Application of Electrolyzed Water in the Food Industry: A Review
Abstract
1. Introduction
2. Application of Electrolyzed Water in the Food Industry
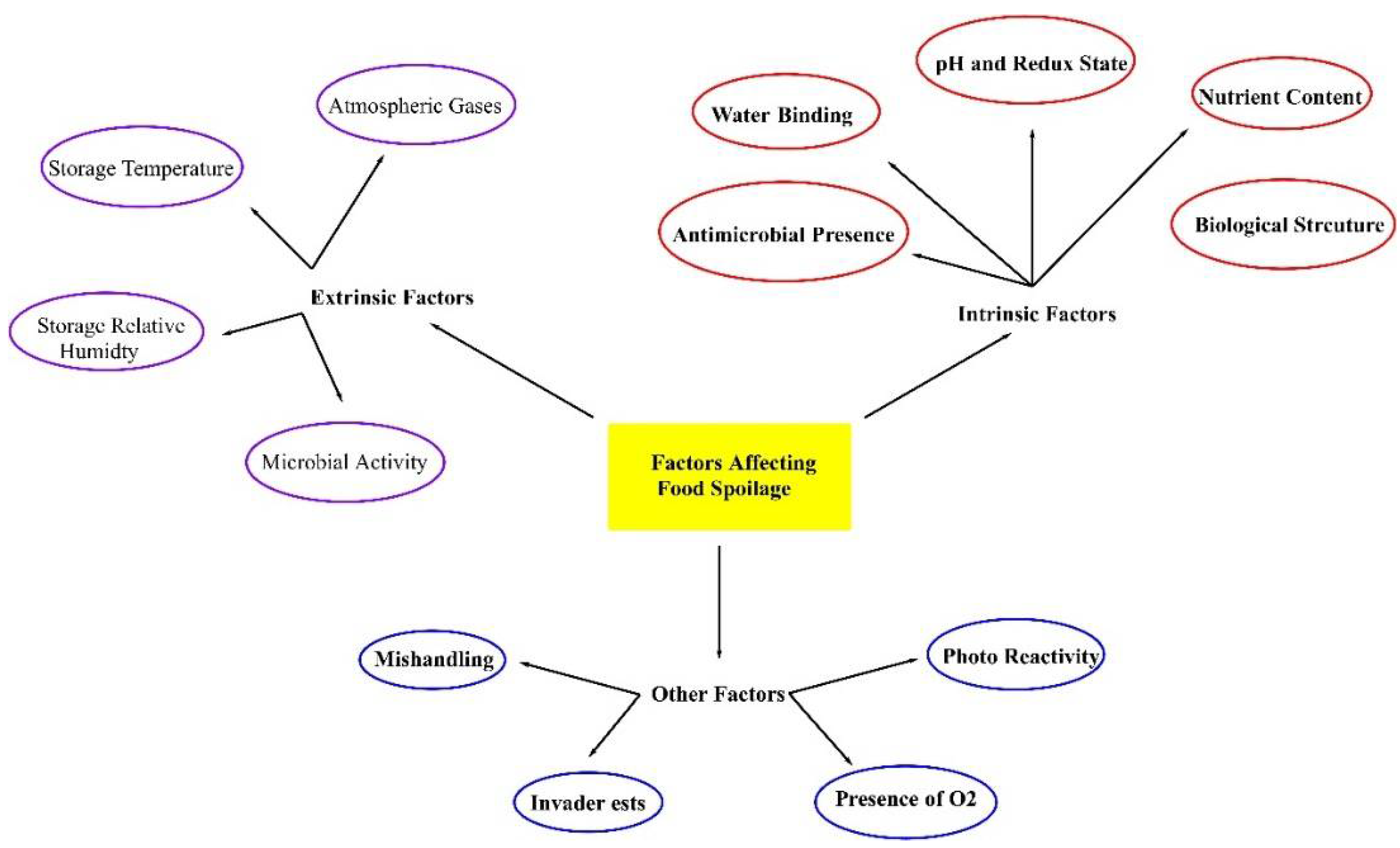
2.1. Main Factors Responsible for Food Spoilage
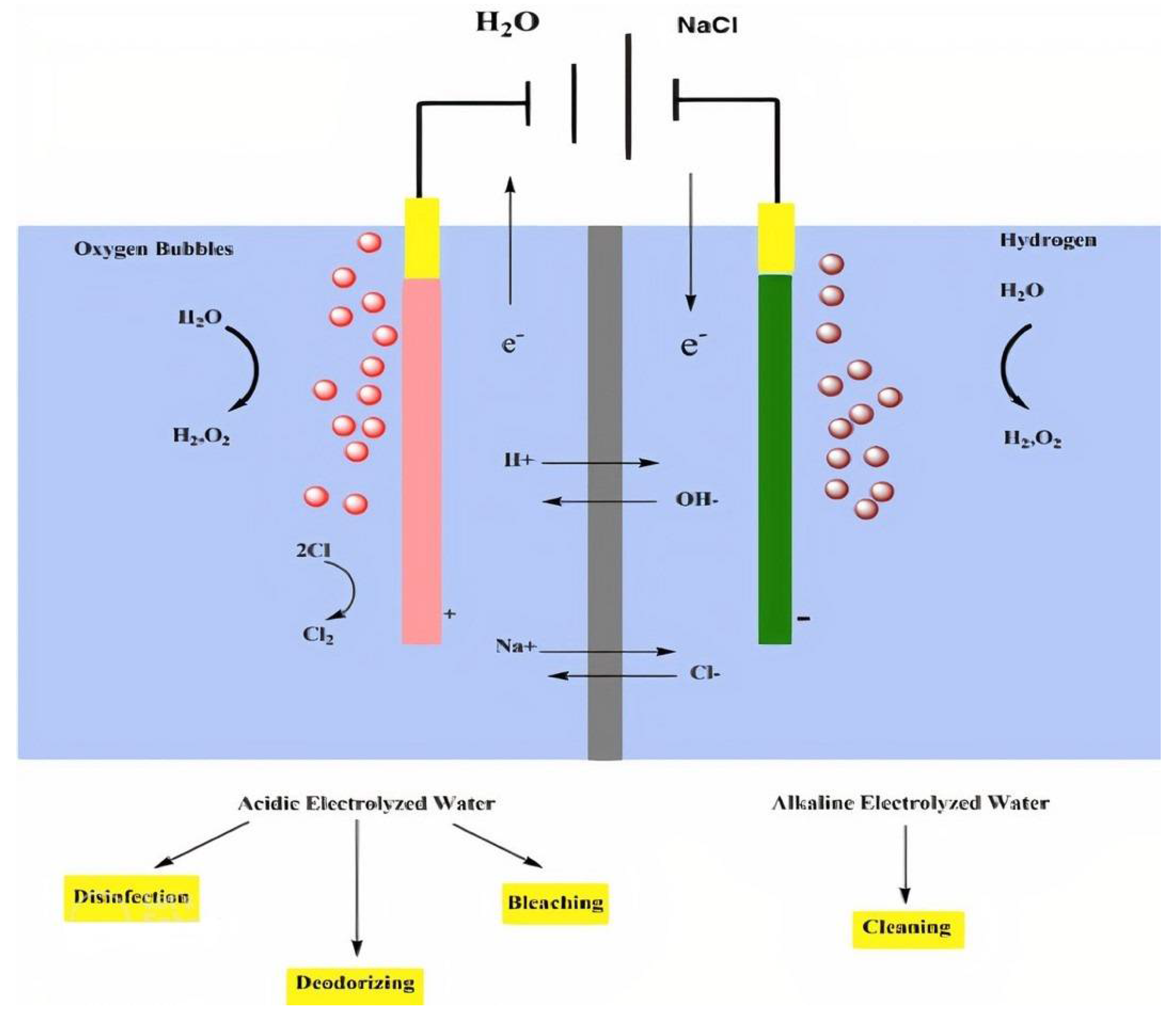
2.2. Types of Electrolyzed Water
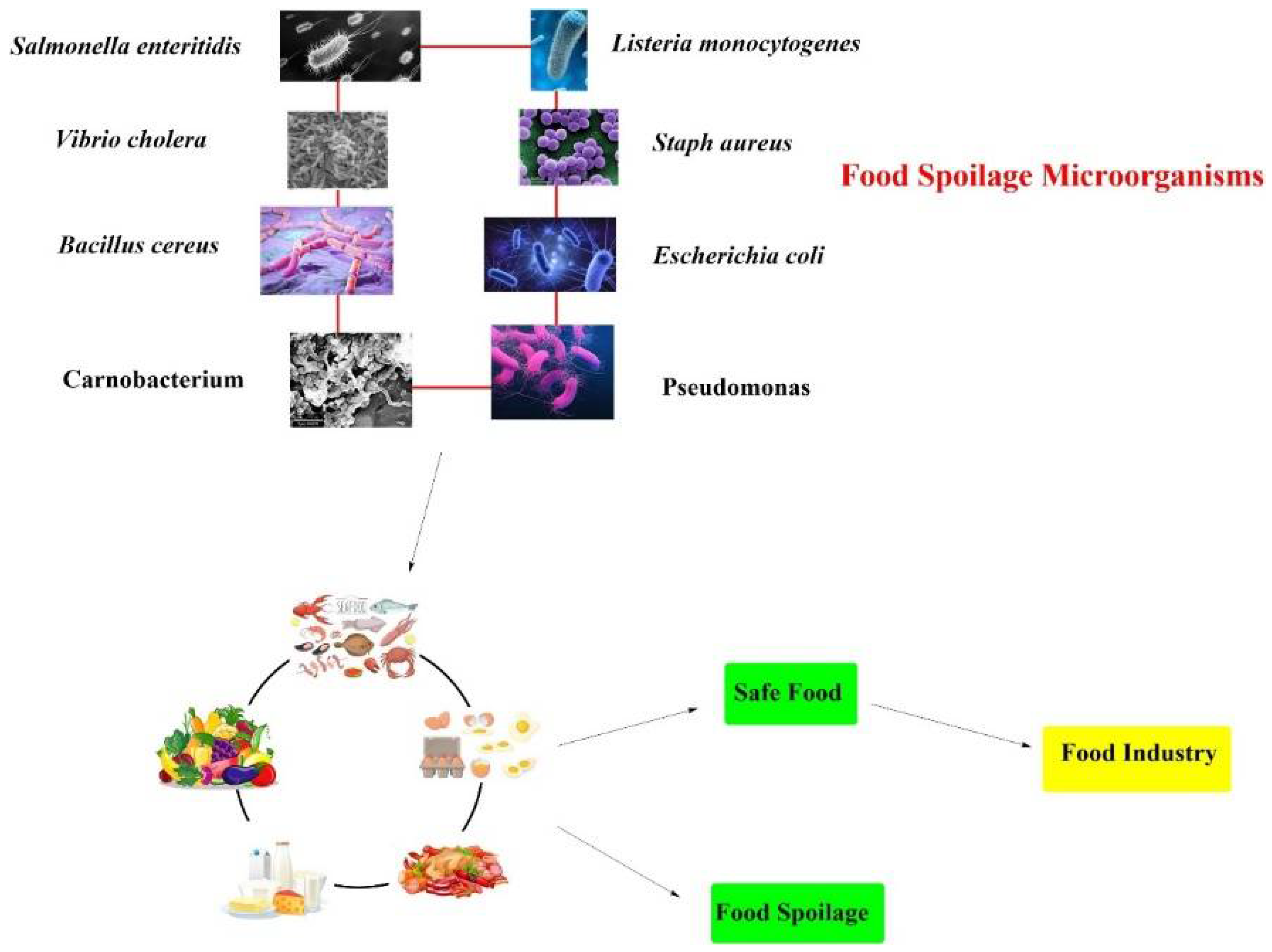
2.3. Antimicrobial Properties of Electrolyzed Water
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ishaq, A.R.; Manzoor, M.; Hussain, A.; Altaf, J.; Javed, Z.; Afzal, I.; Noor, A.; Noor, F. Prospect of microbial food borne diseases in Pakistan: A review. Braz. J. Biol. 2021, 81, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Bisht, A.; Kamble, M.P.; Choudhary, P.; Chaturvedi, K.; Kohli, G.; Juneja, V.K.; Sehgal, S.; Taneja, N.K. A surveillance of food borne disease outbreaks in India: 2009–2018. Food Control 2021, 121, 107630. [Google Scholar] [CrossRef]
- Camino Feltes, M.M.; Arisseto-Bragotto, A.P.; Block, J.M. Food quality, food-borne diseases, and food safety in the Brazilian food industry. Food Qual. Saf. 2017, 1, 13–27. [Google Scholar] [CrossRef]
- Lee, H.; Yoon, Y. Etiological agents implicated in foodborne illness world wide. Food Sci. Anim. Resour. 2021, 41, 1. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Health Observatory Data Repository-Road Traffic Deaths Data by Country. 2020. Available online: https://www.who.int/gho/road_safety/mortality/traffic_deaths_number/en/ (accessed on 28 June 2020).
- Debuisson, N.; Gurevich, R.; Even, R. Bacterial and Viral contamination of table forks, table spoons, dessert forks and teaspoons in restaurants, coffee shops, and university/hospital cafeteria. Int. J. Curr. Microbiol. Appl. Sci. 2021, 1–20. [Google Scholar]
- Fung, F.; Wang, H.S.; Menon, S. Food safety in the 21st century. Biomed. J. 2018, 41, 88–95. [Google Scholar] [CrossRef]
- Kang, Y. Food safety governance in China: Change and continuity. Food Control 2019, 106, 106752. [Google Scholar] [CrossRef]
- Maeda-Yamamoto, M. Development of functional agricultural products and use of a new health claim system in Japan. Trends Food Sci. Technol. 2017, 69, 324–332. [Google Scholar] [CrossRef]
- Tolar, B.; Joseph, L.A.; Schroeder, M.N.; Stroika, S.; Ribot, E.M.; Hise, K.B.; Gerner-Smidt, P. An overview of PulseNet USA databases. Foodborne Pathog. Dis. 2019, 16, 457–462. [Google Scholar] [CrossRef]
- Wu, M.Y.; Hsu, M.Y.; Chen, S.J.; Hwang, D.K.; Yen, T.H.; Cheng, C.M. Point-of-care detection devices for food safety monitoring: Proactive disease prevention. Trends Biotechnol. 2017, 35, 288–300. [Google Scholar] [CrossRef]
- Nayak, R.; Waterson, P. Global food safety as a complex adaptive system: Key concepts and future prospects. Trends Food Sci. Technol. 2019, 91, 409–425. [Google Scholar] [CrossRef]
- King, T.; Cole, M.; Farber, J.M.; Eisenbrand, G.; Zabaras, D.; Fox, E.M.; Hill, J.P. Food safety for food security: Relationship between global megatrends and developments in food safety. Trends Food Sci. Technol. 2017, 68, 160–175. [Google Scholar] [CrossRef]
- Davidson, R.K.; Antunes, W.; Madslien, E.H.; Belenguer, J.; Gerevini, M.; Perez, T.T.; Prugger, R. From food defence to food supply chain integrity. Br. Food J. 2017, 119, 52–66. [Google Scholar] [CrossRef]
