Nanomechanical, Structural and Antioxidant Characterization of Nixtamalized Popcorn Pericarp
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Proximate Chemical Composition
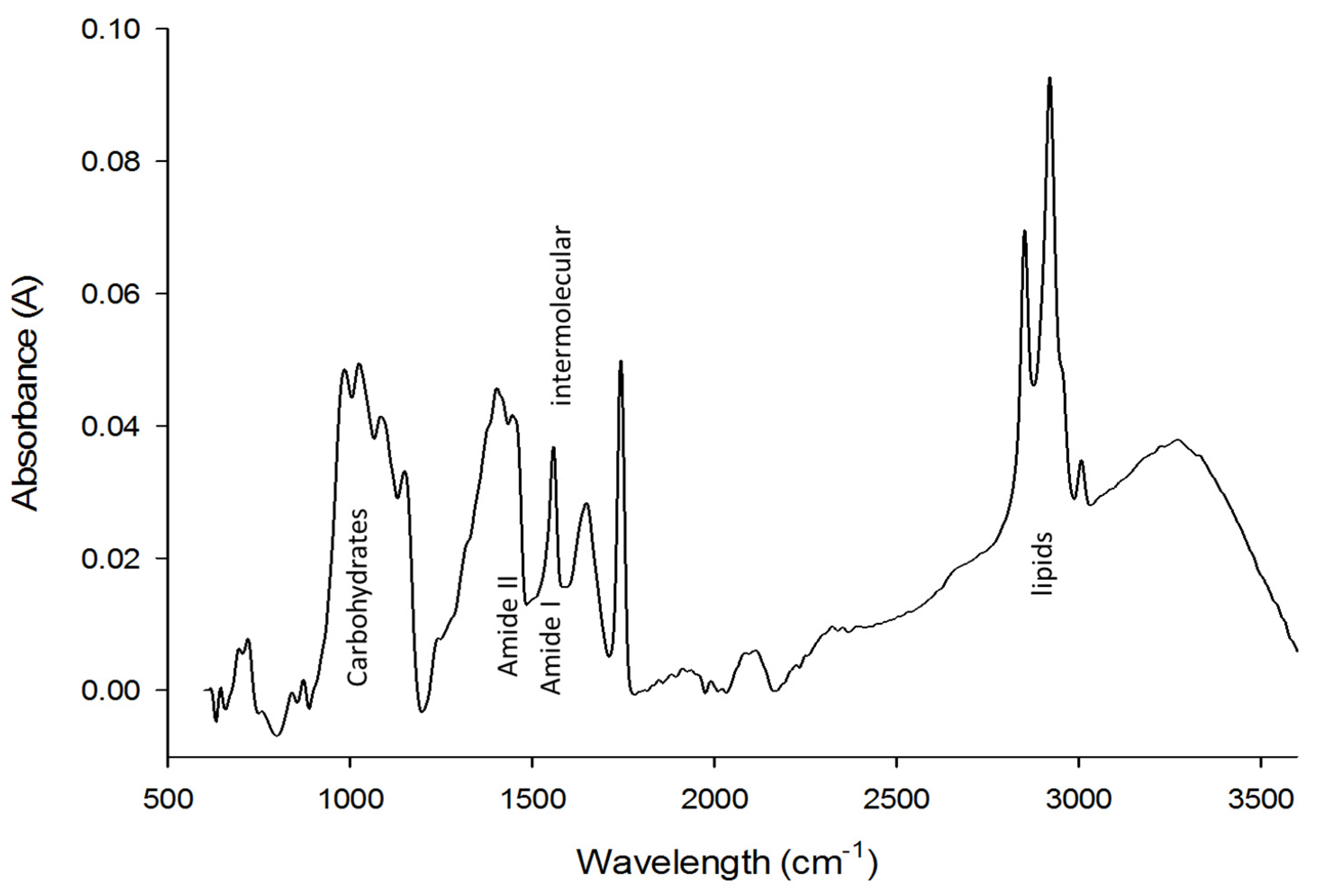
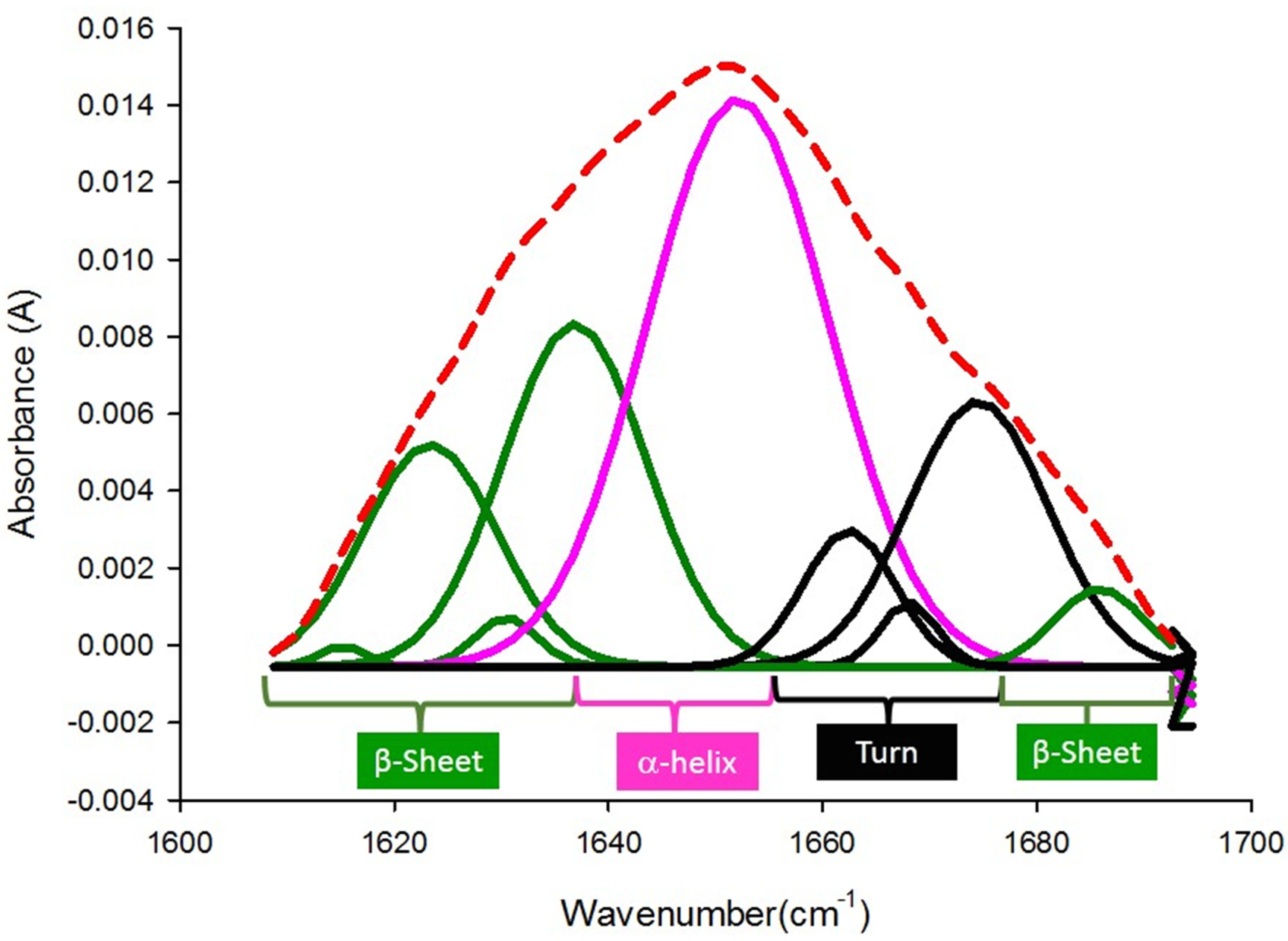
2.3. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.4. Total Phenolic Content
2.5. Antioxidant Activity
2.5.1. ABTS and DPPH
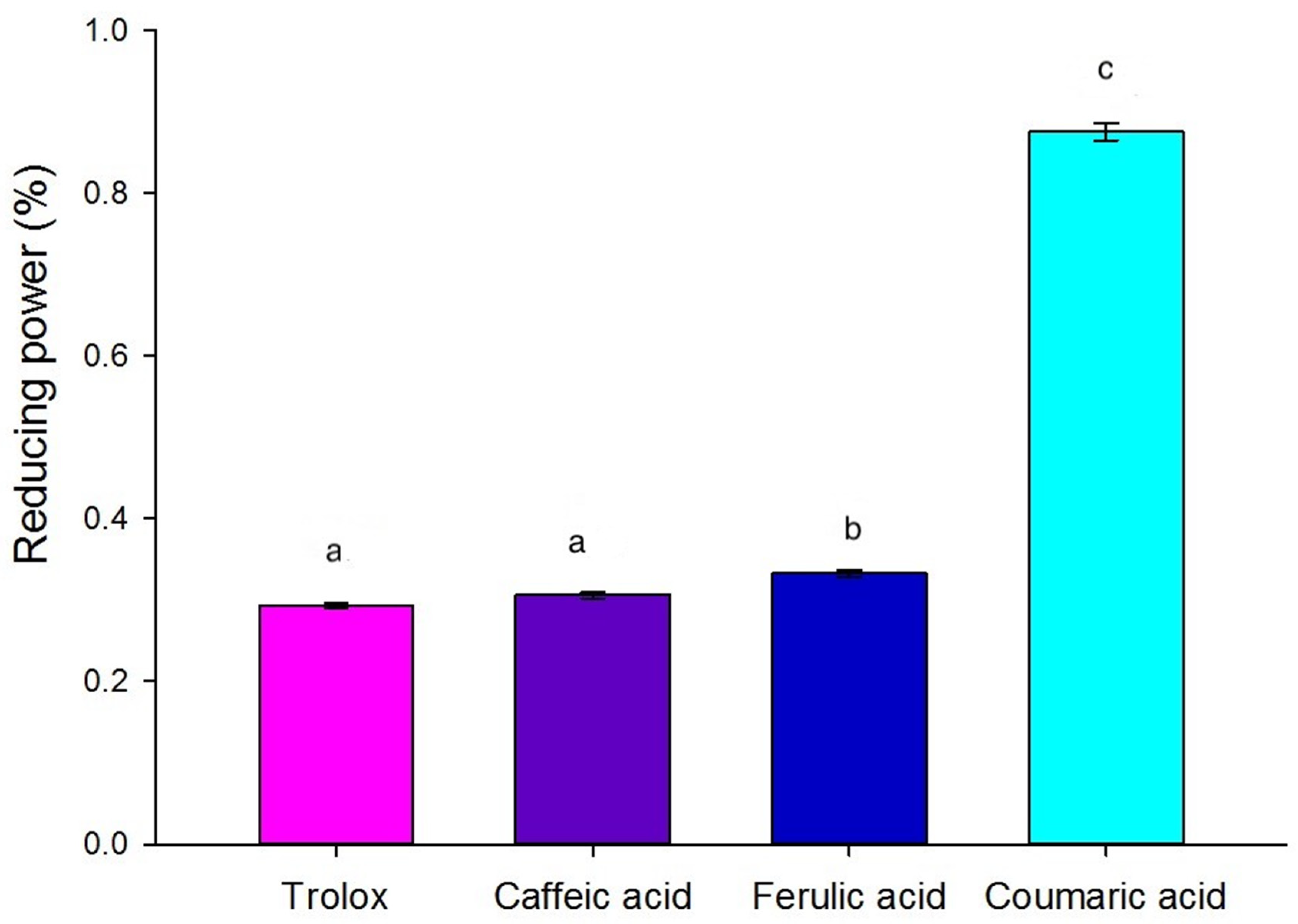
2.5.2. Reducing Power
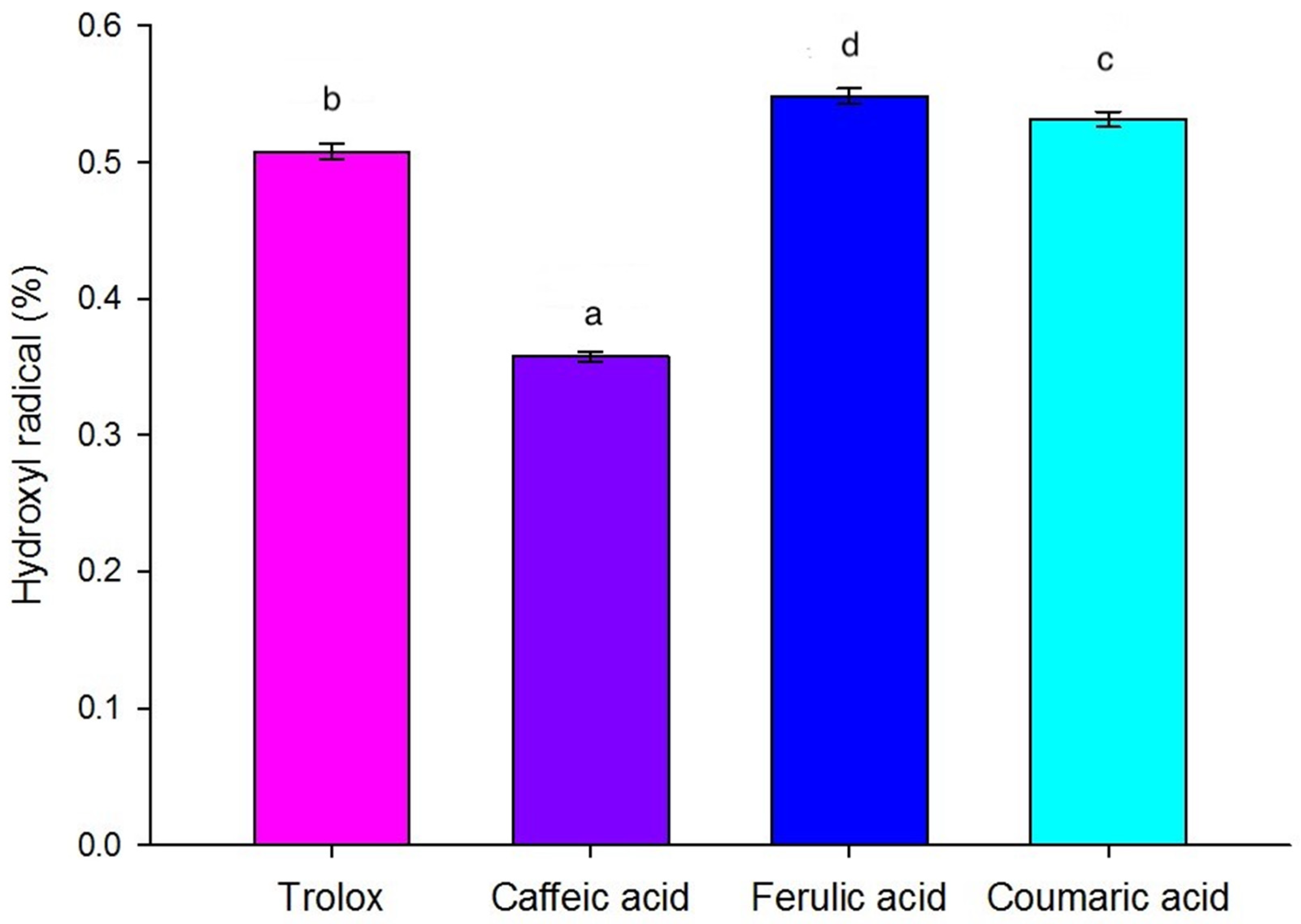
2.5.3. Hydroxyl Radical
2.5.4. Iron Chelation
2.6. Scanning Electron Microscopy (SEM)
2.7. Nanoindentation and Imaging with Atomic Force Microscopy (AFM)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Parameters
3.2. FTIR Spectra of Pericarp
3.3. Total Phenolic Content
3.4. Antioxidant Activity
