.; Mota, C.; Graham, D.W. Anaerobic-aerobic sequencing bioreactors improve energy efficiency for treatment of personal care product industry wastes. Bioresour. Technol. 2013, 139, 73–79. [Google Scholar] [CrossRef]
- Biel-Maeso, M.; Corada-Fernández, C.; Lara-Martín, P.A. Removal of personal care products (PCPs) in wastewater and sludge treatment and their occurrence in receiving soils. Water Res. 2019, 150, 129–139. [Google Scholar] [CrossRef]
- Grandjean, P. Paracelsus Revisited: The Dose Concept in a Complex World. Basic Clin. Pharmacol. Toxicol. 2016, 119, 126–132. [Google Scholar] [CrossRef]
- Hernández, F.; Bakker, J.; Bijlsma, L.; De Boer, J.; Botero-Coy, A.M.; de Bruin, Y.B.; Fischer, S.; Hollender, J.; Kasprzyk-Hordern, B.; Lamoree, M.; et al. The role of analytical chemistry in exposure science: Focus on the aquatic environment. Chemosphere 2019, 222, 564–583. [Google Scholar] [CrossRef]
- Wei, Z.; He, Y.; Wang, X.; Chen, Z.; Wei, X.; Lin, Y.; Cao, C.; Huang, M.; Zheng, B. A comprehensive assessment of upgrading technologies of wastewater treatment plants in Taihu Lake Basin. Environ. Res. 2022, 212, 113398. [Google Scholar] [CrossRef]
- Zhou, Y.; Meng, J.; Zhang, M.; Chen, S.; He, B.; Zhao, H.; Li, Q.; Zhang, S.; Wang, T. Which type of pollutants need to be controlled with priority in wastewater treatment plants: Traditional or emerging pollutants? Environ. Int. 2019, 131, 104982. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Huang, J.; Chen, W.; Wang, B.; Yu, G.; Deng, S. Major pharmaceuticals and personal care products (PPCPs) in wastewater treatment plant and receiving water in Beijing, China, and associated ecological risks. Bull. Environ. Contam. Toxicol. 2014, 92, 655–661. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.; Frihling, B.E.F.; Velasques, J.; Magalhães Filho, F.J.C.; Cavalheri, P.S.; Migliolo, L. Pharmaceuticals residues and xenobiotics contaminants: Occurrence, analytical techniques and sustainable alternatives for wastewater treatment. Sci. Total Environ. 2020, 705, 135568. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Alcalá, I.; Guillén-Navarro, J.M.; Lahora, A. Occurrence and fate of pharmaceuticals in a wastewater treatment plant from southeast of Spain and risk assessment. J. Environ. Manag. 2021, 279, 111565. [Google Scholar] [CrossRef]
- Thomas, A.R.; Kranert, M.; Philip, L. Fate and impact of pharmaceuticals and personal care products during septage co-composting using an in-vessel composter. Waste Manag. 2020, 109, 109–118. [Google Scholar] [CrossRef]
- López, F.A.; Alguacil, F.; Álvarez, T.; Cerpa, A. Utilización de Nanotubos de Carbono para la Eliminación de Metales Tóxicos en Aguas. Available online: http://www.conama2014.conama.org/conama2014/download/files/conama2014/CT%202014/1896711012.pdf (accessed on 16 September 2021).