- Panghal, A.; Chhikara, N.; Sindhu, N.; Jaglan, S. Role of food safety management systems in safe food production: A review. J. Food Saf. 2018, 38, e12464. [Google Scholar] [CrossRef]
- Mun, S.G. The effects of ambient temperature changes on food-borne illness outbreaks associated with the restaurant industry. Int. J. Hosp. Manag. 2020, 85, 102432. [Google Scholar] [CrossRef]
- Aung, M.M.; Chang, Y.S. Traceability in a food supply chain: Safety and quality perspectives. Food Control 2014, 39, 172–184. [Google Scholar] [CrossRef]
- Baloch, M.A.; Mahmood, N.; Zhang, J.W. Effect of natural resources, renewable energy and economic development on CO2 emissions in BRICS countries. Sci. Total Env. 2019, 678, 632–638. [Google Scholar]
- Cai, X.; Wallington, K.; Shafiee-Jood, M.; Marston, L. Understanding and managing the food-energy-water nexus–opportunities for water resources research. Adv. Water Resour. 2018, 111, 259–273. [Google Scholar] [CrossRef]
- Tomomewo, O.S.; Mann, M.D.; Ellafi, A.; Jabbari, H.; Tang, C.; Ba Geri, M.; Kolawole, O.; Ispas, I.; Onwumelu, C.; Alamooti, M. Creating value for the high-saline bakken produced water by optimizing its viscoelastic properties and proppant carrying tendency with high-viscosity friction reducers. In SPE Western Regional Meeting; OnePetro: Richardson, TX, USA, 2021. [Google Scholar]
- Chen, F.; Zhang, M.; Yang, C.H. Application of ultrasound technology in processing of ready-to-eat fresh food: A review. Ultrason. Sonochem. 2020, 63, 104953. [Google Scholar] [CrossRef]
- Leães, Y.S.; Pinton, M.B.; de Aguiar Rosa, C.T.; Robalo, S.S.; Wagner, R.; de Menezes, C.R.; Barin, J.S.; Campagnol, P.C.; Cichoski, A.J. Ultrasound and basic electrolyzed water: A green approach to reduce the technological defects caused by NaCl reduction in meat emulsions. Ultrason. Sonochem. 2020, 61, 104830. [Google Scholar] [CrossRef]
- Seiphetlheng, K.; Steyn, H.J.; Schall, R. Anolyte as an alternative bleach for stained cotton fabrics. J. Consum. Sci. 2017, 2, 12–23. [Google Scholar]
- Xuan, X.T.; Ding, T.; Li, J.; Ahn, J.H.; Zhao, Y.; Chen, S.G.; Ye, X.Q.; Liu, D.H. Estimation of growth parameters of Listeria monocytogenes after sublethal heat and slightly acidic electrolyzed water (SAEW) treatment. Food Control 2017, 71, 17–25. [Google Scholar] [CrossRef]
- Zhao, L.; Li, S.; Yang, H. Recent advances on research of electrolyzed water and its applications. Curr. Opin. Food Sci. 2021, 41, 180–188. [Google Scholar] [CrossRef]
- Yan, P.; Daliri, E.B.; Oh, D.H. New Clinical Applications of Electrolyzed Water: A Review. Microorganisms 2021, 9, 136. [Google Scholar] [CrossRef]
- Rahman, S.M.E.; Khan, I.; Oh, D.H. Electrolyzed water as a novel sanitizer in the food industry: Current trends and future perspectives. Compr. Rev. Food Sci. Food Saf. 2016, 15, 471–490. [Google Scholar] [CrossRef]
- Moghassem Hamidi, R.; Shekarforoush, S.S.; Hosseinzadeh, S.; Basiri, S. Evaluation of the effect of neutral electrolyzed water and peroxyacetic acid alone and in combination on microbiological, chemical, and sensory characteristics of poultry meat during refrigeration storage. Food Sci. Technol. Int. 2020, 27, 499–507. [Google Scholar] [CrossRef]
