Preparation and Performance Enhancements of Low-Heat-Releasing Polyurethane Grouting Materials with Epoxy Resin and Water Glass
Abstract
:1. Introduction
2. Material and Methods Section
2.1. Materials
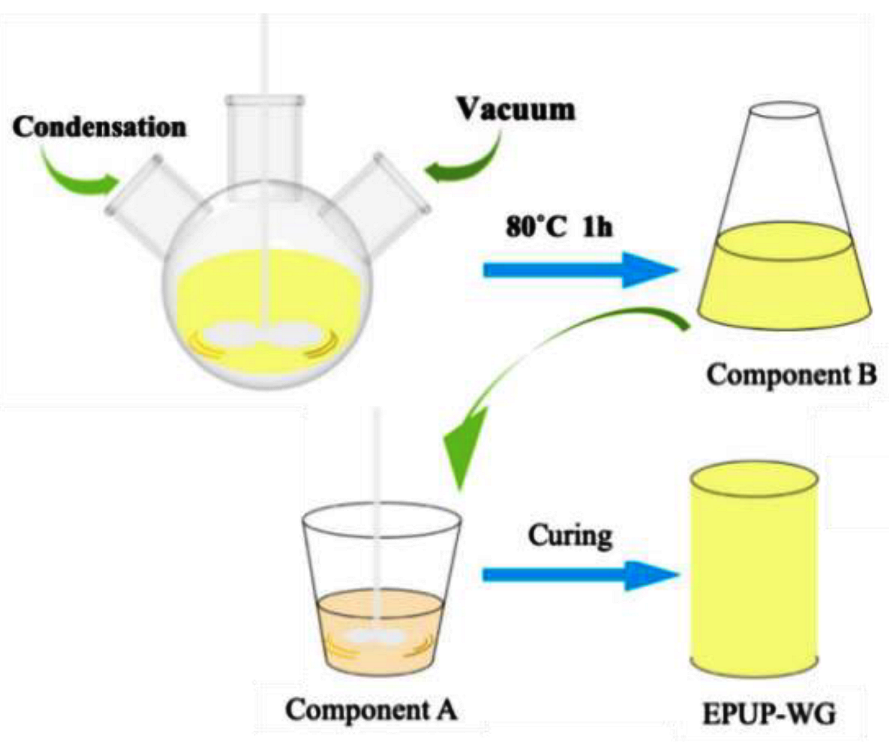
2.2. Methods
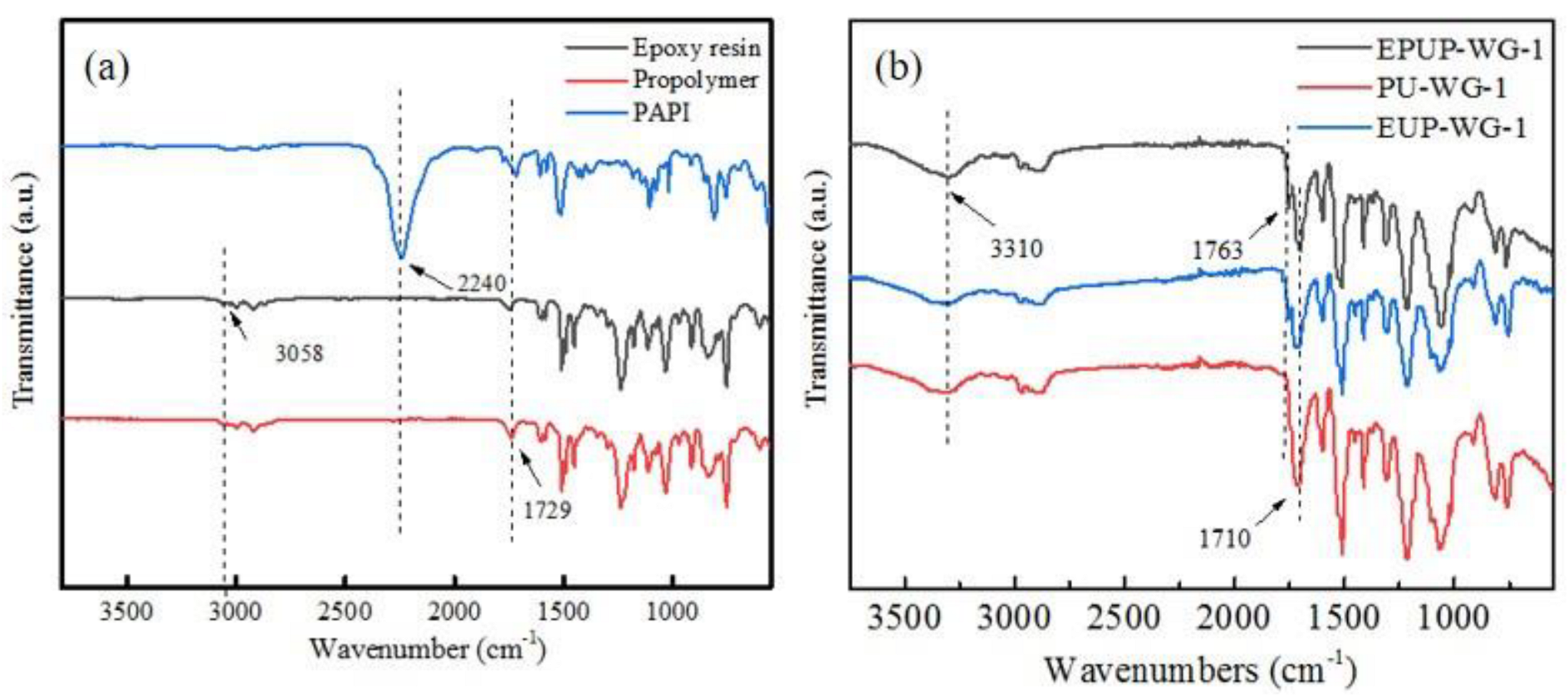