- León, V.M.; Viñas, L.; Concha-Graña, E.; Fernández-González, V.; Salgueiro-González, N.; Moscoso-Pérez, C.; Muniategui-Lorenzo, S.; Campillo, J.A. Identification of contaminants of emerging concern with potential environmental risk in Spanish continental shelf sediments. Sci. Total Environ. 2020, 742, 140505. [Google Scholar] [CrossRef]
- Ilyas, H.; van Hullebusch, E.D. Performance comparison of different constructed wetlands designs for the removal of personal care products. Int. J. Environ. Res. Public Health 2020, 17, 3091. [Google Scholar] [CrossRef]
- Zhao, X.; Tan, W.; Dang, Q.; Li, R.; Xi, B. Enhanced biotic contributions to the dechlorination of pentachlorophenol by humus respiration from different compostable environments. Chem. Eng. J. 2019, 361, 1565–1575. [Google Scholar] [CrossRef]
- Ahmadkhaniha, D.; Fedel, M.; Heydarzadeh Sohi, M.; Deflorian, F. Corrosion behavior of severely plastic deformed magnesium based alloys: A review. Surf. Eng. Appl. Electrochem. 2017, 53, 439–448. [Google Scholar] [CrossRef]
- Basak, S.; Das, M.K.; Duttaroy, A.K. Plastics derived endocrine-disrupting compounds and their effects on early development. Birth Defects Res. 2020, 112, 1308–1325. [Google Scholar] [CrossRef]
- Sikdar, S.; Kundu, M. A Review on Detection and Abatement of Heavy Metals. Chembioeng Rev. 2018, 5, 18–29. [Google Scholar] [CrossRef]
- Zheng, Z.; Peters, G.M.; Arp, H.P.H.; Andersson, P.L. Combining in Silico Tools with Multicriteria Analysis for Alternatives Assessment of Hazardous Chemicals: A Case Study of Decabromodiphenyl Ether Alternatives. Environ. Sci. Technol. 2019, 53, 6341–6351. [Google Scholar] [CrossRef] [PubMed]
- Sonne, C.; Dietz, R.; Jenssen, B.M.; Lam, S.S.; Letcher, R.J. Emerging contaminants and biological effects in Arctic wildlife. Trends Ecol. Evol. 2021, 36, 421–429. [Google Scholar] [CrossRef]
- Negrete-Bolagay, D.; Zamora-Ledezma, C.; Chuya-Sumba, C.; De Sousa, F.B.; Whitehead, D.; Alexis, F.; Guerrero, V.H. Persistent organic pollutants: The trade-off between potential risks and sustainable remediation methods. J. Environ. Manag. 2021, 300, 113737. [Google Scholar] [CrossRef] [PubMed]
- Kristanti, R.A.; Ngu, W.J.; Yuniarto, A.; Hadibarata, T. Rhizofiltration for removal of inorganic and organic pollutants in groundwater: A review. Biointerface Res. Appl. Chem. 2021, 11, 12326–12347. [Google Scholar] [CrossRef]