3.4.1. ABTS and DPPH
3.4.2. Reducing Power
3.4.3. Hydroxyl Radical
3.4.4. Iron Chelation
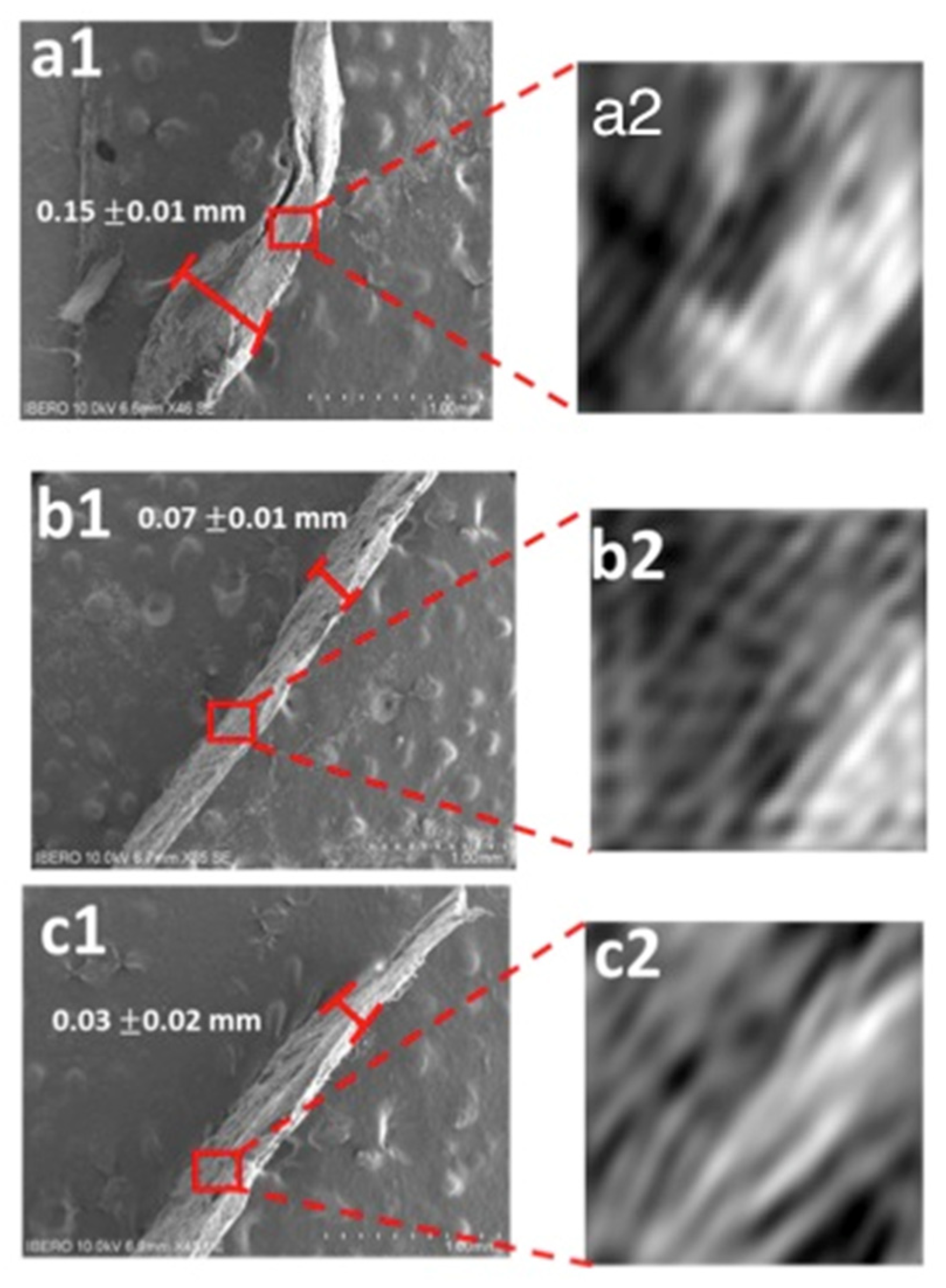
3.5. Scanning Electron Microscopy (SEM)
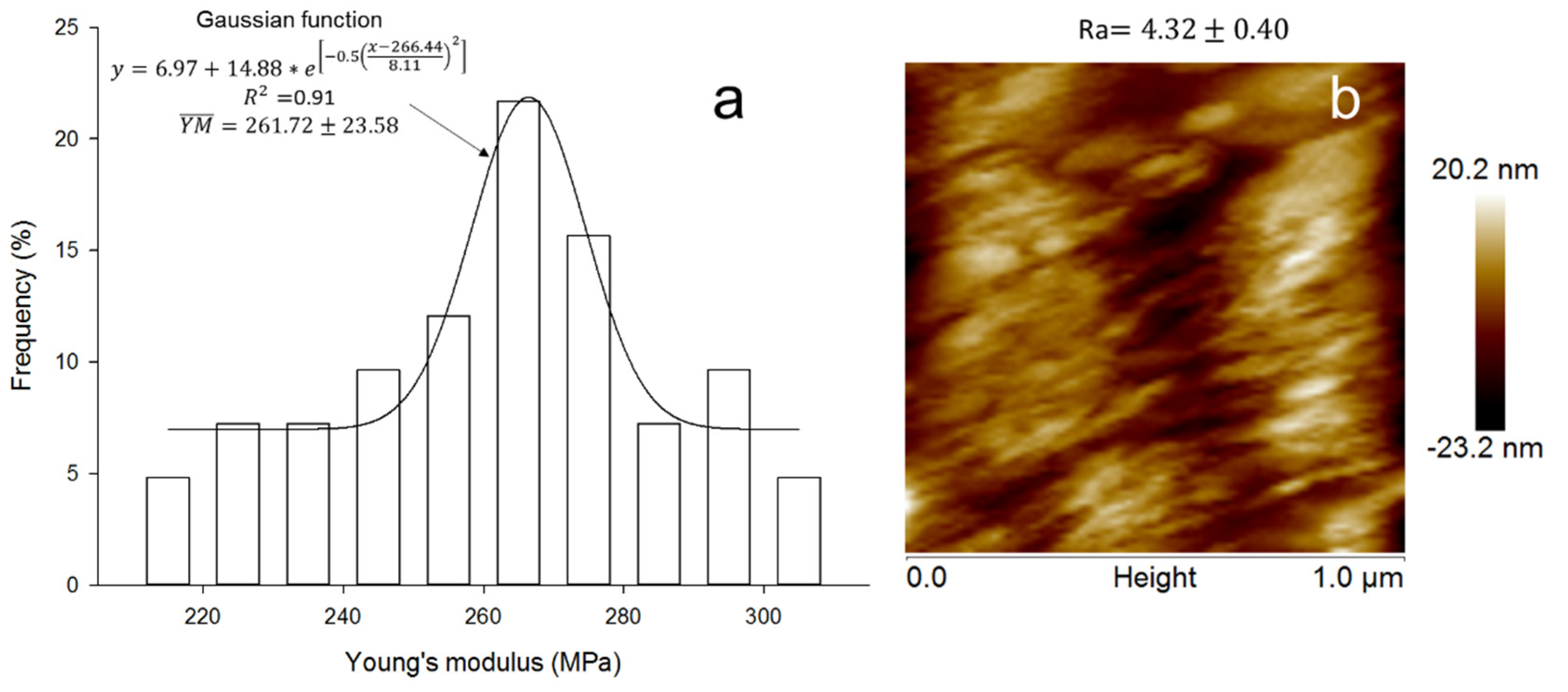
3.6. Nanostructure and Nanoindentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutiérrez-Cortez, E.; Rojas-Molina, I.; Rojas, A.; Arjona, J.L.; Cornejo-Villegas, M.A.; Zepeda-Benítez, Y.; Velázquez-Hernández, R.; Ibarra-Alvarado, C.; Rodríguez-García, M.E. Microstructural Changes in the Maize Kernel Pericarp during Cooking Stage in Nixtamalization Process. J. Cereal Sci. 2010, 51, 81–88. [Google Scholar] [CrossRef]
- Paraginski, R.T.; de Souza, N.L.; Alves, G.H.; Ziegler, V.; de Oliveira, M.; Elias, M.C. Sensory and Nutritional Evaluation of Popcorn Kernels with