- Zang, Y.T.; Bing, S.; Li, Y.J.; Shu, D.Q.; Huang, A.M.; Wu, H.X.; Lan, L.T.; Wu, H.D. Efficacy of slightly acidic electrolyzed water on the microbial safety and shelf life of shelled eggs. Poultry Sci. 2019, 98, 5932–5939. [Google Scholar] [CrossRef]
- Hao, J.; Wang, Q. Application of electrolyzed water in fruits and vegetables industry. In Electrolyzed Water in Food: Fundamentals and Applications; Springer: Singapore, 2019; pp. 67–111. [Google Scholar]
- Afari, G.K.; Hung, Y.C. A meta-analysis on the effectiveness of electrolyzed water treatments in reducing food-borne pathogens on different foods. Food Control. 2018, 93, 150–164. [Google Scholar] [CrossRef]
- Graça, A.; Santo, D.; Quintas, C.; Nunes, C. Growth of Escherichia coli, Salmonella enterica and Listeria spp., and their inactivation using ultraviolet energy and electrolyzed water, on ‘Rocha’fresh-cut pears. Food Control 2017, 77, 41–49. [Google Scholar] [CrossRef]
- Hopkins, D.Z.; Parisi, M.A.; Dawson, P.L.; Northcutt, J.K. Surface Decontamination of Fresh, Whole Peaches (Prunus persica) Using Sodium Hypochlorite or Acidified Electrolyzed Water Solutions. Int. J. Fruit Sci. 2020, 29, 1. [Google Scholar] [CrossRef]
- Li, L.; Hao, J.; Song, S.; Nirasawa, S.; Jiang, Z.; Liu, H. Effect of slightly acidic electrolyzed water on bioactive compounds and morphology of broccoli sprouts. Food Res. Int. 2018, 105, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Shen, X.; Ulloa, O.; Suslow, T.V.; Hanrahan, I.; Zhu, M.J. Evaluation of JC9450 and neutral electrolyzed water in controlling Listeria monocytogenes on fresh apples and preventing cross-contamination. Front. Microbiol. 2020, 10, 3128. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.Z.; Mele, M.A.; Hussein, K.A.; Kang, H.M. Acidic electrolyzed water, hydrogen peroxide, ozone water and sodium hypochlorite influence quality, shelf life and antimicrobial efficacy of cherry tomatoes. Res. J. Biotechnol. 2018, 13, 4. [Google Scholar]
- Mohammad, Z.; Kalbasi-Ashtari, A.; Riskowski, G.; Juneja, V.; Castillo, A. Inactivation of Salmonella and Shiga toxin-producing Escherichia coli (STEC) from the surface of alfalfa seeds and sprouts by combined antimicrobial treatments using ozone and electrolyzed water. Food Res. Int. 2020, 136, 109488. [Google Scholar] [CrossRef]
- Nour, V.; Plesoianu, A.M.; Ionica, M.E. Effect of dip wash treatments with organic acids and acidic electrolyzed water combined with ultraviolet irradiation on quality of strawberry fruit during storage. Bragantia 2021, 80, 1–12. [Google Scholar] [CrossRef]
- Li, Y.; Tan, L.; Guo, L.; Zhang, P.; Malakar, P.K.; Ahmed, F.; Liu, H.; Wang, J.J.; Zhao, Y. Acidic electrolyzed water more effectively breaks down mature Vibrio parahaemolyticus biofilm than DNase I. Food Control 2020, 117, 107312. [Google Scholar] [CrossRef]
- Hsu, Y.F.; Chuang, C.Y.; Huang, H.C.; Yang, S. Applying membrane-less electrolyzed water for inactivating pathogenic microorganisms. Appl. Ecol. Env. Res. 2019, 17, 15019–15027. [Google Scholar] [CrossRef]
- Han, Q.; Song, X.; Zhang, Z.; Fu, J.; Wang, X.; Malakar, P.K.; Liu, H.; Pan, Y.; Zhao, Y. Removal of food-borne pathogen biofilms by acidic electrolyzed water. Front. Microbiol. 2017, 8, 988. [Google Scholar] [CrossRef]