- Ebele, A.J.; Oluseyi, T.; Drage, D.S.; Harrad, S.; Abdallah, M.A.E. Occurrence, seasonal variation and human exposure to pharmaceuticals and personal care products in surface water, groundwater and drinking water in Lagos State, Nigeria. Emerg. Contam. 2020, 6, 124–132. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales, UK. Water Res. 2008, 42, 3498–3518. [Google Scholar] [CrossRef] [PubMed]
- Libralato, G.; Lofrano, G.; Siciliano, A.; Gambino, E.; Boccia, G.; Federica, C.; Francesco, A.; Galdiero, E.; Gesuele, R.; Guida, M. Occurrence and potential risks of emerging contaminants in water. In Visible Light Active Structured Photocatalysts for the Removal of Emerging Contaminants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–25. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Duttagupta, S.; Mukherjee, A. Emerging organic contaminants in global community drinking water sources and supply: A review of occurrence, processes and remediation. J. Environ. Chem. Eng. 2022, 10, 107560. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Shukla, P.; Giri, B.S.; Chowdhary, P.; Chandra, R.; Gupta, P.; Pandey, A. Prevalence and hazardous impact of pharmaceutical and personal care products and antibiotics in environment: A review on emerging contaminants. Environ. Res. 2021, 194, 110664. [Google Scholar] [CrossRef]
- Sahani, S.; Sharma, Y.C.; Kim, T.Y. Emerging Contaminants in Wastewater and Surface Water; Springer: Singapore, 2022; pp. 9–30. [Google Scholar] [CrossRef]
- Xabadia, A.; Esteban, E.; Martinez, Y.; Ortuzar, I. Contaminants of Emerging Concern: A Review of Biological and Economic Principles to Guide Water Management Policies. Int. Rev. Environ. Resour. Econ. 2021, 15, 387–430. [Google Scholar] [CrossRef]
- Vasilachi, I.C.; Asiminicesei, D.M.; Fertu, D.I.; Gavrilescu, M. Occurrence and fate of emerging pollutants in water environment and options for their removal. Water 2021, 13, 181. [Google Scholar] [CrossRef]
