Nanomaterials in Bone Regeneration
Abstract
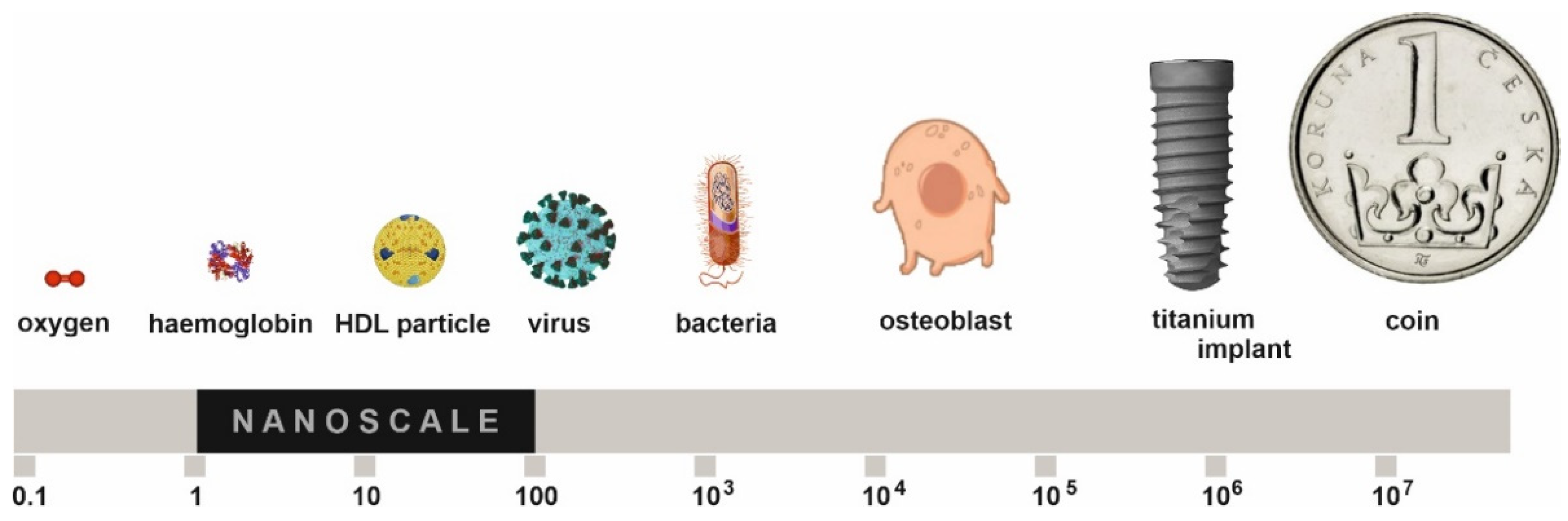
:1. Introduction
2. Bone Biology
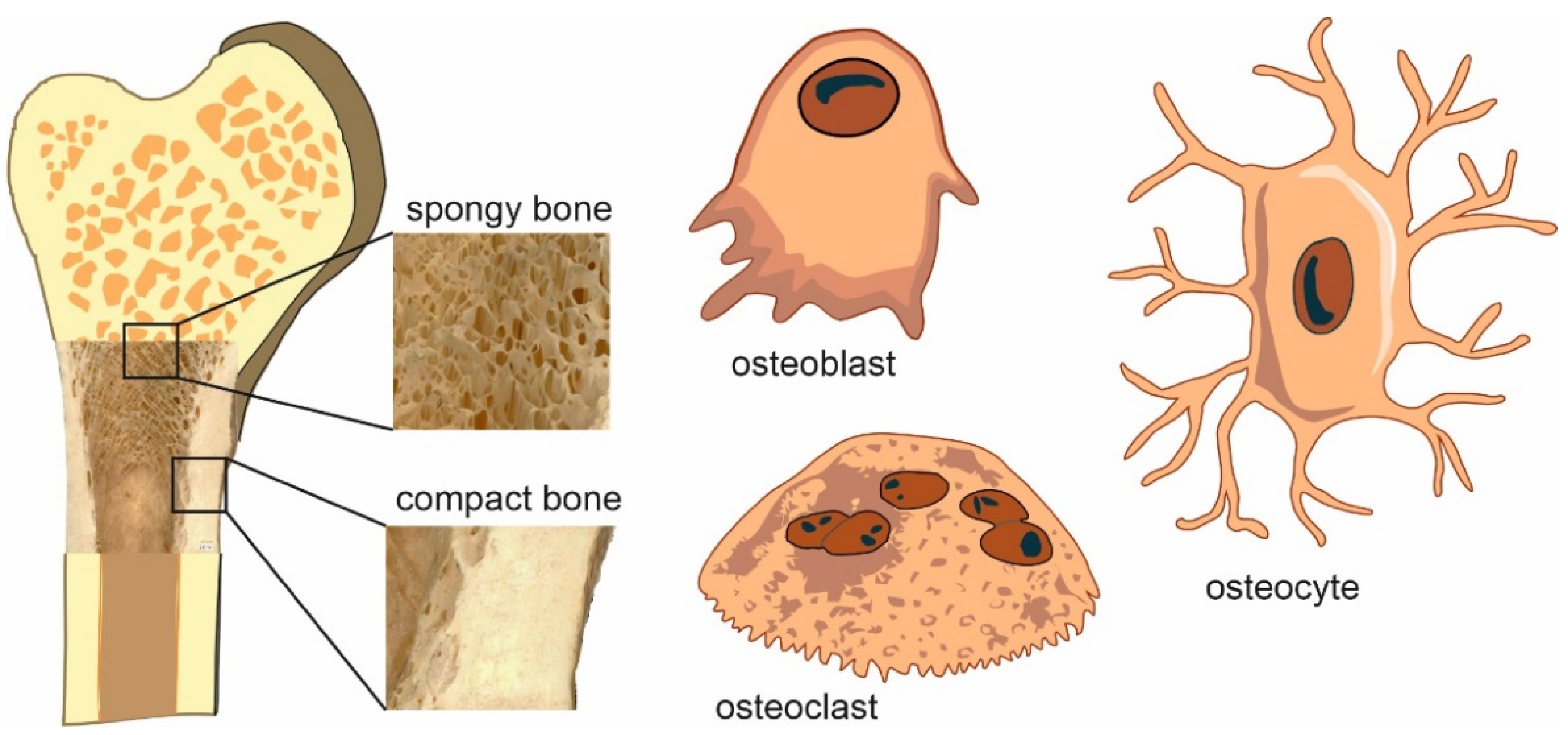
2.1. Bone Cells
2.2. Bone Extracellular Matrix
2.3. Bone Development
3. Nanostructured Materials
Titanium
4. Nanoparticles
4.1. Gold Nanoparticles
4.2. Silver Nanoparticles
4.3. Platinum Nanoparticles
4.4. Palladium Nanoparticles
4.5. Tantalum Nanoparticles
4.6. Iron Oxide Nanoparticles
4.7. Copper Nanoparticles
4.8. Zinc Nanoparticles
4.9. Magnesium Oxide Nanoparticles
4.10. Nickel Oxide Nanoparticles
4.11. Titanium Dioxide Nanoparticles
4.12. Calcium Oxide Nanoparticles
4.13. Aluminum Oxide Nanoparticles
4.14. Cerium Dioxide Nanoparticles
4.15. Strontium Nanoparticles
4.16. Carbon Nanoparticles
4.17. Silicon Dioxide Nanoparticles
4.18. Hydroxyapatite Nanoparticles
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD-MSCs | Adipose-derived mesenchymal stem cells |
| ALP | Alkaline phosphatase |
| BM-MSCs | Bone marrow-derived mesenchymal stem cells |
| BMP | Bone morphogenetic protein |
| CD | Carbon dot |
| CNF | Carbon nanofiber |
| CNT | Carbon nanotube |
| Col1 | Type I collagen |
| CS | Chitosan |
| CT | Calcitonin |
| Cu-CPC | Cu-containing calcium phosphate cement |
| DPSCs | Dental pulp stem cells |
| ECM | Extracellular matrix |
| eNOS | Endothelial nitric oxide synthase |
| FGF | Fibroblast growth factor |
| FGFR3 | Fibroblast growth factor receptor 3 gene |
| GO | Graphene oxide |
| HA | Hyaluronic acid |
| HAp | Hydroxyapatite |
| HIF1α | Hypoxia-inducible factor 1-alpha |
| HOX | Homeobox transcription factor |
| HUVEC | Human umbilical vein endothelial cell |
| IGF-1 | Insulin-like growth factor–1 |
| MC3T3-E1 | Mouse-calvaria-derived osteoprecursor cells |
| MSCs | Mesenchymal stem cells |
| MTT | 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide |
| MW-CNT | Multi-walled nanotube |
| ND | Nanodiamond |
| OC | Osteocalcin |
| ON | Osteonectin |
| OPG | Osteoprotegerin (osteoclastogenesis inhibitory factor) |
| OPN | Osteopontin |
| Osx | Osterix |
| PAX | Paired box transcription factor |
| PCL | Polycaprolactone |
| PEEK | Polyetheretherketone |
| PEG | Polyethylene glycol |
| PLA | Poly-L-lactic acid |
| PLGA | Poly (lactic-co-glycolic) acid |
| PTHrP | Parathyroid hormone-like peptide |
| RANK | Nuclear factor κβ receptor activator |
| RANKL | Nuclear factor κβ receptor activator ligand |
| ROS | Reactive oxigen species |
| RUNX2 | Runt-related transcription factor 2 |
| SBF | Simulated body fluid |
| SN | Silicate nanoplatelet |
| SW-CNT | Single-walled nanotube |
| TGF-β | Transforming growth factor β |
| VEGF | Vascular endothelial growth factor |
| Wnt | Wingless Integration |
| ZFP467 | Zinc finger protein 467 |
| ZIP1 | Zinc transporter protein |
| β-TCP | β–tricalcium phosphate |
References
- Tanaka, M.; Izumiya, M.; Haniu, H.; Ueda, K.; Ma, C.; Ueshiba, K.; Ideta, H.; Sobajima, A.; Uchiyama, S.; Takahashi, J.; et al. Current Methods in the Study of Nanomaterials for Bone Regeneration. Nanomaterials 2022, 12, 1195. [Google Scholar] [CrossRef]
- Natarajan, D.; Ye, Z.; Wang, L.; Ge, L.; Pathak, J.L. Rare Earth Smart Nanomaterials for Bone Tissue Engineering and Implantology: Advances, Challenges, and Prospects. Bioeng. Transl. Med. 2022, 7, e10262. [Google Scholar] [CrossRef]
- Bozorgi, A.; Khazaei, M.; Soleimani, M.; Jamalpoor, Z. Application of Nanoparticles in Bone Tissue Engineering; A Review on the Molecular Mechanisms Driving Osteogenesis. Biomater. Sci. 2021, 9, 4541–4567. [Google Scholar] [CrossRef]
- Lyons, J.G.; Plantz, M.A.; Hsu, W.K.; Hsu, E.L.; Minardi, S. Nanostructured Biomaterials for Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 922. [Google Scholar] [CrossRef]
- Yi, H.; Ur Rehman, F.; Zhao, C.; Liu, B.; He, N. Recent Advances in Nano Scaffolds for Bone Repair. Bone Res. 2016, 4, 16050. [Google Scholar] [CrossRef]
- Bhat, W.F.; Bhat, S.A.; Bano, B. Evaluation of Polyphenols as Possible Therapeutics for Amyloidoses: Comparative Analysis of Kaempferol and Catechin. Int. J. Biol. Macromol. 2015, 81, 60–68. [Google Scholar] [CrossRef]
- Kumar, P.; Saini, M.; Dehiya, B.S.; Sindhu, A.; Kumar, V.; Kumar, R.; Lamberti, L.; Pruncu, C.I.; Thakur, R. Comprehensive Survey on Nanobiomaterials for Bone Tissue Engineering Applications. Nanomaterials 2020, 10, 2019. [Google Scholar] [CrossRef]
- Walmsley, G.G.; McArdle, A.; Tevlin, R.; Momeni, A.; Atashroo, D.; Hu, M.S.; Feroze, A.H.; Wong, V.W.; Lorenz, P.H.; Longaker, M.T.; et al. Nanotechnology in Bone Tissue Engineering. Nanomed.-Nanotechnol. Biol. Med. 2015, 11, 1253–1263. [Google Scholar] [CrossRef] [Green Version]
- Salamanna, F.; Gambardella, A.; Contartese, D.; Visani, A.; Fini, M. Nano-Based Biomaterials as Drug Delivery Systems Against Osteoporosis: A Systematic Review of Preclinical and Clinical Evidence. Nanomaterials 2021, 11, 530. [Google Scholar] [CrossRef]