- Stefanello, A.; Magrini, L.N.; Lemos, J.G.; Garcia, M.V.; Bernardi, A.O.; Cichoski, A.J.; Copetti, M.V. Comparison of electrolized water and multiple chemical sanitizer action against heat-resistant molds (HRM). Int. J. Food Microbiol. 2020, 335, 108856. [Google Scholar] [CrossRef]
- Singh, R.L. (Ed.) Principles and Applications of Environmental Biotechnology for a Sustainable Future; Springer: Singapore, 2017. [Google Scholar]
- Rawat, S. Food Spoilage: Microorganisms and their prevention. Asian J. Plant Science Resear. 2015, 5, 47–56. [Google Scholar]
- Hammond, S.T.; Brown, J.H.; Burger, J.R.; Flanagan, T.P.; Fristoe, T.S.; Mercado-Silva, N.; Nekola, J.C.; Okie, J.G. Food spoilage, storage, and transport: Implications for a sustainable future. BioScience 2015, 65, 758–768. [Google Scholar] [CrossRef]
- Alvarenga, V.O.; Campagnollo, F.B.; do Prado-Silva, L.; Horita, C.N.; Caturla, M.Y.; Pereira, E.P.; Crucello, A.; Sant’Ana, A.S. Impact of unit operations from farm to fork on microbial safety and quality of foods. In Advances in Food and Nutrition Research; Academic Press: Cambridge, UK, 2018; Volume 85, pp. 131–175. [Google Scholar]
- Li, G.; Rabe, K.S.; Nielsen, J.; Engqvist, M.K. Machine learning applied to predicting microorganism growth temperatures and enzyme catalytic optima. ACS Synth. Biol. 2019, 8, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Devanthi, P.V.; Gkatzionis, K. Soy sauce fermentation: Microorganisms, aroma formation, and process modification. Food Res. Int. 2019, 120, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Abatenh, E.; Gizaw, B.; Tsegaye, Z.; Wassie, M. The role of microorganisms in bioremediation-A review. Open J. Env. Biol. 2017, 2, 38–46. [Google Scholar] [CrossRef]
- Manhart, M.; Adkar, B.V.; Shakhnovich, E.I. Trade-offs between microbial growth phases lead to frequency-dependent and non-transitive selection. Proc. Royal Soc. B Biol. Sci. 2018, 285, 20172459. [Google Scholar] [CrossRef]
- Mannaa, M.; Kim, K.D. Influence of temperature and water activity on deleterious fungi and mycotoxin production during grain storage. Mycobiology 2017, 45, 240–254. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, H.U. Systems strategies for developing industrial microbial strains. Nature Biotech. 2015, 33, 1061–1072. [Google Scholar] [CrossRef]
- Odeyemi, O.A.; Alegbeleye, O.O.; Strateva, M.; Stratev, D. Understanding spoilage microbial community and spoilage mechanisms in foods of animal origin. Compr. Rev. Food Sci. Food Saf. 2020, 19, 311–331. [Google Scholar] [CrossRef]
- Rolfe, C.; Daryaei, H. Intrinsic and Extrinsic Factors Affecting Microbial Growth in Food Systems. In Food Safety Engineering; Springer: Cham, Switzerland, 2020; pp. 3–24. [Google Scholar]
- Gonzales-Barron, U.; Coelho-Fernandes, S.; Santos-Rodrigues, G.; Choupina, A.; Piedra, R.B.; Osoro, K.; Celaya, R.; García, R.R.; Peric, T.; Del Bianco, S.; et al. Microbial deterioration of lamb meat from European local breeds as affected by its intrinsic properties. Small Rumin. Res. 2021, 195, 106298. [Google Scholar] [CrossRef]