- Zhu, X.; Qi, J.; Cheng, L.; Zhen, G.; Lu, X.; Zhang, X. Depolymerization and conversion of waste-activated sludge to value-added bioproducts by fungi. Fuel 2022, 320, 123890. [Google Scholar] [CrossRef]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Saratale, R.G.; Ferreira, L.F.R.; Bilal, M.; Chandra, R.; Bharagava, R.N. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J. Environ. Chem. Eng. 2021, 9, 105012. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.K.; Chandra, R. Recent Advances in Physicochemical and Biological Approaches for Degradation and Detoxification of Industrial Wastewater. In Emerging Treatment Technologies for Waste Management; Springer: Singapore, 2021; pp. 1–28. [Google Scholar] [CrossRef]
- Varjani, S.J.; Chaithanya Sudha, M. Treatment Technologies for Emerging Organic Contaminants Removal from Wastewater. In Energy, Environment, and Sustainability; Springer Nature: Berlin/Heidelberg, Germany, 2018; pp. 91–115. [Google Scholar] [CrossRef]
- Samal, K.; Bandyopadhyay, R.; Dash, R.R. Biological Treatment of Contaminants of Emerging Concern in Wastewater: A Review. J. Hazard. Toxic Radioact. Waste 2022, 26, 04022002. [Google Scholar] [CrossRef]
- Taher, M.N.; Al-Mutwalli, S.A.; Sapmaz, T.; Koseoglu-Imer, D.Y. Potentials and performance of biological processes for treatment of pharmaceuticals and personal care products in wastewater. In The Future of Effluent Treatment Plants; Elsevier: Amsterdam, The Netherlands, 2021; pp. 523–550. [Google Scholar] [CrossRef]
- Saaty, T.L. The Analytic Hierarchy Process (AHP); Elsevier: Amsterdam, The Netherelands, 1980; Volume 41. [Google Scholar]
- Chen, C.F. Applying the analytical hierarchy process (AHP) approach to convention site selection. J. Travel Res. 2006, 45, 167–174. [Google Scholar] [CrossRef]
- Yang, M.; Khan, F.I.; Sadiq, R. Prioritization of environmental issues in offshore oil and gas operations: A hybrid approach using fuzzy inference system and fuzzy analytic hierarchy process. Process Saf. Environ. Prot. 2011, 89, 22–34. [Google Scholar] [CrossRef]
- Buckley, J.; Feuring, T.; Hayashi, Y. Fuzzy hierarchical analysis revisited. Eur. J. Oper. Res. 2001, 129, 48–64. [Google Scholar] [CrossRef]
- Karimi, A.R.; Mehrdadi, N.; Hashemian, S.J.; Bidhendi, G.R.; Moghaddam, R.T. Selection of wastewater treatment process based on the analytical hierarchy process and fuzzy analytical hierarchy process methods. Int. J. Environ. Sci. Technol. 2011, 8, 267–280. [Google Scholar] [CrossRef] [Green Version]