- Brannigan, K.; Griffin, M. An Update into the Application of Nanotechnology in Bone Healing. Open Orthop. J. 2016, 10, 808–823. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.-G.; Li, Z.-H.; Mao, X.-Z.; Wang, W.-C. Advances in Bone Repair with Nanobiomaterials: Mini-Review. Cytotechnology 2011, 63, 437–443. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Lee, P.; Lui, V.C.H.; Chen, Y.; Liu, X.; Lok, C.N.; To, M.; Yeung, K.W.K.; Wong, K.K.Y. Silver Nanoparticles Promote Osteogenesis of Mesenchymal Stem Cells and Improve Bone Fracture Healing in Osteogenesis Mechanism Mouse Model. Nanomed.-Nanotechnol. Biol. Med. 2015, 11, 1949–1959. [Google Scholar] [CrossRef]
- Sargazi, S.; Siddiqui, B.; Qindeel, M.; Rahdar, A.; Bilal, M.; Behzadmehr, R.; Mirinejad, S.; Pandey, S. Chitosan Nanocarriers for MicroRNA Delivery and Detection: A Preliminary Review with Emphasis on Cancer. Carbohydr. Polym. 2022, 290, 119489. [Google Scholar] [CrossRef]
- Pandey, S.; Son, N.; Kang, M. Synergistic Sorption Performance of Karaya Gum Crosslink Poly(Acrylamide-Co-Acrylonitrile) @ Metal Nanoparticle for Organic Pollutants. Int. J. Biol. Macromol. 2022, 210, 300–314. [Google Scholar] [CrossRef]
- Barani, M.; Hosseinikhah, S.M.; Rahdar, A.; Farhoudi, L.; Arshad, R.; Cucchiarini, M.; Pandey, S. Nanotechnology in Bladder Cancer: Diagnosis and Treatment. Cancers 2021, 13, 2214. [Google Scholar] [CrossRef]
- Hasan, A.; Morshed, M.; Memic, A.; Hassan, S.; Webster, T.; Marei, H. Nanoparticles in Tissue Engineering: Applications, Challenges and Prospects. Int. J. Nanomed. 2018, 13, 5637–5655. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Gajraj, V.; Das, A.; Sen, D.; Xu, H.; Mariappan, C.R. Silver, Copper, Magnesium and Zinc Contained Electroactive Mesoporous Bioactive S53P4 Glass–Ceramics Nanoparticle for Bone Regeneration: Bioactivity, Biocompatibility and Antibacterial Activity. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2309–2321. [Google Scholar] [CrossRef]
- Karkeh-Abadi, F.; Safardoust-Hojaghan, H.; Jasim, L.S.; Abdulsahib, W.K.; Mahdi, M.A.; Salavati-Niasari, M. Synthesis and Characterization of Cu2Zn1.75Mo3O12 Ceramic Nanoparticles with Excellent Antibacterial Property. J. Mol. Liq. 2022, 356, 119035. [Google Scholar] [CrossRef]
- Yu, N.; Zhao, L.; Cheng, D.; Ding, M.; Lyu, Y.; Zhao, J.; Li, J. Radioactive Organic Semiconducting Polymer Nanoparticles for Multimodal Cancer Theranostics. J. Colloid Interface Sci. 2022, 619, 219–228. [Google Scholar] [CrossRef]
- Wang, N.; Qi, D.; Liu, L.; Zhu, Y.; Liu, H.; Zhu, S. Fabrication of In Situ Grown Hydroxyapatite Nanoparticles Modified Porous Polyetheretherketone Matrix Composites to Promote Osteointegration and Enhance Bone Repair. Front. Bioeng. Biotechnol. 2022, 10, 831288. [Google Scholar] [CrossRef]
- Covarrubias, C.; Bejarano, J.; Maureira, M.; Tapia, C.; Díaz, M.; Rodríguez, J.P.; Palza, H.; Lund, F.; Von Marttens, A.; Caviedes, P.; et al. Preparation of Osteoinductive—Antimicrobial Nanocomposite Scaffolds Based on Poly (D,L-Lactide-Co-Glycolide) Modified with Copper–Doped Bioactive Glass Nanoparticles. Polym. Polym. Compos. 2022, 30, 096739112210982. [Google Scholar] [CrossRef]
- Mosher, C.Z.; Brudnicki, P.A.P.; Gong, Z.; Childs, H.R.; Lee, S.W.; Antrobus, R.M.; Fang, E.C.; Schiros, T.N.; Lu, H.H. Green Electrospinning for Biomaterials and Biofabrication. Biofabrication 2021, 13, 035049. [Google Scholar] [CrossRef]
- Ma, Q.; Song, Y.; Sun, W.; Cao, J.; Yuan, H.; Wang, X.; Sun, Y.; Shum, H.C. Cell-Inspired All-Aqueous Microfluidics: From Intracellular Liquid–Liquid Phase Separation toward Advanced Biomaterials. Adv. Sci. 2020, 7, 1903359. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P.; Murab, S.; Karmakar, S.; Chowdhury, P.K.; Ghosh, S. Modulation of Self-Assembly Process of Fibroin: An Insight for Regulating the Conformation of Silk Biomaterials. Biomacromolecules 2015, 16, 3936–3944. [Google Scholar] [CrossRef] [PubMed]
- Carradὸ, A.; Pelletier, H.; Faerber, J.; Versini, G.; Mihailescu, I.N. Pulsed Laser Deposition of Thin Coatings: Applications on Biomaterials. Mater. Sci. Forum 2010, 638–642, 530–535. [Google Scholar] [CrossRef]
- Vasudev, M.C.; Anderson, K.D.; Bunning, T.J.; Tsukruk, V.V.; Naik, R.R. Exploration of Plasma-Enhanced Chemical Vapor Deposition as a Method for Thin-Film Fabrication with Biological Applications. ACS Appl. Mater. Interfaces 2013, 5, 3983–3994. [Google Scholar] [CrossRef]
- Brenckle, M.A.; Tao, H.; Kim, S.; Paquette, M.; Kaplan, D.L.; Omenetto, F.G. Protein-Protein Nanoimprinting of Silk Fibroin Films. Adv. Mater. 2013, 25, 2409–2414. [Google Scholar] [CrossRef] [Green Version]
- Martin, T.A.; Caliari, S.R.; Williford, P.D.; Harley, B.A.; Bailey, R.C. The Generation of Biomolecular Patterns in Highly Porous Collagen-GAG Scaffolds Using Direct Photolithography. Biomaterials 2011, 32, 3949–3957. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Qin, N.; Tao, T.H. Nanomanufacturing of Biopolymers Using Electron and Ion Beams. J. Micromech. Microeng. 2020, 30, 033001. [Google Scholar] [CrossRef]
- Vasita, R.; Katti, D.S. Nanofibers and Their Applications in Tissue Engineering. Int. J. Nanomed. 2006, 1, 15–30. [Google Scholar] [CrossRef]