- Smet, C.; Baka, M.; Dickenson, A.; Walsh, J.L.; Valdramidis, V.P.; Van Impe, J.F. Antimicrobial efficacy of cold atmospheric plasma for different intrinsic and extrinsic parameters. Plasma Processes Polym. 2018, 15, 1700048. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Holley, R.A. Factors influencing the microbial safety of fresh produce: A review. Food Microbiol. 2012, 32, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Møretrø, T.; Langsrud, S. Residential bacteria on surfaces in the food industry and their implications for food safety and quality. Comp. Rev. Food Sci. Food Saf. 2017, 16, 1022–1041. [Google Scholar] [CrossRef] [PubMed]
- Hamad, S.H. 20 factors affecting the growth of microorganisms in food. Prog. Food Preserv. 2012, 10, 405. [Google Scholar]
- Zhao, P.; Ndayambaje, J.P.; Liu, X.; Xia, X. Microbial spoilage of fruits: A review on causes and prevention methods. Food Rev. Int. 2020, 38, 1–22. [Google Scholar] [CrossRef]
- Pangloli, P.; Hung, Y.C. Effects of water hardness and pH on efficacy of chlorine-based sanitizers for inactivating Escherichia coli O157: H7 and Listeria monocytogenes. Food Control 2013, 32, 626–631. [Google Scholar] [CrossRef]
- Xie, J.; Sun, X.H.; Pan, Y.J.; Zhao, Y. Physicochemical properties and bactericidal activities of acidic electrolyzed water used or stored at different temperatures on shrimp. Food Res. Int. 2012, 47, 331–336. [Google Scholar] [CrossRef]
- Wang, H.; Feng, H.; Luo, Y. Microbial reduction and storage quality of fresh-cut cilantro washed with acidic electrolyzed water and aqueous ozone. Food Res. Int. 2004, 37, 949–956. [Google Scholar] [CrossRef]
- Rivera-Garcia, A.; Santos-Ferro, L.; Ramirez-Orejel, J.C.; Agredano-Moreno, L.T.; Jimenez-Garcia, L.F.; Paez-Esquiliano, D.; Andrade-Esquivel, E.; Cano-Buendia, J.A. The effect of neutral electrolyzed water as a disinfectant of eggshells artificially contaminated with Listeria monocytogenes. Food Sci. Nut. 2019, 7, 2252–2260. [Google Scholar] [CrossRef]
- Hsu, S.Y. Effects of flow rate, temperature and salt concentration on chemical and physical properties of electrolyzed oxidizing water. J. Food Eng. 2005, 66, 171–176. [Google Scholar] [CrossRef]
- Rahman, S.M.; Ding, T.; Oh, D.H. Effectiveness of low concentration electrolyzed water to inactivate food-borne pathogens under different environmental conditions. Int. J. Food Microbiol. 2010, 139, 147–153. [Google Scholar] [CrossRef]
- Xuan, X.T.; Fan, Y.F.; Ling, J.G.; Hu, Y.Q.; Liu, D.H.; Chen, S.G.; Ye, X.Q.; Ding, T. Preservation of squid by slightly acidic electrolyzed water ice. Food Control 2017, 73, 1483–1489. [Google Scholar] [CrossRef]
- Bansal, V.; Prasad, P.; Mehta, D.; Siddiqui, M.W. Ultrasound techniques in postharvest disinfection of fruits and vegetables. In Postharvest Disinfection of Fruits and Vegetables; Academic Press: Cambridge, UK, 2018; pp. 159–177. [Google Scholar]
- Shiroodi, S.G.; Ovissipour, M. Electrolyzed water application in fresh produce sanitation. In Postharvest Disinfection of Fruits and Vegetables; Academic Press: Cambridge, MA, USA, 2018; pp. 67–89. [Google Scholar]
- Xuan, X.; Ling, J. Generation of electrolyzed water. In Electrolyzed Water in Food: Fundamentals and Applications; Springer: Singapore, 2019; pp. 1–6. [Google Scholar]