- Mikhailov, L.; Tsvetinov, P. Evaluation of services using a fuzzy analytic hierarchy process. Appl. Soft Comput. J. 2004, 5, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.-H.; Mon, D.-L. Evaluating weapon system by Analytical Hierarchy Process based on fuzzy scales. Fuzzy Sets Syst. 1994, 63, 1–10. [Google Scholar] [CrossRef]
- Buckley, J. Fuzzy Hierarchical Analysis. Fuzzy Sets Syst. 1985, 17, 233. [Google Scholar] [CrossRef]
- Hájek, P. What is mathematical fuzzy logic. Fuzzy Sets Syst. 2006, 157, 597–603. [Google Scholar] [CrossRef]
- Novák, V. Which logic is the real fuzzy logic? Fuzzy Sets Syst. 2006, 157, 635–641. [Google Scholar] [CrossRef]
- Zadeh, A. Fuzzy logic—A personal perspective. Fuzzy Sets Syst. 2015, 281, 4–20. [Google Scholar] [CrossRef]
- Bone, C.; Padilla-Almeida, O.; Ananganó, P.; Guamán, S.; Kirby, E.; Toulkeridis, T. Use of Multitemporal Indexes in the Identification of Forest Fires-A Case Study of Southern Chile. In Proceedings of the 2019 Sixth International Conference on eDemocracy & eGovernment, Quito, Ecuador, 24–26 April 2019. [Google Scholar]
- Carrera, B.; Padilla Almeida, O.; Toulkeridis, T. Modeling of the spatial distribution of the vector Aedes Aegypti, transmitter of the Zika Virus in continental Ecuador by the application of GIS tools. Bionatura 2020, 5, 1314–1327. [Google Scholar] [CrossRef]
- Herrera-Enríquez, G.; Toulkeridis, T.; Castillo-Montesdeoca, E.; Rodríguez-Rodríguez, G. Critical Factors of Business Adaptability During Resilience in Baños de Agua Santa, Ecuador, Due to Volcanic Hazards; Springer: Cham, Switzerland, 2021; pp. 283–297. [Google Scholar] [CrossRef]
- Padilla, O.; Rosas, P.; Moreno, W.; Toulkeridis, T. Modeling of the ecological niches of the anopheles spp in Ecuador by the use of geo-informatic tools. Spat. Spatio-Temporal Epidemiol. 2017, 21, 1–11. [Google Scholar] [CrossRef]
- Dhanirama, D.; Gronow, J.; Voulvoulis, N. Cosmetics as a potential source of environmental contamination in the UK. Environ. Technol. 2012, 33, 1597–1608. [Google Scholar] [CrossRef]
- Khan, K.; Sanderson, H.; Roy, K. Ecotoxicological QSARs of Personal Care Products and Biocides. In Ecotoxicological QSARs; Humana: New York, NY, USA, 2020; pp. 357–386. [Google Scholar] [CrossRef]
- Khetan, S.K.; Collins, T.J. Human pharmaceuticals in the aquatic environment: A challenge to green chemisty. Chem. Rev. 2007, 107, 2319–2364. [Google Scholar] [CrossRef]