- Komatsu, T. Protein-Based Smart Microtubes and Nanotubes as Ultrasmall Biomaterials. Chem. Lett. 2020, 49, 1245–1255. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; da Silvia Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, B. Normal Bone Anatomy and Physiology. Clin. J. Am. Soc. Nephrol. 2008, 3, S131–S139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenkre, J.; Bassett, J. The Bone Remodelling Cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.W.; Romas, E.; Donnan, L.; Findlay, D.M. Bone Biology. Baillieres Clin. Endocrinol. Metab. 1997, 11, 1–22. [Google Scholar] [CrossRef]
- Blumer, M.J.F. Bone Tissue and Histological and Molecular Events during Development of the Long Bones. Ann. Anat.-Anat. Anz. 2021, 235, 151704. [Google Scholar] [CrossRef]
- Keith, A. Concerning the Origin and Nature of Osteoblasts. Proc. R. Soc. Med. 1927, 21, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Jensen, E.D.; Gopalakrishnan, R.; Westendorf, J.J. Regulation of Gene Expression in Osteoblasts. BioFactors 2010, 36, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Rutkovskiy, A.; Stensløkken, K.-O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Suda, T.; Takahashi, N.; Martin, T.J. Modulation of Osteoclast Differentiation. Endocr. Rev. 1992, 13, 66–80. [Google Scholar] [CrossRef]
- Baron, R.; Chakraborty, M.; Chatterjee, D.; Horne, W.; Lomri, A.; Ravesloot, J.-H. Biology of the Osteoclast. In Physiology and Pharmacology of Bone; Springer: Berlin/Heidelberg, Germany, 1993; Volume 107, pp. 111–147. ISBN 978-3-642-77993-0. [Google Scholar]
- Gay, C.V.; Mueller, W.J. Carbonic Anhydrase and Osteoclasts: Localization by Labeled Inhibitor Autoradiography. Science 1974, 183, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, G.C.; Moseley, J.M.; Sexton, P.M.; Mendelsohn, F.A.; Martin, T.J. Abundant Calcitonin Receptors in Isolated Rat Osteoclasts. Biochemical and Autoradiographic Characterization. J. Clin. Investig. 1986, 78, 355–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, C.H.; Forwood, M.R.; Otter, M.W. Mechanotransduction in Bone: Do Bone Cells Act as Sensors of Fluid Flow? FASEB J. 1994, 8, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Weinbaum, S.; Cowin, S.C.; Zeng, Y. A Model for the Excitation of Osteocytes by Mechanical Loading-Induced Bone Fluid Shear Stresses. J. Biomech. 1994, 27, 339–360. [Google Scholar] [CrossRef]
- Mezuk, B. Affective Disorders, Bone Metabolism, and Osteoporosis. Clinic. Rev. Bone Miner. Metab. 2008, 6, 101–113. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.-G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef]
- Stock, M.; Schett, G. Vitamin K-Dependent Proteins in Skeletal Development and Disease. Int. J. Mol. Sci. 2021, 22, 9328. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Xu, Z.; Wu, F.; Zhang, H.; Yang, C.; Chen, J.; Ding, B.; Sui, X.; Guo, Z.; et al. Undercarboxylated Osteocalcin Inhibits the Early Differentiation of Osteoclast Mediated by Gprc6a. PeerJ 2021, 9, e10898. [Google Scholar] [CrossRef]
- Zoch, M.L.; Clemens, T.L.; Riddle, R.C. New Insights into the Biology of Osteocalcin. Bone 2016, 82, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Florez, H.; Hernández-Rodríguez, J.; Carrasco, J.L.; Filella, X.; Prieto-González, S.; Monegal, A.; Guañabens, N.; Peris, P. Low Serum Osteocalcin Levels Are Associated with Diabetes Mellitus in Glucocorticoid Treated Patients. Osteoporos. Int. 2021, 33, 745–750. [Google Scholar] [CrossRef]
- Kratz, M.; Zelnick, L.R.; Trenchevska, O.; Jeffs, J.W.; Borges, C.R.; Tseng, H.-H.; Booth, S.L.; Kestenbaum, B.R.; Utzschneider, K.M.; de Boer, I.H. Relationship Between Chronic Kidney Disease, Glucose Homeostasis, and Plasma Osteocalcin Carboxylation and Fragmentation. J. Renal Nutr. 2021, 31, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Yue, J.; Liu, W. Broadening the Role of Osteocalcin in the Hypothalamic-Pituitary-Gonadal Axis. J. Endocrinol. 2021, 249, R43–R51. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Gill, G.; Kaur, H.; Amhmed, M.; Jakhu, H. Role of Osteopontin in Bone Remodeling and Orthodontic Tooth Movement: A Review. Prog. Orthod. 2018, 19, 18. [Google Scholar] [CrossRef]
- Young, M.F. Bone Matrix Proteins: Their Function, Regulation, and Relationship to Osteoporosis. Osteoporos. Int. 2003, 14, 35–42. [Google Scholar] [CrossRef]
- Dey, P. Bone Mineralisation. In Contemporary Topics about Phosphorus in Biology and Materials; Churchill, G.D., Dutour Sikirić, M., Čolović, B., Füredi Milhofer, H., Eds.; IntechOpen: London, UK, 2020; ISBN 978-1-78985-039-0. [Google Scholar]
- Berendsen, A.D.; Olsen, B.R. Bone Development. Bone 2015, 80, 14–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, B.K. The Embryonic Development of Bone. Am. Sci. 1988, 76, 174–181. [Google Scholar]
- Day, T.F.; Guo, X.; Garrett-Beal, L.; Yang, Y. Wnt/β-Catenin Signaling in Mesenchymal Progenitors Controls Osteoblast and Chondrocyte Differentiation during Vertebrate Skeletogenesis. Dev. Cell 2005, 8, 739–750. [Google Scholar] [CrossRef] [Green Version]
- Kronenberg, H.M. Developmental Regulation of the Growth Plate. Nature 2003, 423, 332–336. [Google Scholar] [CrossRef]
- Olsen, B.R.; Reginato, A.M.; Wang, W. Bone Development. Annu. Rev. Cell Dev. Biol. 2000, 16, 191–220. [Google Scholar] [CrossRef]