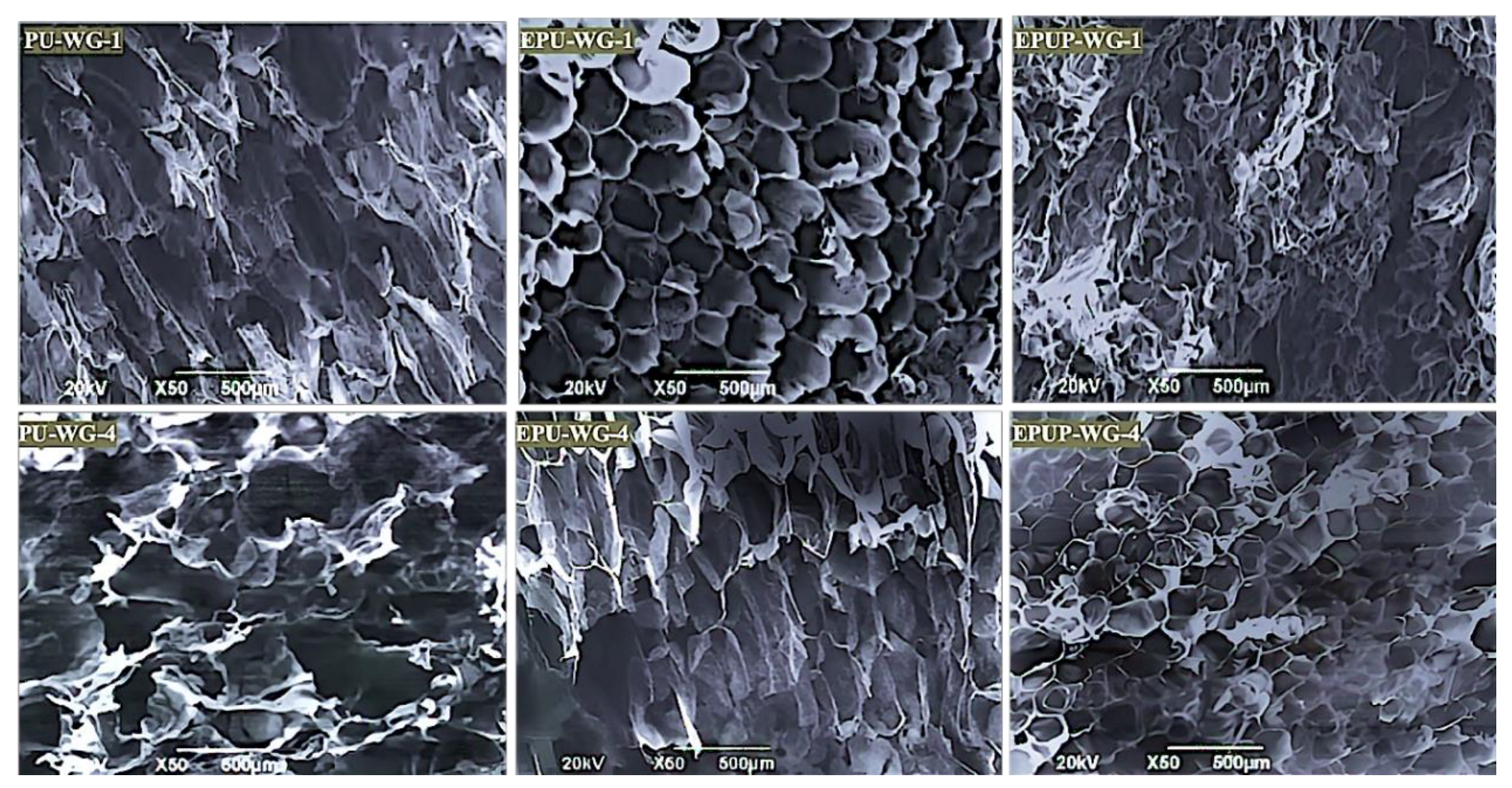
2.3. Characterization
- n is the expansion rate, and the unit is times;
- is the average density of each liquid component of the material, in grams per cubic centimeter (g/cm3);
- is the apparent core density of the cured product, in grams per cubic centimeter (g/cm3).