- Lin, N.; Ding, N.; Meza-Wilson, E.; Devasurendra, A.M.; Godwin, C.; Park, S.K.; Batterman, S. Volatile organic compounds in feminine hygiene products sold in the US market: A survey of products and health risks. Environ. Int. 2020, 144, 105740. [Google Scholar] [CrossRef]
- da Costa, W.K.O.C.; da Silva, C.S.; Figueiredo, J.F.D.; Nóbrega, J.A.; Paim, A.P.S. Direct analysis of deodorants for determination of metals by inductively coupled plasma optical emission spectrometry. J. Pharm. Biomed. Anal. 2018, 155, 247–252. [Google Scholar] [CrossRef]
- Silva, A.L.P.; Prata, J.C.; Mouneyrac, C.; Barcelò, D.; Duarte, A.C.; Rocha-Santos, T. Risks of Covid-19 face masks to wildlife: Present and future research needs. Sci. Total Environ. 2021, 792, 148505. [Google Scholar] [CrossRef] [PubMed]
- Archer, E.; Wolfaardt, G.M.; van Wyk, J.H. Review: Pharmaceutical and personal care products (PPCPs) as endocrine disrupting contaminants (EDCs) in South African surface waters. Water SA 2017, 43, 684. [Google Scholar] [CrossRef] [Green Version]
- Klaschka, U.; von der Ohe, P.C.; Bschorer, A.; Krezmer, S.; Sengl, M.; Letzel, M. Occurrences and potential risks of 16 fragrances in five German sewage treatment plants and their receiving waters. Environ. Sci. Pollut. Res. 2013, 20, 2456–2471. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef]
- Juliano, C.; Magrini, G. Cosmetic Ingredients as Emerging Pollutants of Environmental and Health Concern. A Mini-Review. Cosmetics 2017, 4, 11. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S.; Show, P.L. A review on effective removal of emerging contaminants from aquatic systems: Current trends and scope for further research. J. Hazard. Mater. 2021, 409, 124413. [Google Scholar] [CrossRef]
- Qiu, F.; Chastain, B.; Zhou, Y.; Zhang, C.; Sridharan, H. Modeling land suitability/capability using fuzzy evaluation. GeoJournal 2014, 79, 167–182. [Google Scholar] [CrossRef]
- Zhang, Y.; Geißen, S.U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef]
- Thomas, A.R.; Kranert, M.; Philip, L. In-vessel co-composting—A rapid resource recovery option for septage treatment in Indian cities. J. Water Sanit. Hyg. Dev. 2018, 8, 688–697. [Google Scholar] [CrossRef]
- Gašpariková, E.; Kapusta, Š.; Bodík, I.; Derco, J.; Kratochvíl, K. Evaluation of Anaerobic-Aerobic Wastewater Treatment Plant Operations. Pol. J. Environ. Stud. 2005, 14, 29–34. [Google Scholar]