- Hricova, D.; Stephan, R.; Zweifel, C. Electrolyzed water and its application in the food industry. J. Food Prot. 2008, 71, 1934–1947. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Orejel, J.C.; Cano-Buendía, J.A. Applications of electrolyzed water as a sanitizer in the food and animal-by products industry. Processes 2020, 8, 534. [Google Scholar] [CrossRef]
- Rahman, S.M.; Ding, T.; Oh, D.H. Inactivation effect of newly developed low concentration electrolyzed water and other sanitizers against microorganisms on spinach. Food Control 2010, 21, 1383–1387. [Google Scholar] [CrossRef]
- Keskinen, L.A.; Burke, A.; Annous, B.A. Efficacy of chlorine, acidic electrolyzed water and aqueous chlorine dioxide solutions to decontaminate Escherichia coli O157: H7 from lettuce leaves. Int. J. Food Microbiol. 2009, 132, 134–140. [Google Scholar] [CrossRef]
- Forghani, F.; Park, J.H.; Oh, D.H. Effect of water hardness on the production and microbicidal efficacy of slightly acidic electrolyzed water. Food Microbiol. 2015, 48, 28–34. [Google Scholar] [CrossRef]
- Cao, T.T.; Wang, Y.J.; Zhang, Y.Q. Effect of strongly alkaline electrolyzed water on silk degumming and the physical properties of the fibroin fiber. PLoS ONE 2013, 8, e65654. [Google Scholar] [CrossRef]
- Quan, Y.; Choi, K.D.; Chung, D.; Shin, I.S. Evaluation of bactericidal activity of weakly acidic electrolyzed water (WAEW) against Vibrio vulnificus and Vibrio parahaemolyticus. Int. J. Food Microbiol. 2010, 136, 255–260. [Google Scholar] [CrossRef]
- Rahman, S.M.; Jin, Y.G.; Oh, D.H. Combination treatment of alkaline electrolyzed water and citric acid with mild heat to ensure microbial safety, shelf-life and sensory quality of shredded carrots. Food Microbiol. 2011, 28, 484–491. [Google Scholar] [CrossRef]
- Liang, D.; Wang, Q.; Zhao, D.; Han, X.; Hao, J. Systematic application of slightly acidic electrolyzed water (SAEW) for natural microbial reduction of buckwheat sprouts. LWT 2019, 108, 14–20. [Google Scholar] [CrossRef]
- Ming, R.; Zhu, Y.; Deng, L.; Zhang, A.; Wang, J.; Han, Y.; Ren, Z. Effect of electrode material and electrolysis process on the preparation of electrolyzed oxidizing water. N. J. Chem. 2018, 42, 12143–12151. [Google Scholar] [CrossRef]
- Cao, W.; Zhu, Z.W.; Shi, Z.X.; Wang, C.Y.; Li, B.M. Efficiency of slightly acidic electrolyzed water for inactivation of Salmonella enteritidis and its contaminated shell eggs. Int. J. Food Microbiol. 2009, 130, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Fabrizio, K.A.; Cutter, C.N. Stability of electrolyzed oxidizing water and its efficacy against cell suspensions of Salmonella Typhimurium and Listeria monocytogenes. J. Food Prot. 2003, 66, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Nan, S.; Li, Y.; Li, B.; Wang, C.; Cui, X.; Cao, W. Effect of slightly acidic electrolyzed water for inactivating Escherichia coli O157: H7 and Staphylococcus aureus analyzed by transmission electron microscopy. J. Food Protect. 2010, 73, 2211–2216. [Google Scholar] [CrossRef]
- Zheng, W.; Xie, C.; Liang, J.; Yu, Q.D.; Bai, D.; Huang, J. Effects of weak acidic electrolytic water ice and modified packaging on shrimp quality of Litopenaeus vannamei. Sci. Technol. Food Ind. 2018, 39, 183–187. [Google Scholar]