- Schipani, E.; Ryan, H.E.; Didrickson, S.; Kobayashi, T.; Knight, M.; Johnson, R.S. Hypoxia in Cartilage: HIF-1α Is Essential for Chondrocyte Growth Arrest and Survival. Genes Dev. 2001, 15, 2865–2876. [Google Scholar] [CrossRef]
- Karsenty, G.; Wagner, E.F. Reaching a Genetic and Molecular Understanding of Skeletal Development. Dev. Cell 2002, 2, 389–406. [Google Scholar] [CrossRef] [Green Version]
- Baker, J.; Liu, J.-P.; Robertson, E.J.; Efstratiadis, A. Role of Insulin-like Growth Factors in Embryonic and Postnatal Growth. Cell 1993, 75, 73–82. [Google Scholar] [CrossRef]
- Smits, P.; Li, P.; Mandel, J.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B.; Lefebvre, V. The Transcription Factors L-Sox5 and Sox6 Are Essential for Cartilage Formation. Dev. Cell 2001, 1, 277–290. [Google Scholar] [CrossRef] [Green Version]
- Ortega, N.; Behonick, D.J.; Werb, Z. Matrix Remodeling during Endochondral Ossification. Trends Cell Biol. 2004, 14, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Kingsley, D.M. What Do BMPs Do in Mammals? Clues from the Mouse Short-Ear Mutation. Trends Genet. 1994, 10, 16–21. [Google Scholar] [CrossRef]
- Mishnaevsky, L.; Levashov, E.; Valiev, R.Z.; Segurado, J.; Sabirov, I.; Enikeev, N.; Prokoshkin, S.; Solov’yov, A.V.; Korotitskiy, A.; Gutmanas, E.; et al. Nanostructured Titanium-Based Materials for Medical Implants: Modeling and Development. Mater. Sci. Eng. R-Rep. 2014, 81, 1–19. [Google Scholar] [CrossRef]
- Koch, C.C.; Langdon, T.G.; Lavernia, E.J. Bulk Nanostructured Materials. Metall. Mater. Trans. A-Phys. Metall. Mater. Sci. 2017, 48, 5181–5199. [Google Scholar] [CrossRef] [Green Version]
- Browne, M.; Gregson, P.J. Effect of Mechanical Surface Pretreatment on Metal Ion Release. Biomaterials 2000, 21, 385–392. [Google Scholar] [CrossRef]
- Estrin, Y.; Ivanova, E.P.; Michalska, A.; Truong, V.K.; Lapovok, R.; Boyd, R. Accelerated Stem Cell Attachment to Ultrafine Grained Titanium. Acta Biomater. 2011, 7, 900–906. [Google Scholar] [CrossRef]
- Bindu, S.; Sanosh, K.P.; Smetana, K.; Balakrishnan, A.; Kim, T.N. An in Vivo Evaluation of Ultra-Fine Grained Titanium Implants. J. Mater. Sci. Technol. 2009, 2009, 556–560. [Google Scholar]
- Babuska, V.; Dobra, J.; Kulda, V.; Kripnerova, M.; Moztarzadeh, A.; Bolek, L.; Lahoda, J.; Hrusak, D. Comparison of Fibroblast and Osteoblast Response to Cultivation on Titanium Implants with Different Grain Sizes. J. Nanomater. 2015, 2015, 920893. [Google Scholar] [CrossRef] [Green Version]
- Medvedev, A.E.; Neumann, A.; Ng, H.P.; Lapovok, R.; Kasper, C.; Lowe, T.C.; Anumalasetty, V.N.; Estrin, Y. Combined Effect of Grain Refinement and Surface Modification of Pure Titanium on the Attachment of Mesenchymal Stem Cells and Osteoblast-like SaOS-2 Cells. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 71, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Babuska, V.; Palan, J.; Kolaja Dobra, J.; Kulda, V.; Duchek, M.; Cerny, J.; Hrusak, D. Proliferation of Osteoblasts on Laser-Modified Nanostructured Titanium Surfaces. Materials 2018, 11, 1827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Zhang, K.; Wu, C.; Lei, X.; Ding, J.; Shi, X.; Liu, C. Micro-Arc Oxidation Enhances the Blood Compatibility of Ultrafine-Grained Pure Titanium. Materials 2017, 10, 1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazarov, D.; Zemtsova, E.; Solokhin, A.; Valiev, R.; Smirnov, V. Modification of the Surface Topography and Composition of Ultrafine and Coarse Grained Titanium by Chemical Etching. Nanomaterials 2017, 7, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhukova, Y.; Hiepen, C.; Knaus, P.; Osterland, M.; Prohaska, S.; Dunlop, J.W.C.; Fratzl, P.; Skorb, E.V. The Role of Titanium Surface Nanostructuring on Preosteoblast Morphology, Adhesion, and Migration. Adv. Healthc. Mater. 2017, 6, 1601244. [Google Scholar] [CrossRef] [Green Version]
- Chappuis, V.; Maestre, L.; Bürki, A.; Barré, S.; Buser, D.; Zysset, P.; Bosshardt, D. Osseointegration of Ultrafine-Grained Titanium with a Hydrophilic Nano-Patterned Surface: An in Vivo Examination in Miniature Pigs. Biomater. Sci. 2018, 6, 2448–2459. [Google Scholar] [CrossRef] [Green Version]
- Masrouri, M.; Faraji, G.; Pedram, M.S.; Sadrkhah, M. In-Vivo Study of Ultrafine-Grained CP-Ti Dental Implants Surface Modified by SLActive with Excellent Wettability. Int. J. Adhes. Adhes. 2020, 102, 102684. [Google Scholar] [CrossRef]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. Biomed Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef] [Green Version]
- Asri, R.I.M.; Harun, W.S.W.; Samykano, M.; Lah, N.A.C.; Ghani, S.A.C.; Tarlochan, F.; Raza, M.R. Corrosion and Surface Modification on Biocompatible Metals: A Review. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 77, 1261–1274. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Zheng, Z. Biocompatibility of Osteoblasts in Titanium Morphology Created by Dc Voltage. Oxid. Antioxid. Med. Sci. 2021, 10, 1–10. [Google Scholar]
- Lee, K.; Mazare, A.; Schmuki, P. One-Dimensional Titanium Dioxide Nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef] [Green Version]
- Baker, E.A.; Fleischer, M.M.; Vara, A.D.; Salisbury, M.R.; Baker, K.C.; Fortin, P.T.; Friedrich, C.R. Local and Systemic In Vivo Responses to Osseointegrative Titanium Nanotube Surfaces. Nanomaterials 2021, 11, 583. [Google Scholar] [CrossRef] [PubMed]