Yellow, White and Red Pericarps Expanded in Different Ways. J. Cereal Sci. 2016, 69, 383–391. [Google Scholar] [CrossRef]
- Shavandi, M.; Javanmard, M.; Basiri, A. Novel Popping through Infrared: Effect on Some Physicochemical Properties of Popcorn (Zea Mays L. Var. Everta). LWT 2022, 155, 112955. [Google Scholar] [CrossRef]
- Gwirtz, J.A.; Garcia-Casal, M.N. Processing Maize Flour and Corn Meal Food Products. Ann. N. Y. Acad. Sci. 2014, 1312, 66–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, A.A.; Ashman, R.B.; Kirleis, A.W. Pericarp Thickness and Other Kernel Physical Characteristics Relate to Microwave Popping Quality of Popcorn. J. Food Sci. 1993, 58, 342–346. [Google Scholar] [CrossRef]
- Escalante-Aburto, A.; Mariscal-Moreno, R.M.; Santiago-Ramos, D.; Ponce-García, N. An Update of Different Nixtamalization Technologies, and Its Effects on Chemical Composition and Nutritional Value of Corn Tortillas. Food Rev. Int. 2020, 36, 456–498. [Google Scholar] [CrossRef]
- Park, D.; Maga, J.A. Effects of Storage Temperature and Kernel Physical Condition on Popping Qualities of Popcorn Hybrids. Cereal Chem. 2002, 79, 572–575. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Lazo-Vélez, M.A.; Nava-Valdez, Y.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Improvement of Dietary Fiber, Ferulic Acid and Calcium Contents in Pan Bread Enriched with Nejayote Food Additive from White Maize (Zea Mays). J. Cereal Sci. 2014, 60, 264–269. [Google Scholar] [CrossRef]
- Narváez-González, E.D.; Figueroa-Cárdenas, J.D.D.; Taba, S.; Tostado, E.C.; Peniche, R.Á.M.; Sánchez, F.R. Relationships between the Microstructure, Physical Features, and Chemical Composition of Different Maize Accessions from Latin America. Cereal Chem. 2006, 83, 595–604. [Google Scholar] [CrossRef]
- Junior, C.V.D.; Godoy, S.; Gonela, A.; Scapim, C.A.; Grandis, A.; Dos Santos, W.D.; Maria de Fátima, P.S. Biochemical composition of the pericarp cell wall of popcorn inbred lines with different popping expansion. Curr. Res. Food Sci. 2022, 5, 102–106. [Google Scholar] [CrossRef]
- Bocharova, O.; Reshta, S.; Bocharova, M. Investigation of the chemical safety of microwaved popcorn in respect of acrylamide formation. Int. Food Res. J. 2017, 24, 2274–2277. Available online: https://www.proquest.com/openview/f407abf46e96081584d9295290092f3a (accessed on 20 May 2022).