- Zhang, C.; Ning, K.; Guo, Y.; Chen, J.; Liang, C.; Zhang, X.; Wang, R.; Guo, L. Cosmetic wastewater treatment by a combined anaerobic/aerobic (ABR+UBAF) biological system. Desalin. Water Treat. 2015, 53, 1606–1612. [Google Scholar] [CrossRef]
- Popli, S.; Patel, U.D. Destruction of azo dyes by anaerobic–aerobic sequential biological treatment: A review. Int. J. Environ. Sci. Technol. 2015, 12, 405–420. [Google Scholar] [CrossRef] [Green Version]
- Ong, Y.T.; Ahmad, A.L.; Zein, S.H.S.; Tan, S.H. A review on carbon nanotubes in an environmental protection and green engineering perspective. Braz. J. Chem. Eng. 2010, 27, 227–242. [Google Scholar] [CrossRef]
- Gui, X.; Zeng, Z.; Lin, Z.; Gan, Q.; Xiang, R.; Zhu, Y.; Cao, A.; Tang, Z. Magnetic and highly recyclable macroporous carbon nanotubes for spilled oil sorption and separation. ACS Appl. Mater. Interfaces 2013, 5, 5845–5850. [Google Scholar] [CrossRef] [PubMed]
- Haiba, E.; Nei, L.; Kutti, S.; Lillenberg, M.; Herodes, K.; Ivask, M.; Kipper, K.; Aro, R.; Laaniste, A. Degradation of diclofenac and triclosan residues in sewage sludge compost. Agron. Res. 2017, 15, 395–405. [Google Scholar]
- Butkovskyi, A.; Ni, G.; Leal, L.H.; Rijnaarts, H.H.M.; Zeeman, G. Mitigation of micropollutants for black water application in agriculture via composting of anaerobic sludge. J. Hazard. Mater. 2016, 303, 41–47. [Google Scholar] [CrossRef] [PubMed]
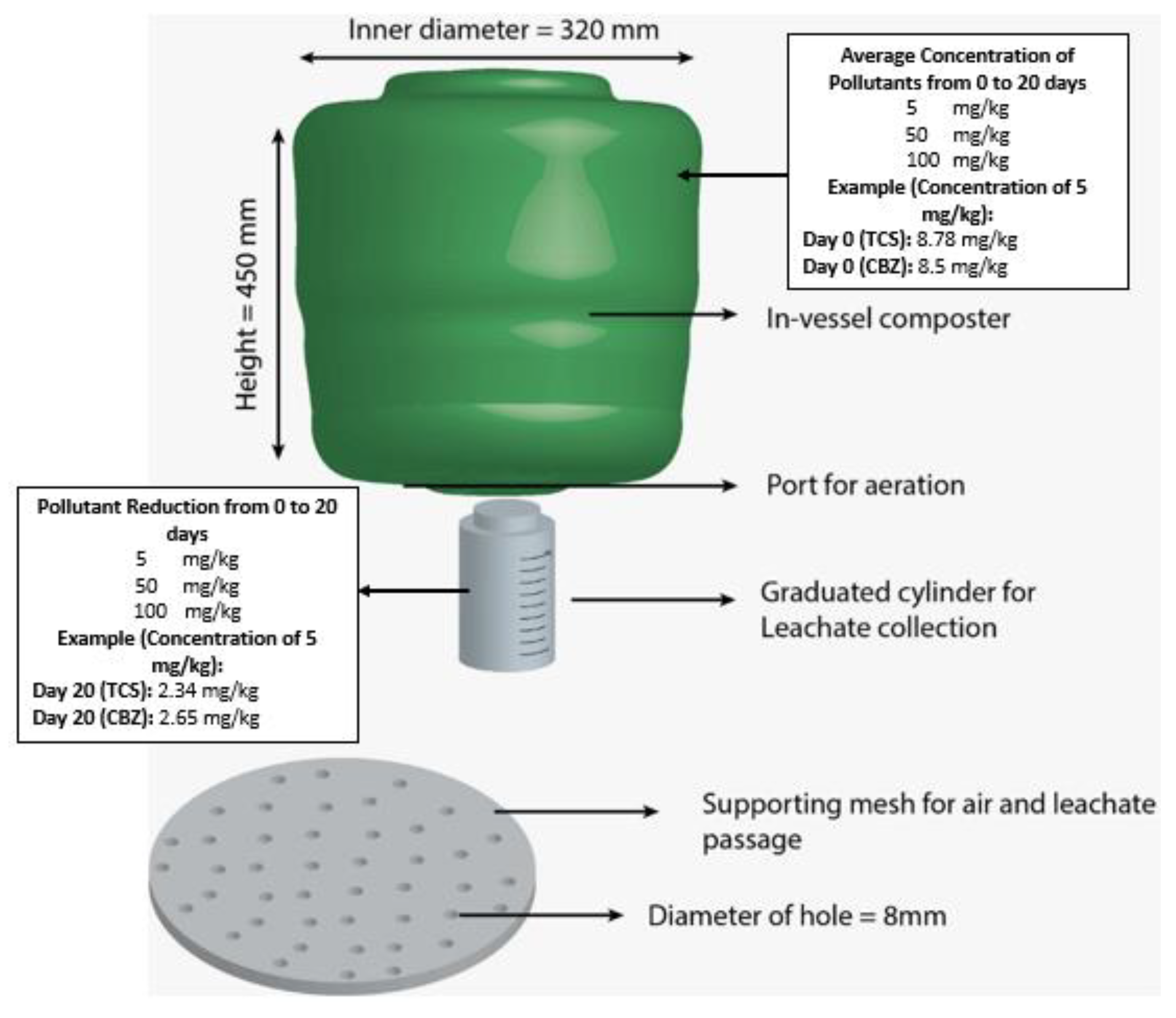
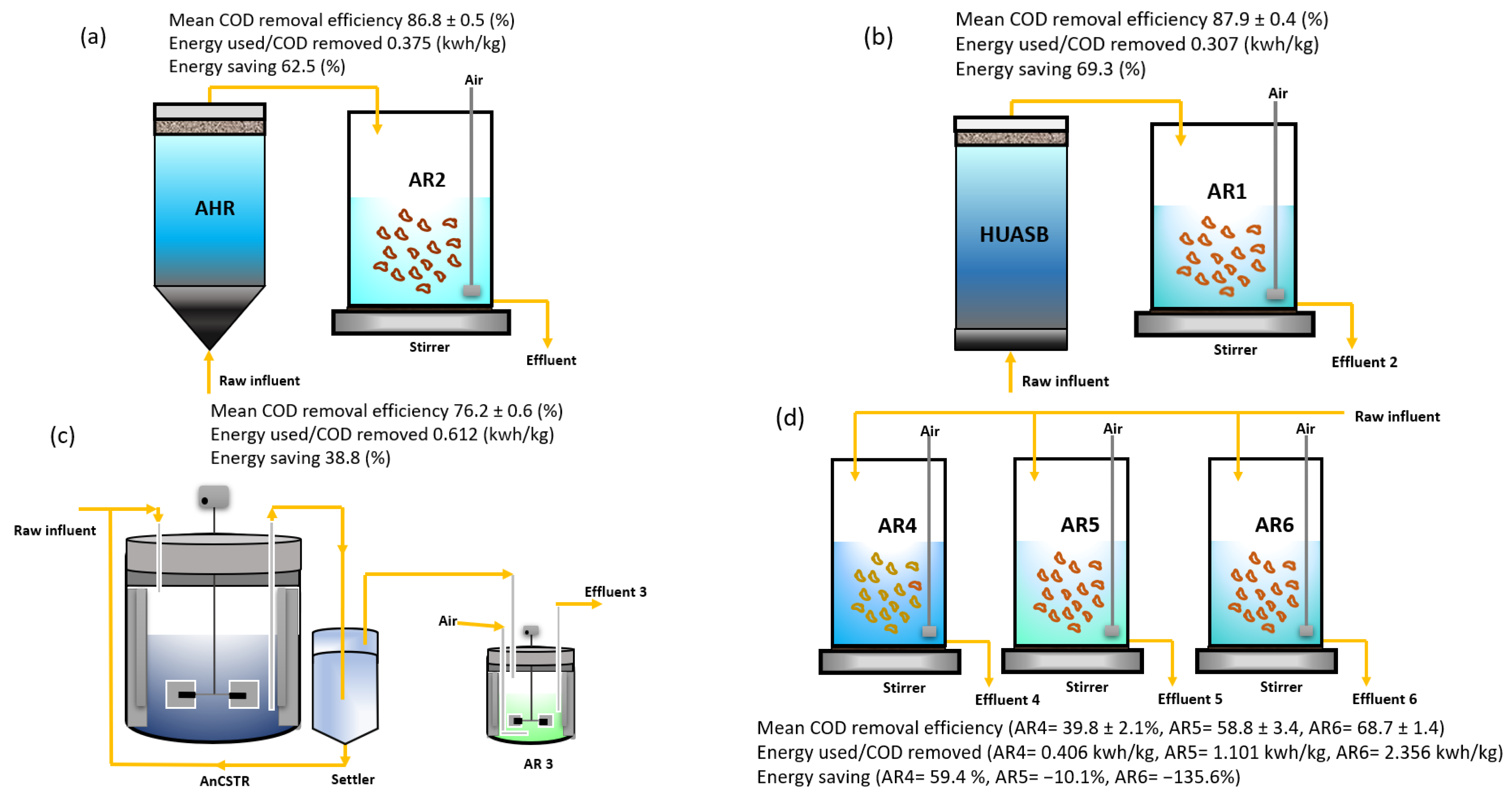

| PCPs | Toxic Components | Environmental Impact |
|---|---|---|
| Feminine Sanitary Napkins | Aldehydes, Alkanes, Halohydrocarbons, Terpenes, Ketones, PAHs | They generate toxic and mutagenic waste, produce cancer at the time of postuse exposure [80]. |
| Deodorants | Heavy metals | They are bioaccumulative, cause several diseases [81]. |
| Disposable Masks | Microplastics | When receiving UV rays, they release chemical substances that cause adverse effects on the marine ecosystem [82]. |
| Condoms | Parabens (Methylparaben, Propylparaben and Octylparaben) | Endocrine disturbances. Both estrogenic and antiandrogenic effects [83]. |
| Fragrances | Musk Xylene, Musk Ketone, Galaxolide | Musk causes endocrine disruption in aquatic biota and galaxolide is bioaccumulative [84,85]. |