- Camargo, S.E.A.; Xia, X.; Fares, C.; Ren, F.; Hsu, S.-M.; Budei, D.; Aravindraja, C.; Kesavalu, L.; Esquivel-Upshaw, J.F. Nanostructured Surfaces to Promote Osteoblast Proliferation and Minimize Bacterial Adhesion on Titanium. Materials 2021, 14, 4357. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.; Elie, A.-M.; Plawinski, L.; Serro, A.P.; Botelho do Rego, A.M.; Almeida, A.; Urdaci, M.C.; Durrieu, M.-C.; Vilar, R. Femtosecond Laser Surface Texturing of Titanium as a Method to Reduce the Adhesion of Staphylococcus Aureus and Biofilm Formation. Appl. Surf. Sci. 2016, 360, 485–493. [Google Scholar] [CrossRef]
- Uhlmann, E.; Schweitzer, L.; Cunha, A.; Polte, J.; Huth-Herms, K.; Kieburg, H.; Hesse, B. Application of Laser Surface Nanotexturing for the Reduction of Peri-Implantitis on Biomedical Grade 5 Ti-6Al-4V Dental Abutments. In Proceedings of the Frontiers in Ultrafast Optics: Biomedical, Scientific, and Industrial Applications XIX; Herman, P.R., Meunier, M., Osellame, R., Eds.; SPIE: San Francisco, CA, USA, 2019; p. 9. [Google Scholar]
- Schnell, G.; Staehlke, S.; Duenow, U.; Nebe, J.B.; Seitz, H. Femtosecond Laser Nano/Micro Textured Ti6Al4V Surfaces—Effect on Wetting and MG-63 Cell Adhesion. Materials 2019, 12, 2210. [Google Scholar] [CrossRef] [Green Version]
- Nikolova, M.P.; Chavali, M.S. Metal Oxide Nanoparticles as Biomedical Materials. Biomimetics 2020, 5, 27. [Google Scholar] [CrossRef]
- Biswas, A.K.; Islam, M.R.; Choudhury, Z.S.; Mostafa, A.; Kadir, M.F. Nanotechnology Based Approaches in Cancer Therapeutics. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 043001. [Google Scholar] [CrossRef]
- Kurhade, P.; Kodape, S.; Choudhury, R. Overview on Green Synthesis of Metallic Nanoparticles. Chem. Pap. 2021, 75, 5187–5222. [Google Scholar] [CrossRef]
- Thakur, P.K.; Verma, V. A Review on Green Synthesis, Characterization and Anticancer Application of Metallic Nanoparticles. Appl. Biochem. Biotechnol. 2021, 193, 2357–2378. [Google Scholar] [CrossRef]
- Nejati, K.; Dadashpour, M.; Gharibi, T.; Mellatyar, H.; Akbarzadeh, A. Biomedical Applications of Functionalized Gold Nanoparticles: A Review. J. Clust. Sci. 2022, 33, 1–16. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, A.; Qayoom, I.; Teotia, A.K.; Gupta, S.; Padmanabhan, P.; Dev, A.; Kumar, A. Biofabrication of Gold Nanoparticles with Bone Remodeling Potential: An in Vitro and in Vivo Assessment. J. Nanopart. Res. 2020, 22, 152. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, P.; Wang, Y.; Li, J.; Qiao, D.; Chen, R.; Yang, W.; Yan, F. Gold Nanoparticles Promote the Bone Regeneration of Periodontal Ligament Stem Cell Sheets Through Activation of Autophagy. Int. J. Nanomed. 2021, 16, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kong, N.; Zhang, Y.; Yang, W.; Yan, F. Size-Dependent Effects of Gold Nanoparticles on Osteogenic Differentiation of Human Periodontal Ligament Progenitor Cells. Theranostics 2017, 7, 1214–1224. [Google Scholar] [CrossRef]
- Choi, S.Y.; Song, M.S.; Ryu, P.D.; Lam, A.T.N.; Joo, S.-W.; Lee, S.Y. Gold Nanoparticles Promote Osteogenic Differentiation in Human Adipose-Derived Mesenchymal Stem Cells through the Wnt/β-Catenin Signaling Pathway. Int. J. Nanomed. 2015, 10, 4383–4392. [Google Scholar] [CrossRef] [Green Version]
- Yi, C.; Liu, D.; Fong, C.-C.; Zhang, J.; Yang, M. Gold Nanoparticles Promote Osteogenic Differentiation of Mesenchymal Stem Cells through P38 MAPK Pathway. ACS Nano 2010, 4, 6439–6448. [Google Scholar] [CrossRef]
- Rosi, N.L.; Giljohann, D.A.; Thaxton, C.S.; Lytton-Jean, A.K.R.; Han, M.S.; Mirkin, C.A. Oligonucleotide-Modified Gold Nanoparticles for Intracellular Gene Regulation. Science 2006, 312, 1027–1030. [Google Scholar] [CrossRef]
- Heo, D.N.; Ko, W.-K.; Bae, M.S.; Lee, J.B.; Lee, D.-W.; Byun, W.; Lee, C.H.; Kim, E.-C.; Jung, B.-Y.; Kwon, I.K. Enhanced Bone Regeneration with a Gold Nanoparticle–Hydrogel Complex. J. Mat. Chem. B 2014, 2, 1584–1593. [Google Scholar] [CrossRef]
- Alexander, J.W. History of the Medical Use of Silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef] [Green Version]
- Gour, A.; Jain, N.K. Advances in Green Synthesis of Nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef] [Green Version]
- Jo, D.H.; Kim, J.H.; Lee, T.G.; Kim, J.H. Size, Surface Charge, and Shape Determine Therapeutic Effects of Nanoparticles on Brain and Retinal Diseases. Nanomed.-Nanotechnol. Biol. Med. 2015, 11, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.A.; Katare, Y.K.; Mitragotri, S. Particle Shape: A New Design Parameter for Micro- and Nanoscale Drug Delivery Carriers. J. Control Release 2007, 121, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, S.; Ramontja, J. Sodium Alginate Stabilized Silver Nanoparticles–Silica Nanohybrid and Their Antibacterial Characteristics. Int. J. Biol. Macromol. 2016, 93, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef] [PubMed]