- Srichomporn, S.; Pothisoong, T.; Boonsri, N.; Srichomporn, K.; Malumpong, C. Effects of pericarp thickness of popcorn kernel on popping quality in cooked-oil popper. In Proceedings of the 51st Kasetsart University Annual Conference, Bangkok, Thailand, 5–7 February 2013; Kasetsart University: Bangkok, Thailand, 2013. Available online: https://www.cabdirect.org/cabdirect/abstract/20133409620 (accessed on 20 May 2022).
- Paes, G.P.; Viana, J.M.S.; Silva, F.F.; Mundim, G.B. Linkage disequilibrium, SNP frequency change due to selection, and association mapping in popcorn chromosome regions containing QTLs for quality traits. Genet. Mol. Biol. 2016, 39, 97–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noman, A.S.M.; Hoque, M.A.; Haque, M.M.; Pervin, F.; Karim, M.R. Nutritional and Anti-Nutritional Components in Pachyrhizus Erosus L. Tuber. Food Chem. 2007, 102, 1112–1118. [Google Scholar] [CrossRef]
- González-Vázquez, M.; Calderón-Domínguez, G.; Mora-Escobedo, R.; Salgado-Cruz, M.P.; Arreguín-Centeno, J.H.; Monterrubio-López, R. Polysaccharides of Nutritional Interest in Jicama (Pachyrhizus Erosus) during Root Development. Food Sci. Nutr. 2022, 10, 1146–1158. [Google Scholar] [CrossRef] [PubMed]
- Pedrol, N.; Ramos, P. Protein Content Quantification By Bradford Method. In Handbook of Plant Ecophysiology Techniques; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 283–295. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Rojas-Candelas, L.E.; Chanona-Pérez, J.J.; Méndez, J.V.M.; Morales-Hernández, J.A.; Benavides, H.A.C. Characterization of Structural Changes of Casein Micelles at Different PH Using Microscopy and Spectroscopy Techniques. Microsc. Microanal. 2022, 28, 527–536. [Google Scholar] [CrossRef]
- Carbonaro, M.; Nucara, A. Secondary Structure of Food Proteins by Fourier Transform Spectroscopy in the Mid-Infrared Region. Amino Acids 2010, 38, 679–690. [Google Scholar] [CrossRef]
- Jiang, Y.; Bai, X.; Lang, S.; Zhao, Y.; Liu, C.; Yu, L. Optimization of Ultrasonic-Microwave Assisted Alkali Extraction of Arabinoxylan from the Corn Bran Using Response Surface Methodology. Int. J. Biol. Macromol. 2019, 128, 452–458. [Google Scholar] [CrossRef]
- Bobo-García, G.; Davidov-Pardo, G.; Arroqui, C.; Vírseda, P.; Marín-Arroyo, M.R.; Navarro, M. Intra-Laboratory Validation of Microplate Methods for Total Phenolic Content and Antioxidant Activity on Polyphenolic Extracts, and Comparison with Conventional Spectrophotometric Methods. J. Sci. Food Agric. 2015, 95, 204–209. [Google Scholar] [CrossRef]
- Leite, A.V.; Malta, L.G.; Riccio, M.F.; Eberlin, M.N.; Pastore, G.M.; Maróstica Júnior, M.R. Antioxidant potential of rat plasma by administration of freeze-dried jaboticaba peel (Myrciaria jaboticaba Vell Berg). J. Agric. Food Chem. 2011, 59, 2277–2283. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on Products of Browning Reaction. Antioxidative Activities of Products of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Han, L.; Chen, L. In Vitro Antioxidant Activity of Protein Hydrolysates Prepared from Corn Gluten Meal. J. Sci. Food Agric. 2008, 88, 1660–1666. [Google Scholar] [CrossRef]
- Carter, P. Spectrophotometric Determination of Serum Iron at the Submicrogram Level with a New Reagent (Ferrozine). Anal. Biochem. 1971, 40, 450–458. [Google Scholar] [CrossRef]
- Rojas-Candelas, L.E.; Chanona-Pérez, J.J.; Méndez Méndez, J.V.; Perea-Flores, M.J.; Cervantes-Sodi, H.F.; Hernández-Hernández, H.M.; Marin-Bustamante, M.Q. Physicochemical, Structural and Nanomechanical Study Elucidating the Differences in Firmness among Four Apple Cultivars. Postharvest Biol. Technol. 2021, 171, 111342. [Google Scholar] [CrossRef]