- Huang, Y.R.; Hung, Y.C.; Hsu, S.Y.; Huang, Y.W.; Hwang, D.F. Application of electrolyzed water in the food industry. Food Control 2008, 19, 329–345. [Google Scholar] [CrossRef]
- Izumi, H. Electrolyzed water as a disinfectant for fresh-cut vegetables. J. Food Sci. 1999, 64, 536–539. [Google Scholar] [CrossRef]
- Al-Haq, M.I.; Sugiyama, J.; Isobe, S. Applications of electrolyzed water in agriculture & food industries. Food Sci. Technol. Res. 2005, 11, 135–150. [Google Scholar]
- Koseki, S.; Yoshida, K.; Isobe, S.; Itoh, K. Decontamination of lettuce using acidic electrolyzed water. J. Food Protect. 2001, 64, 652–658. [Google Scholar] [CrossRef]
- Northcutt, J.; Smith, D.; Ingram, K.D.; Hinton, A., Jr.; Musgrove, M. Recovery of bacteria from broiler carcasses after spray washing with acidified electrolyzed water or sodium hypochlorite solutions. Poultry Sci. 2007, 86, 2239–2244. [Google Scholar] [CrossRef]
- Abadias, M.; Usall, J.; Oliveira, M.; Alegre, I.; Viñas, I. Efficacy of neutral electrolyzed water (NEW) for reducing microbial contamination on minimally-processed vegetables. Int. J. Food Microbiol. 2008, 123, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Shang, Y.; Shi, Z.; Xin, H.; Cao, W. Physicochemical properties and bactericidal efficiency of neutral and acidic electrolyzed water under different storage conditions. J. Food Eng. 2009, 91, 582–586. [Google Scholar] [CrossRef]
- Koseki, S.; Yoshida, K.; Isobe, S.; Itoh, K. Efficacy of acidic electrolyzed water for microbial decontamination of cucumbers and strawberries. J. Food Prot. 2004, 67, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Issa-Zacharia, A.; Kamitani, Y.; Morita, K.; Iwasaki, K. Sanitization potency of slightly acidic electrolyzed water against pure cultures of Escherichia coli and Staphylococcus aureus, in comparison with that of other food sanitizers. Food Control 2010, 21, 740–745. [Google Scholar] [CrossRef]
- Deza, M.A.; Araujo, M.; Garrido, M.J. Efficacy of neutral electrolyzed water to inactivate Escherichia coli, Listeria monocytogenes, Pseudomonas aeruginosa, and Staphylococcus aureus on plastic and wooden kitchen cutting boards. J. Food Prot. 2007, 70, 102–108. [Google Scholar] [CrossRef]
- Wang, H.; Duan, D.; Wu, Z.; Xue, S.; Xu, X.; Zhou, G. Primary concerns regarding the application of electrolyzed water in the meat industry. Food Control 2019, 95, 50–56. [Google Scholar] [CrossRef]
- Koide, S.; Takeda, J.I.; Shi, J.; Shono, H.; Atungulu, G.G. Disinfection efficacy of slightly acidic electrolyzed water on fresh cut cabbage. Food Control 2009, 20, 294–297. [Google Scholar] [CrossRef]
- Graca, A.; Abadias, M.; Salazar, M.; Nunes, C. The use of electrolyzed water as a disinfectant for minimally processed apples. Postharvest Biol. Technol. 2011, 61, 172–177. [Google Scholar] [CrossRef]
- Nakayama, M.; Kabayama, S.; Nakano, H.; Zhu, W.J.; Terawaki, H.; Nakayama, K.; Katoh, K.; Satoh, T.; Ito, S. Biological effects of electrolyzed water in hemodialysis. Nephron Clin. Pract. 2009, 112, c9–c15. [Google Scholar] [CrossRef]