- Qing, T.; Mahmood, M.; Zheng, Y.; Biris, A.S.; Shi, L.; Casciano, D.A. A Genomic Characterization of the Influence of Silver Nanoparticles on Bone Differentiation in MC3T3-E1 Cells. J. Appl. Toxicol. 2018, 38, 172–179. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zheng, Y.; Feng, Q.; Elkhooly, T.A.; Liu, X.; Yang, X.; Wang, Y.; Xie, Y. Silver Nanoparticles Stimulate Osteogenesis of Human Mesenchymal Stem Cells through Activation of Autophagy. Nanomedicine 2020, 15, 337–353. [Google Scholar] [CrossRef]
- Oei, J.D.; Zhao, W.W.; Chu, L.; DeSilva, M.N.; Ghimire, A.; Rawls, H.R.; Whang, K. Antimicrobial Acrylic Materials with in Situ Generated Silver Nanoparticles. J. Biomed. Mater. Res. Part B 2012, 100B, 409–415. [Google Scholar] [CrossRef]
- Cao, X.; Zhu, L.; Bai, Y.; Li, F.; Yu, X. Green One-Step Synthesis of Silver Nanoparticles and Their Biosafety and Antibacterial Properties. Green Chem. Lett. Rev. 2022, 15, 28–34. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Birla, S.; Yadav, A.; Santos, C.A.D. Strategic Role of Selected Noble Metal Nanoparticles in Medicine. Crit. Rev. Microbiol. 2015, 42, 696–719. [Google Scholar] [CrossRef]
- Kim, W.-K.; Kim, J.-C.; Park, H.-J.; Sul, O.-J.; Lee, M.-H.; Kim, J.-S.; Choi, H.-S. Platinum Nanoparticles Reduce Ovariectomy-Induced Bone Loss by Decreasing Osteoclastogenesis. Exp. Mol. Med. 2012, 44, 432. [Google Scholar] [CrossRef]
- Eid, K.; Eldesouky, A.; Fahmy, A.; Shahat, A.; AbdElaal, R. Calcium Phosphate Scaffold Loaded with Platinum Nanoparticles for Bone Allograft. Am. J. Biomed. Sci. 2013, 5, 242–249. [Google Scholar] [CrossRef]
- Radwan-Pragłowska, J.; Janus, Ł.; Piątkowski, M.; Bogdał, D.; Matysek, D. 3D Hierarchical, Nanostructured Chitosan/PLA/HA Scaffolds Doped with TiO2/Au/Pt NPs with Tunable Properties for Guided Bone Tissue Engineering. Polymers 2020, 12, 792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murugesan, B.; Pandiyan, N.; Arumugam, M.; Sonamuthu, J.; Samayanan, S.; Yurong, C.; Juming, Y.; Mahalingam, S. Fabrication of Palladium Nanoparticles Anchored Polypyrrole Functionalized Reduced Graphene Oxide Nanocomposite for Antibiofilm Associated Orthopedic Tissue Engineering. Appl. Surf. Sci. 2020, 510, 145403. [Google Scholar] [CrossRef]
- Calabrese, G.; Petralia, S.; Fabbi, C.; Forte, S.; Franco, D.; Guglielmino, S.; Esposito, E.; Cuzzocrea, S.; Traina, F.; Conoci, S. Au, Pd and Maghemite Nanofunctionalized Hydroxyapatite Scaffolds for Bone Regeneration. Regen. Biomater. 2020, 7, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Heidari, F.; Tabatabaei, F.S.; Razavi, M.; Lari, R.B.; Tavangar, M.; Romanos, G.E.; Vashaee, D.; Tayebi, L. 3D Construct of Hydroxyapatite/Zinc Oxide/Palladium Nanocomposite Scaffold for Bone Tissue Engineering. J. Mater. Sci.-Mater. Med. 2020, 31, 85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheng, G.; Xing, X.; Liu, J.; Cheng, Y.; Ye, T.; Wang, Q.; Xiao, X.; Li, Z.; Deng, H. Near-Infrared Light-Triggered Porous AuPd Alloy Nanoparticles to Produce Mild Localized Heat to Accelerate Bone Regeneration. J. Phys. Chem. Lett. 2019, 10, 4185–4191. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Pan, S.-T.; Qiu, J.-X. The Clinical Application of Porous Tantalum and Its New Development for Bone Tissue Engineering. Materials 2021, 14, 2647. [Google Scholar] [CrossRef]
- Wang, P.; Qiao, P.; Xing, H.; Zhang, R.; Lingling, E.; Liu, H. Cytotoxicity, Oxidative Stress, and Autophagy Effects of Tantalum Nanoparticles on MC3T3-E1 Mouse Osteoblasts. J. Nanosci. Nanotechnol. 2020, 20, 1417–1424. [Google Scholar] [CrossRef]
- Zhang, L.; Haddouti, E.-M.; Beckert, H.; Biehl, R.; Pariyar, S.; Rüwald, J.M.; Li, X.; Jaenisch, M.; Burger, C.; Wirtz, D.C.; et al. Investigation of Cytotoxicity, Oxidative Stress, and Inflammatory Responses of Tantalum Nanoparticles in THP-1-Derived Macrophages. Mediat. Inflamm. 2020, 2020, 3824593. [Google Scholar] [CrossRef]
- Lu, T.; Wen, J.; Qian, S.; Cao, H.; Ning, C.; Pan, X.; Jiang, X.; Liu, X.; Chu, P.K. Enhanced Osteointegration on Tantalum-Implanted Polyetheretherketone Surface with Bone-like Elastic Modulus. Biomaterials 2015, 51, 173–183. [Google Scholar] [CrossRef]
- Zhu, H.; Ji, X.; Guan, H.; Zhao, L.; Zhao, L.; Liu, C.; Cai, C.; Li, W.; Tao, T.; Reseland, J.E.; et al. Tantalum Nanoparticles Reinforced Polyetheretherketone Shows Enhanced Bone Formation. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 101, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ye, D.; Li, M.; Ma, M.; Gu, N. Adaptive Materials Based on Iron Oxide Nanoparticles for Bone Regeneration. ChemPhysChem 2018, 19, 1965–1979. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.R.; Xu, S.; Stains, J.P.; Bennett, C.H.; Lovering, R.M. Superparamagnetic Iron Oxide Nanoparticles in Musculoskeletal Biology. Tissue Eng. Part B-Rev. 2017, 23, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Zahn, D.; Landers, J.; Buchwald, J.; Diegel, M.; Salamon, S.; Müller, R.; Köhler, M.; Ecke, G.; Wende, H.; Dutz, S. Ferrimagnetic Large Single Domain Iron Oxide Nanoparticles for Hyperthermia Applications. Nanomaterials 2022, 12, 343. [Google Scholar] [CrossRef] [PubMed]