- Wellner, N. Fourier Transform Infrared (FTIR) and Raman Microscopy: Principles and Applications to Food Microstructures; Woodhead Publishing Limited: Thorston, UK, 2013; ISBN 9780857095251. [Google Scholar]
- Chateigner-Boutin, A.L.; Ordaz-Ortiz, J.J.; Alvarado, C.; Bouchet, B.; Durand, S.; Verhertbruggen, Y.; Barrière, Y.; Saulnier, L. Developing Pericarp of Maize: A Model to Study Arabinoxylan Synthesis and Feruloylation. Front. Plant Sci. 2016, 7, 1476. [Google Scholar] [CrossRef] [Green Version]
- Buitimea-Cantúa, N.E.; Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A.; del Refugio Rocha-Pizaña, M.; de la Rosa-Millán, J.; Torres-Chávez, P.I. Protein-Phenolic Aggregates with Anti-Inflammatory Activity Recovered from Maize Nixtamalization Wastewaters (Nejayote). LWT 2020, 134, 109881. [Google Scholar] [CrossRef]
- Ogbaga, C.; Miller, M.; Athar, H.; Johnso, G. Fourier Transform Infrared Spectroscopic Analysis of Maize (Zea Mays) Subjected to Progressive Drought Reveals Involvement of Lipids, Amides and Carbohydrates. Afr. J. Biotechnol. 2017, 16, 1061–1066. [Google Scholar] [CrossRef] [Green Version]
- de la Rosa-Millán, J.; Orona-Padilla, J.L.; Flores-Moreno, V.M.; Serna-Saldívar, S.O. Physicochemical, Functional AndATR-FTIR Molecular Analysis of Protein Extracts Derived from Starchy Pulses. Int. J. Food Sci. Technol. 2018, 53, 1414–1424. [Google Scholar] [CrossRef]
- Li, P.; Huo, L.; Su, W.; Lu, R.; Deng, C.; Liu, L.; Deng, Y.; Guo, N.; Lu, C.; He, C. Free radical-scavenging capacity, antioxidant activity and phenolic content of Pouzolzia zeylanica. J. Serb. Chem. Soc. 2011, 76, 709–717. [Google Scholar] [CrossRef]
- Ivanišová, E.; Ondrejovid, M.; Šilhár, S. Antioxidant Activity of Milling Fractions of Selected Cereals. Nov. Biotechnol. Chim. 2012, 11, 45–56. [Google Scholar] [CrossRef]
- Ragaee, S.; Abdel-Aal, E.S.M.; Noaman, M. Antioxidant Activity and Nutrient Composition of Selected Cereals for Food Use. Food Chem. 2006, 98, 32–38. [Google Scholar] [CrossRef]
- Lopez-Martinez, L.X.; Parkin, K.L.; Garcia, H.S. Phase II-Inducing, Polyphenols Content and Antioxidant Capacity of Corn (Zea Mays L.) from Phenotypes of White, Blue, Red and Purple Colors Processed into Masa and Tortillas. Plant Foods Hum. Nutr. 2011, 66, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Balandrano, D.D.; Báez-González, J.G.; Carvajal-Millán, E.; Muy-Rangel, D.; Urías-Orona, V.; Martínez-López, A.L.; Márquez-Escalante, J.A.; Heredia, J.B.; Beta, T.; Niño-Medina, G. Alkali-Extracted Feruloylated Arabinoxylans from Nixtamalized Maize Bran Byproduct: A Synonymous with Soluble Antioxidant Dietary Fiber. Waste Biomass Valorization 2018, 11, 403–409. [Google Scholar] [CrossRef]
- Oboh, G.; Ademiluyi, A.O.; Akindahunsi, A.A. The Effect of Roasting on the Nutritional and Antioxidant Properties of Yellow and White Maize Varieties. Int. J. Food Sci. Technol. 2010, 45, 1236–1242. [Google Scholar] [CrossRef]
- Martínez-Tomé, M.; Murcia, M.A.; Frega, N.; Ruggieri, S.; Jiménez, A.M.; Roses, F.; Parras, P. Evaluation of Antioxidant Capacity of Cereal Brans. J. Agric. Food Chem. 2004, 52, 4690–4699. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, B.; Zhang, T.; Mu, W.; Liu, J. Antioxidant and Free Radical-Scavenging Activities of Chickpea Protein Hydrolysate (CPH). Food Chem. 2008, 106, 444–450. [Google Scholar] [CrossRef]
- Sri Kantha, S.; Wada, S.; Takeuche, M.; Watabe, S.; Ochi, H. A Sensitive Method to Screen for Hydroxyl Radical Scavenging Activity in Natural Food Extracts Using Competitive Inhibition ELISA for 8-Hydroxydexyguanosine. Biotechnol. Tech. 1996, 10, 713–716. [Google Scholar] [CrossRef]
- Wong, F.C.; Yong, A.L.; Ting, E.P.S.; Khoo, S.C.; Ong, H.C.; Chai, T.T. Antioxidant, Metal Chelating, Anti-Glucosidase Activities and Phytochemical Analysis of Selected Tropical Medicinal Plants. Iran. J. Pharm. Res. 2014, 13, 1407–1413. [Google Scholar] [CrossRef]