- Tango, C.N.; Khan, I.; Kounkeu, P.F.; Momna, R.; Hussain, M.S.; Oh, D.H. Slightly acidic electrolyzed water combined with chemical and physical treatments to decontaminate bacteria on fresh fruits. Food Microbiol. 2017, 67, 97–105. [Google Scholar] [CrossRef]
- Athayde, D.R.; Flores, D.R.; Silva, J.S.; Silva, M.S.; Genro, A.L.; Wagner, R.; Campagnol, P.C.; Menezes, C.R.; Cichoski, A.J. Characteristics and use of electrolyzed water in food industries. Int. Food Res. J. 2018, 25, 11–16. [Google Scholar]
- Dong, H.; Nagamatsu, Y.; Chen, K.K.; Tajima, K.; Kakigawa, H.; Shi, S.; Kozono, Y. Corrosion behavior of dental alloys in various types of electrolyzed water. Dental Mat. J. 2003, 22, 482–493. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Water | Detected Elements | Ca+2 (mg) | Mg+2 (mg) | K+ (mg) | Na+ (mg) |
|---|---|---|---|---|---|
| Ordinary tap-water | pH 8.00 | 24.42 | 4.98 | 5.04 | 46.60 |
| Ultrapure water | pH 8.23 | 5.32 | 0.83 | 0.94 | 2.88 |
| Acid electrolyzed water | pH 3.00 | 13.55 | 2.92 | 1.71 | 19.47 |
| SAEW | pH 5–6.5 | 16.53 | 3.41 | 4.96 | 56.03 |
| EW Generating Machine | Salt/Acid Used as Substrate | pH | Reported Food Safety Application | Target Pathogen | Reference |
|---|---|---|---|---|---|
| NEW | NaCl (1.0%) | 8.6 | Lettuce, corn salad, shredded carrots, freshly cut iceberg lettuce | Salmonella, Escherichia coli | [90] |
| AEW | NaCl (0.1–0.2%) | 2.5 | Alfalfa seeds and sprouts, tomatoes | E. coli O157:H7, Listeria monocytogenes | [93] |
| NEW | NaCl (25%) | 8.27 | Plastic and wood cutting boards | Staphylococcus aureus, Listeria monocytogene, Pseudomonas | [94] |
| SAEW | NaCl (0.1%) | 5.9 | Pure culture | Vibrio vulnificus | [77] |
| SAEW | HCl (2%) | 5.8 | Pure culture | Escherichia coli, Salmonella, S. aureus | [83] |
| SALcEW | NaCl (0.9%) | 6.2–6.3 | Freshly cut spinach | Total bacteria, yeast, molds, E. coli O157:H7, Listeria monocytogenes | [73] |
| SAEW | NaCl (0.6%) and HCl (0.15%) | 6–6.5 | Pure culture, Lettuce, pork | Total bacteria, Listeria monocytogenes | [67,70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebezov, M.; Saeed, K.; Khaliq, A.; Rahman, S.J.U.; Sameed, N.; Semenova, A.; Khayrullin, M.; Dydykin, A.; Abramov, Y.; Thiruvengadam, M.; et al. Application of Electrolyzed Water in the Food Industry: A Review. Appl. Sci. 2022, 12, 6639. https://doi.org/10.3390/app12136639
Rebezov M, Saeed K, Khaliq A, Rahman SJU, Sameed N, Semenova A, Khayrullin M, Dydykin A, Abramov Y, Thiruvengadam M, et al. Application of Electrolyzed Water in the Food Industry: A Review. Applied Sciences. 2022; 12(13):6639. https://doi.org/10.3390/app12136639
Chicago/Turabian StyleRebezov, Maksim, Kanza Saeed, Adnan Khaliq, Syed Junaid Ur Rahman, Nimra Sameed, Anastasia Semenova, Mars Khayrullin, Andrey Dydykin, Yury Abramov, Muthu Thiruvengadam, and et al. 2022. "Application of Electrolyzed Water in the Food Industry: A Review" Applied Sciences 12, no. 13: 6639. https://doi.org/10.3390/app12136639
APA StyleRebezov, M., Saeed, K., Khaliq, A., Rahman, S. J. U., Sameed, N., Semenova, A., Khayrullin, M., Dydykin, A., Abramov, Y., Thiruvengadam, M., Shariati, M. A., Bangar, S. P., & Lorenzo, J. M. (2022). Application of Electrolyzed Water in the Food Industry: A Review. Applied Sciences, 12(13), 6639. https://doi.org/10.3390/app12136639