- Scharf, A.; Holmes, S.; Thoresen, M.; Mumaw, J.; Stumpf, A.; Peroni, J. Superparamagnetic Iron Oxide Nanoparticles as a Means to Track Mesenchymal Stem Cells in a Large Animal Model of Tendon Injury: SPIOs to Track MSCs in Tendon Injury. Contrast Media Mol. Imaging 2015, 10, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, D.; Chen, J.; Liu, W.; Duan, L.; You, W.; Zhu, W.; Xiong, J.; Wang, D. Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells by Magnetic Nanoparticle Composite Scaffolds under a Pulsed Electromagnetic Field. Saudi Pharm. J. 2017, 25, 575–579. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, Y.; Zhu, C.; Zhang, W.; Mao, Z.; Gao, C. Fe3O4/BSA Particles Induce Osteogenic Differentiation of Mesenchymal Stem Cells under Static Magnetic Field. Acta Biomater. 2016, 46, 141–150. [Google Scholar] [CrossRef]
- Yun, H.-M.; Ahn, S.-J.; Park, K.-R.; Kim, M.-J.; Kim, J.-J.; Jin, G.-Z.; Kim, H.-W.; Kim, E.-C. Magnetic Nanocomposite Scaffolds Combined with Static Magnetic Field in the Stimulation of Osteoblastic Differentiation and Bone Formation. Biomaterials 2016, 85, 88–98. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Yao, Q. Copper-Based Biomaterials for Bone and Cartilage Tissue Engineering. J. Orthop. Transl. 2021, 29, 60–71. [Google Scholar] [CrossRef]
- Ghosh, S.; Webster, T.J. Metallic Nanoscaffolds as Osteogenic Promoters: Advances, Challenges and Scope. Metals 2021, 11, 1356. [Google Scholar] [CrossRef]
- D’Mello, S.; Elangovan, S.; Hong, L.; Ross, R.D.; Sumner, D.R.; Salem, A.K. Incorporation of Copper into Chitosan Scaffolds Promotes Bone Regeneration in Rat Calvarial Defects: Copper-Loaded Chitosan Scaffolds for Bone Regeneration. J. Biomed. Mater. Res. Part B 2015, 103, 1044–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.; Cao, Y.; Zou, J.; Zhu, F.; Gao, Y.; Zheng, X.; Wang, H.; Zhang, T.; Wu, T. Improved Osteogenesis and Angiogenesis of a Novel Copper Ions Doped Calcium Phosphate Cement. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 114, 111032. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Y.; Qing, Y.; Li, R.; Tang, X.; Guo, D.; Qin, Y. Enhancing ZnO-NP Antibacterial and Osteogenesis Properties in Orthopedic Applications: A Review. Int. J. Nanomed. 2020, 15, 6247–6262. [Google Scholar] [CrossRef]
- Khader, A.; Arinzeh, T.L. Biodegradable Zinc Oxide Composite Scaffolds Promote Osteochondral Differentiation of Mesenchymal Stem Cells. Biotechnol. Bioeng. 2020, 117, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Lin, S.; Feng, Q.; Dong, C.; Yang, Y.; Li, G.; Bian, L. Nanocomposite Hydrogels Stabilized by Self-Assembled Multivalent Bisphosphonate-Magnesium Nanoparticles Mediate Sustained Release of Magnesium Ion and Promote in-Situ Bone Regeneration. Acta Biomater. 2017, 64, 389–400. [Google Scholar] [CrossRef]
- Safari, N.; Golafshan, N.; Kharaziha, M.; Reza Toroghinejad, M.; Utomo, L.; Malda, J.; Castilho, M. Stable and Antibacterial Magnesium–Graphene Nanocomposite-Based Implants for Bone Repair. ACS Biomater. Sci. Eng. 2020, 6, 6253–6262. [Google Scholar] [CrossRef]
- Park, K.-S.; Kim, B.-J.; Lih, E.; Park, W.; Lee, S.-H.; Joung, Y.K.; Han, D.K. Versatile Effects of Magnesium Hydroxide Nanoparticles in PLGA Scaffold–Mediated Chondrogenesis. Acta Biomater. 2018, 73, 204–216. [Google Scholar] [CrossRef]
- Petretta, M.; Gambardella, A.; Boi, M.; Berni, M.; Cavallo, C.; Marchiori, G.; Maltarello, M.C.; Bellucci, D.; Fini, M.; Baldini, N.; et al. Composite Scaffolds for Bone Tissue Regeneration Based on PCL and Mg-Containing Bioactive Glasses. Biology 2021, 10, 398. [Google Scholar] [CrossRef]
- Raura, N.; Garg, A.; Arora, A.; Roma, M. Nanoparticle Technology and Its Implications in Endodontics: A Review. Biomater. Res. 2020, 24, 21. [Google Scholar] [CrossRef]
- Wetteland, C.L.; Nguyen, N.-Y.T.; Liu, H. Concentration-Dependent Behaviors of Bone Marrow Derived Mesenchymal Stem Cells and Infectious Bacteria toward Magnesium Oxide Nanoparticles. Acta Biomater. 2016, 35, 341–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahlström, M.G.; Thyssen, J.P.; Wennervaldt, M.; Menné, T.; Johansen, J.D. Nickel Allergy and Allergic Contact Dermatitis: A Clinical Review of Immunology, Epidemiology, Exposure, and Treatment. Contact Dermat. 2019, 81, 227–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- More, S.L.; Kovochich, M.; Lyons-Darden, T.; Taylor, M.; Schulte, A.M.; Madl, A.K. Review and Evaluation of the Potential Health Effects of Oxidic Nickel Nanoparticles. Nanomaterials 2021, 11, 642. [Google Scholar] [CrossRef] [PubMed]
- Bano, S.; Nazir, S.; Munir, S.; Al-Ajmi, M.F.; Afzal, M.; Mazhar, K. “Smart” Nickel Oxide Based Core-Shell Nanoparticles for Combined Chemo and Photodynamic Cancer Therapy. Int. J. Nanomed. 2016, 11, 3159–3166. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.A.; Shahid, S.; Ayaz, A.; Alkahtani, J.; Elshikh, M.S.; Riaz, T. Phytomolecules-Coated NiO Nanoparticles Synthesis Using Abutilon Indicum Leaf Extract: Antioxidant, Antibacterial, and Anticancer Activities. Int. J. Nanomed. 2021, 16, 1757–1773. [Google Scholar] [CrossRef]
- Hong, Y.C.; Kim, J.H.; Bang, C.U.; Uhm, H.S. Gas-Phase Synthesis of Nitrogen-Doped TiO2 Nanorods by Microwave Plasma Torch at Atmospheric Pressure. Phys. Plasmas 2005, 12, 114501. [Google Scholar] [CrossRef]