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, W.; Fang, Q.; Long, Z. Quantitative identification and analysis on hazard sources of roof fall accident in coal mine. Procedia Eng. 2012, 45, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Li, Q.F.; Zhao, Y.H.; Wang, J.W.; Kang, M.Q. Effect of surface silanization of carbon fiber on mechanical properties of carbon fiber reinforced polyurethane composites. Compos. Sci. Technol. 2015, 110, 87–94. [Google Scholar] [CrossRef]
- He, W.; Zhou, X.W.; Xu, R. Experimental research on the new type of sodium silicate chemical grouting material. J. China Coal Soc. 2011, 36, 1812–1815. [Google Scholar]
- Jun, K.H.; Park, J.; Na, H.; Lim, H.M.; Chang, G. Improvement of flame-retardant performance of polyurethane foam coated with water glass. Fire Sci. Eng. 2020, 34, 7–13. [Google Scholar]
- Cheng, Y.Y.; Wu, B.; Ma, X.F.; Lu, S.X.; Xu, W.G.; Szunerits, S.; Boukherroub, R. Facile preparation of high density polyethylene superhydrophobic/superoleophilic coatings on glass, copper and polyurethane sponge for self-cleaning, corrosion resistance and efficient oil/water separation. J. Colloid Interface Sci. 2018, 525, 76–85. [Google Scholar] [CrossRef]
- Butrón, C.; Axelsson, M.; Gustafson, G. Silica sol for rock grouting: Laboratory testing of strength, fracture behaviour and hydraulic conductivity. Tunn. Undergr. Space Technol. 2009, 24, 603–607. [Google Scholar] [CrossRef]
- He, Z.L.; Li, Q.F.; Wang, J.W.; Yin, N.; Jiang, S.; Kang, M.Q. Effect of silane treatment on the mechanical properties of polyurethane/water glass grouting materials. Constr. Build. Mater. 2016, 116, 110–120. [Google Scholar] [CrossRef]
- Yang, Z.P.; Zhang, X.F.; Liu, X.; Guan, X.M.; Zhang, C.J.; Niu, Y.T. Flexible and stretchable polyurethane/waterglass grouting material. Constr. Build. Mater. 2017, 138, 240–246. [Google Scholar] [CrossRef]
- Anagnostopoulos, C.A. Effect of different superplasticisers on the physical and mechanical properties of cement grouts. Constr. Build. Mater. 2014, 50, 162–168. [Google Scholar] [CrossRef]
- Xiang, S.C.; Tan, Y.S.; Gao, Y.L.; Li, L.L. Influence of a polyurethane-modified polycarboxylate on properties of cement mortar. J. Appl. Polym. Sci. 2022, 139, 51793. [Google Scholar] [CrossRef]