- Kladnik, A.; Chamusco, K.; Dermastia, M.; Chourey, P. Evidence of Programmed Cell Death in Post-Phloem Transport Cells of the Maternal Pedicel Tissue in Developing Caryopsis of Maize. Plant Physiol. 2004, 136, 3572–3581. [Google Scholar] [CrossRef] [Green Version]
- Figueroa, J.D.C.; Véles-Medina, J.J.; Tolentino-Lõpez, E.M.; Gaytán-Martínez, M.; Aragõn-Cuevas, F.; Palacios, N.; Willcox, M. Effect of Traditional Nixtamalization Process on Starch Annealing and the Relation to Pozole Quality. J. Food Process Eng. 2013, 36, 704–714. [Google Scholar] [CrossRef]
- Li, S.; Chen, S.; Han, F.; Xv, Y.; Sun, H.; Ma, Z.; Chen, J.; Wu, W. Development and Optimization of Cold Plasma Pretreatment for Drying on Corn Kernels. J. Food Sci. 2019, 84, 2181–2189. [Google Scholar] [CrossRef] [PubMed]
- Arzate-Vázquez, I.; Méndez-Méndez, J.V.; Flores-Johnson, E.A.; Nicolás-Bermúdez, J.; Chanona-Pérez, J.J.; Santiago-Cortés, E. Study of the Porosity of Calcified Chicken Eggshell Using Atomic Force Microscopy and Image Processing. Micron 2019, 118, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Antoine, C.; Peyron, S.; Mabille, F.; Lapierre, C.; Bouchet, B.; Abecassis, J.; Rouau, X. Individual Contribution of Grain Outer Layers and Their Cell Wall Structure to the Mechanical Properties of Wheat Bran. J. Agric. Food Chem. 2003, 51, 2026–2033. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G.N.; Méndez-Méndez, J.; Arzate-Vázquez, I.; Calderón-Domínguez, G.; Ribotta, P.D. Nano- and Micro-Mechanical Properties of Wheat Grain by Atomic Force Microscopy (AFM) and Nano-Indentation (IIT) and Their Relationship with the Mechanical Properties Evaluated by Uniaxial Compression Test. J. Cereal Sci. 2019, 90, 102830. [Google Scholar] [CrossRef]

| Parameter | |
|---|---|
| Ash (%) | 67.77 ± 3.13 |
| Lipid (%) | 6.76 ± 0.13 |
| Total sugars (%) | 10.93 ± 0.13 |
| Reducing sugars (%) | 3.93 ± 0.14 |
| Non-reducing sugars (%) | 6.99 ± 0.23 |
| Moisture (%) | 5.71 ± 0.53 |
| Nitrogen (%) | 1.15 ± 0.04 |
| Crude protein total (%) | 7.2 ± 0.33 |
| Crude protein soluble (%) | 0.11 ± 0.005 |
| Crude protein insoluble (%) | 7.10 ± 0.30 |
| Sample | Total Phenolic Content | ABTS | DPPH |
|---|---|---|---|
| Pericarp | 0.21 ± 0.008 | 550.1 ± 2.9 | 44.2 ± 1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Candelas, L.E.; Díaz-Ramírez, M.; Rayas-Amor, A.A.; Cruz-Monterrosa, R.G.; Méndez-Méndez, J.V.; Villanueva-Carvajal, A.; Cortés-Sánchez, A.d.J. Nanomechanical, Structural and Antioxidant Characterization of Nixtamalized Popcorn Pericarp. Appl. Sci. 2022, 12, 6789. https://doi.org/10.3390/app12136789
Rojas-Candelas LE, Díaz-Ramírez M, Rayas-Amor AA, Cruz-Monterrosa RG, Méndez-Méndez JV, Villanueva-Carvajal A, Cortés-Sánchez AdJ. Nanomechanical, Structural and Antioxidant Characterization of Nixtamalized Popcorn Pericarp. Applied Sciences. 2022; 12(13):6789. https://doi.org/10.3390/app12136789
Chicago/Turabian StyleRojas-Candelas, Liliana Edith, Mayra Díaz-Ramírez, Adolfo Armando Rayas-Amor, Rosy Gabriela Cruz-Monterrosa, Juan Vicente Méndez-Méndez, Adriana Villanueva-Carvajal, and Alejandro de Jesús Cortés-Sánchez. 2022. "Nanomechanical, Structural and Antioxidant Characterization of Nixtamalized Popcorn Pericarp" Applied Sciences 12, no. 13: 6789. https://doi.org/10.3390/app12136789
APA StyleRojas-Candelas, L. E., Díaz-Ramírez, M., Rayas-Amor, A. A., Cruz-Monterrosa, R. G., Méndez-Méndez, J. V., Villanueva-Carvajal, A., & Cortés-Sánchez, A. d. J. (2022). Nanomechanical, Structural and Antioxidant Characterization of Nixtamalized Popcorn Pericarp. Applied Sciences, 12(13), 6789. https://doi.org/10.3390/app12136789