| Soap, shampoo, detergent, toothpaste, sunscreen and deodorant | Triclosan (TCS, 5-chloro-2-(2,4-dichlorophenoxy)phenol) | Alters the composition of benthic bacteria. Toxic for algae species, it presents endocrine disruption in fish [86]. |
| Sunscreen | Octocrylene Oxybenzone | Benzophenones cause DNA alteration, bleaching and death of corals and reefs [86]. |
| Hair dyes | Triclosan (TCS, 5-chloro-2-(2,4-dichlorophenoxy)phenol) | Dye residues accumulate on the seafloor, entering the food chain [86]. |
| Criteria | CM | CC | Ef | ED | Ci | Wi | |
|---|---|---|---|---|---|---|---|
| CM | 1.00 | 1.29 | 1.80 | 3.00 | 1.62 | 0.38 | 1.00 |
| CC | 0.78 | 1.00 | 1.40 | 2.33 | 1.26 | 0.29 | 1.00 |
| Ef | 0.56 | 0.71 | 1.00 | 1.67 | 0.90 | 0.21 | 1.00 |
| ED | 0.33 | 0.43 | 0.60 | 1.00 | 0.54 | 0.13 | 1.00 |
| Summation | 2.67 | 3.43 | 4.80 | 8.00 | 4.33 | 1.00 | 4.00 |
| Technique | Criterion | |||||
|---|---|---|---|---|---|---|
| Inversely Proportional | Directly Proportional | |||||
| Calculated | Normalized | Calculated | ||||
| CM | CC | CM | CC | EF | ED | |
| Co-Composting | 0 | 0 | 1 | 1 | 0.924 | 0 |
| Anaerobic-aerobic | 0.707 | 1 | 0.293 | 0 | 0.924 | 0 |
| CNT | 0 | 1 | 1 | 0 | 1 | 0 |
| Technique | = | + | + | + | = | Results | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Co-composting | = | 0.3750 | + | 0.2917 | + | 0.1925 | + | 0.000 | = | 0.86 |
| Anaerobic–Aerobic | = | 0.0855 | + | 0.0000 | + | 0.1155 | + | 0.000 | = | 0.20 |
| CNT | = | 0.2083 | + | 0.0000 | + | 0.0000 | + | 0.000 | = | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dueñas-Muñoz, D.; Guevara, O.; Oviedo, G.-R.; Crisanto-Perrazo, T.; Toulkeridis, T. Sustainable Treatment Techniques for Emerging Pollutants—The Case of Personal Hygiene Products. Appl. Sci. 2022, 12, 6330. https://doi.org/10.3390/app12136330
Dueñas-Muñoz D, Guevara O, Oviedo G-R, Crisanto-Perrazo T, Toulkeridis T. Sustainable Treatment Techniques for Emerging Pollutants—The Case of Personal Hygiene Products. Applied Sciences. 2022; 12(13):6330. https://doi.org/10.3390/app12136330
Chicago/Turabian StyleDueñas-Muñoz, Deysi, Odalis Guevara, Galo-Rafael Oviedo, Tania Crisanto-Perrazo, and Theofilos Toulkeridis. 2022. "Sustainable Treatment Techniques for Emerging Pollutants—The Case of Personal Hygiene Products" Applied Sciences 12, no. 13: 6330. https://doi.org/10.3390/app12136330