- Zhang, K.X.; Sun, Q.S. The use of wire mesh-polyurethane cement (WM-PUC) composite to strengthen RC T-beams under flexure. J. Struct. Eng. 2018, 15, 122–136. [Google Scholar] [CrossRef]
- Sadowski, L.; Jerzy, H.; Zak, A.; Chowaniec, A. Microstructural and mechanical assessment of the causes of failure of floors made of polyurethane-cement composites. Compos. Struct. 2020, 238, 112002. [Google Scholar]
- Sideris, K.K.; Anagnostopoulos, N.S. Durability of normal strength self-compacting concretes and their impact on service life of reinforced concrete structures. Constr. Build. Mater. 2013, 41, 491–497. [Google Scholar] [CrossRef]
- Speranza, G.P.; Peppel, W.J. Preparation of substituted 2-oxazolidones from 1,2-epoxides and isocyanates. J. Org. Chem. 1958, 23, 1922–1924. [Google Scholar] [CrossRef]
- Hong, X.D.; Dong, W.; Yang, S.B.; Mu, B.Y.; Liang, B. Study on structure and performance of reactive silicate reinforced polyurethane composite. Polym. Eng. Sci. 2015, 55, 2322–2327. [Google Scholar] [CrossRef]
- Feng, G.D.; Hu, L.H.; Ma, Y.; Zhang, M.; Liu, C.G.; Zhou, Y.H. Rigid polyisocyanurate–waterglass foam composite: Preparation, mechanism, and thermal and flame-retardant properties. J. Appl. Polym. Sci. 2018, 135, 46182. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, X.M.; Wu, M.Y.; Zhao, Y.Y.; Yu, C. Effects of different catalysts on the structure and properties of polyurethane/water glass grouting materials. J. Appl. Polym. Sci. 2018, 135, 46460. [Google Scholar] [CrossRef]
- Wang, W.Z.; Zhao, W.Q.; Zhang, J.J.; Zhou, J.H. Epoxy-based grouting materials with super-low viscosities and improved toughness. Constr. Build. Mater. 2020, 267, 121104. [Google Scholar] [CrossRef]
- Chen, K.P.; Tian, C.R.; Cao, F.; Liang, S.E.; Jia, X.R.; Wang, J.H. Preparation and characterization of highly thermostable polyisocyanurate foams modified with epoxy resin. J. Appl. Polym. Sci. 2016, 133, 43085. [Google Scholar] [CrossRef]
- Li, Z.K.; Jia, Y.B.; Bai, S.B. Polysulfone foam with high expansion ratio prepared by supercritical carbon dioxide assisted molding foaming method. RSC Adv. 2018, 8, 2880. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.F.; Lu, Q.; Lu, X.L.; Jiang, L.Q.; Cao, T. Preparation and properties of toluene-diisocyanate-trimer-modified epoxy resin. Polymers 2019, 11, 416. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Sample | Component A | Component B | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HEP-330N(g) | HSH-303 (g) | Glycerin (g) | AK8805 (g) | H2O (g) | DMP-30 (g) | DBTDL (g) | WG (phr) | KH560 (g) | NPEF-170 (g) | PAPI (g) | |
| PU-WG-1 | 8.4 | 13.1 | 10 | 1.5 | 1.7 | 0.3 | 0.1 | 0 | 1.5 | 0 | 64.9 |
| PU-WG-2 | 8.4 | 13.1 | 10 | 15 | 1.7 | 0.3 | 0.1 | 5 | 1.5 | 0 | 64.9 |
| PU-WG-3 | 8.4 | 13.1 | 10 | 1.5 | 1.7 | 0.3 | 0.1 | 10 | 1.5 | 0 | 64.9 |
| PU-WG-4 | 8.4 | 13.1 | 10 | 1.5 | 1.7 | 0.3 | 0.1 | 15 | 1.5 | 0 | 64.9 |
| PU-WG-5 | 8.4 | 13.1 | 10 | 1.5 | 1.7 | 0.3 | 0.1 | 20 | 1.5 | 0 | 64.9 |
| EPU-WG-1 | 8.4 | 13.1 | 10 | 1.5 | 1.7 | 0.3 | 0.1 | 0 | 1.5 | 28 | 64.9 |
| EPU-WG-2 | 8.4 | 13.1 | 10 | 1.5 | 1.7 | 0.3 | 0.1 | 5 | 1.5 | 28 | 64.9 |
| EPU-WG-3 | 8.4 | 13.1 | 10 | 1.5 | 1.7 | 0.3 | 0.1 | 10 | 1.5 | 28 | 64.9 |
| EPU-WG-4 | 8.4 | 13.1 | 10 | 1.5 | 1.7 | 0.3 | 0.1 | 15 | 1.5 | 28 | 64.9 |
| EPU-WG-5 | 8.4 | 13.1 | 10 | 1.5 | 1.7 | 0.3 | 0.1 | 20 | 1.5 | 28 | 64.9 |
| Component A | Component B | ||||||||||
| EPUP-WG-1 | 8.4 | 10 | 10 | 1.5 | 1.7 | 0.3 | 0.1 | 0 | 1.5 | 28 | 64.9 |
| EPUP-WG-2 | 8.4 | 10 | 10 | 1.5 | 1.7 | 0.3 | 0.1 | 5 | 1.5 | 28 | 64.9 |
| EPUP-WG-3 | 8.4 | 10 | 10 | 1.5 | 1.7 | 0.3 | 0.1 | 10 | 1.5 | 28 | 64.9 |
| EPUP-WG-4 | 8.4 | 10 | 10 | 1.5 | 1.7 | 0.3 | 0.1 | 15 | 1.5 | 28 | 64.9 |
| EPUP-WG-5 | 8.4 | 10 | 10 | 1.5 | 1.7 | 0.3 | 0.1 | 20 | 1.5 | 28 | 64.9 |
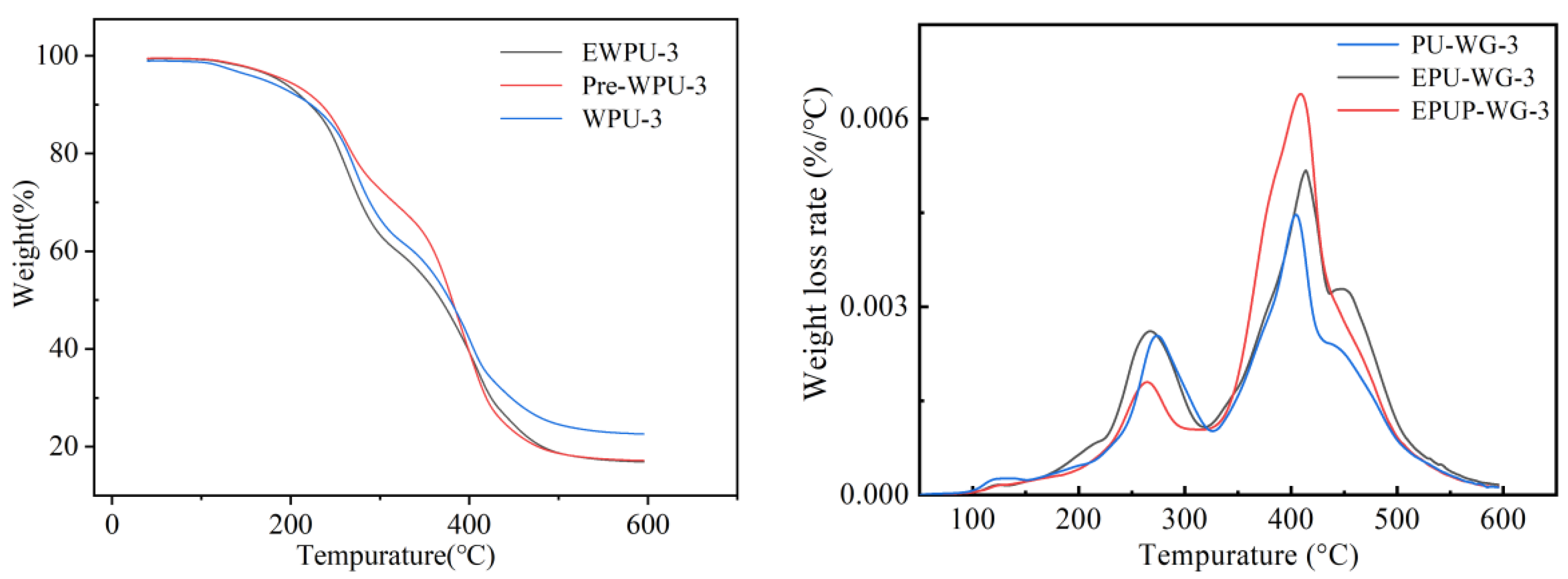
| Sample | T5% (°C) | T50% (°C) |
|---|---|---|
| PU-WG-3 | 170.77 | 378.4 |
| EPU-WG-3 | 187.52 | 368.1 |
| EPUP-WG-3 | 194.06 | 380.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, F.; Wang, S.; Dong, X.; Ye, W.; Ding, Y. Preparation and Performance Enhancements of Low-Heat-Releasing Polyurethane Grouting Materials with Epoxy Resin and Water Glass. Appl. Sci. 2022, 12, 6397. https://doi.org/10.3390/app12136397
Mei F, Wang S, Dong X, Ye W, Ding Y. Preparation and Performance Enhancements of Low-Heat-Releasing Polyurethane Grouting Materials with Epoxy Resin and Water Glass. Applied Sciences. 2022; 12(13):6397. https://doi.org/10.3390/app12136397
Chicago/Turabian StyleMei, Fanghua, Shufen Wang, Xiaoyu Dong, Wujin Ye, and Yunsheng Ding. 2022. "Preparation and Performance Enhancements of Low-Heat-Releasing Polyurethane Grouting Materials with Epoxy Resin and Water Glass" Applied Sciences 12, no. 13: 6397. https://doi.org/10.3390/app12136397