- Johari, N.; Madaah Hosseini, H.R.; Samadikuchaksaraei, A. Optimized Composition of Nanocomposite Scaffolds Formed from Silk Fibroin and Nano-TiO2 for Bone Tissue Engineering. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 79, 783–792. [Google Scholar] [CrossRef]
- Nechifor, G.; Eftimie Totu, E.; Nechifor, A.C.; Isildak, I.; Oprea, O.; Cristache, C.M. Non-Resorbable Nanocomposite Membranes for Guided Bone Regeneration Based on Polysulfone-Quartz Fiber Grafted with Nano-TiO2. Nanomaterials 2019, 9, 985. [Google Scholar] [CrossRef] [Green Version]
- Bressan, E.; Ferroni, L.; Gardin, C.; Bellin, G.; Sbricoli, L.; Sivolella, S.; Brunello, G.; Schwartz-Arad, D.; Mijiritsky, E.; Penarrocha, M.; et al. Metal Nanoparticles Released from Dental Implant Surfaces: Potential Contribution to Chronic Inflammation and Peri-Implant Bone Loss. Materials 2019, 12, 2036. [Google Scholar] [CrossRef] [Green Version]
- Weaver, C.M.; Peacock, M. Calcium. Adv. Nutr. 2011, 2, 290–292. [Google Scholar] [CrossRef]
- Baird, G.S. Ionized Calcium. Clin. Chim. Acta 2011, 412, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Bae, D.-H.; Yeon, J.-H.; Park, S.-Y.; Lee, D.-H.; Ha, S.-D. Bactericidal Effects of CaO (Scallop-Shell Powder) on Foodborne Pathogenic Bacteria. Arch. Pharm. Res. 2006, 29, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.S.; Park, J.S.; Song, S.H.; Jang, S.B. Characterization of Antibacterial Nanoparticles from the Scallop, Ptinopecten Yessoensis. Biosci. Biotechnol. Biochem. 2007, 71, 2242–2247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawai, J.; Shiga, H. Kinetic Analysis of the Antifungal Activity of Heated Scallop-Shell Powder against Trichophyton and Its Possible Application to the Treatment of Dermatophytosis. Biocontrol Sci. 2006, 11, 125–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, C.; Bobillier, F.; Canales, D.; Antonella Sepúlveda, F.; Cament, A.; Amigo, N.; Rivas, L.M.; Ulloa, M.T.; Reyes, P.; Ortiz, J.A.; et al. Mechanical and Antimicrobial Polyethylene Composites with CaO Nanoparticles. Polymers 2020, 12, 2132. [Google Scholar] [CrossRef]
- Münchow, E.A.; Pankajakshan, D.; Albuquerque, M.T.P.; Kamocki, K.; Piva, E.; Gregory, R.L.; Bottino, M.C. Synthesis and Characterization of CaO-Loaded Electrospun Matrices for Bone Tissue Engineering. Clin. Oral Investig. 2016, 20, 1921–1933. [Google Scholar] [CrossRef] [Green Version]
- Piattelli, A. Histological Evaluation of Bone Reactions to Aluminium Oxide Dental Implants in Man: A Case Report. Biomaterials 1996, 17, 711–714. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Bahojb Noruzi, E.; Khanmohammadi Chenab, K.; Jafari, A.; Radinekiyan, F.; Hashemi, S.M.; Ahmadpour, F.; Behboudi, A.; Mosafer, J.; Mokhtarzadeh, A.; et al. Metal-based Nanoparticles for Bone Tissue Engineering. J. Tissue Eng. Regen. Med. 2020, 14, 1687–1714. [Google Scholar] [CrossRef]
- Yu, B.; Fu, S.; Kang, Z.; Zhu, M.; Ding, H.; Luo, T.; Zhu, Y.; Zhang, Y. Enhanced Bone Regeneration of 3D Printed β-Ca2SiO4 Scaffolds by Aluminum Ions Solid Solution. Ceram. Int. 2020, 46, 7783–7791. [Google Scholar] [CrossRef]
- Toloue, E.; Karbasi, S.; Salehi, H.; Rafienia, M. Evaluation of Mechanical Properties and Cell Viability of Poly (3-Hydroxybutyrate)-Chitosan/Al2O3 Nanocomposite Scaffold for Cartilage Tissue Engineering. J. Med. Signals Sens. 2019, 9, 111. [Google Scholar] [CrossRef]
- Li, H.; Xia, P.; Pan, S.; Qi, Z.; Fu, C.; Yu, Z.; Kong, W.; Chang, Y.; Wang, K.; Wu, D.; et al. The Advances of Ceria Nanoparticles for Biomedical Applications in Orthopaedics. Int. J. Nanomed. 2020, 15, 7199–7214. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Neal, C.J.; Sakthivel, T.S.; Kean, T.; Seal, S.; Coathup, M.J. Multi-Functional Cerium Oxide Nanoparticles Regulate Inflammation and Enhance Osteogenesis. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 124, 112041. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kang, F.; Gong, X.; Bai, Y.; Dai, J.; Zhao, C.; Dou, C.; Cao, Z.; Liang, M.; Dong, R.; et al. Ceria Nanoparticles Enhance Endochondral Ossification–Based Critical-sized Bone Defect Regeneration by Promoting the Hypertrophic Differentiation of BMSCs via DHX15 Activation. FASEB J. 2019, 33, 6378–6389. [Google Scholar] [CrossRef]
- Li, X.; Qi, M.; Sun, X.; Weir, M.D.; Tay, F.R.; Oates, T.W.; Dong, B.; Zhou, Y.; Wang, L.; Xu, H.H.K. Surface Treatments on Titanium Implants via Nanostructured Ceria for Antibacterial and Anti-Inflammatory Capabilities. Acta Biomater. 2019, 94, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Purohit, S.D.; Singh, H.; Bhaskar, R.; Yadav, I.; Chou, C.-F.; Gupta, M.K.; Mishra, N.C. Gelatin—Alginate—Cerium Oxide Nanocomposite Scaffold for Bone Regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111111. [Google Scholar] [CrossRef] [PubMed]
- Lukin, A.V.; Lukina, G.I.; Volkov, A.V.; Baranchikov, A.E.; Ivanov, V.K.; Prokopov, A.A. Morphometry Results of Formed Osteodefects When Using Nanocrystalline CeO2 in the Early Stages of Regeneration. J. Dent. 2019, 2019, 9416381. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mishra, M. Application of Strontium-Based Nanoparticles in Medicine and Environmental Sciences. Nanotechnol. Environ. Eng. 2021, 6, 25. [Google Scholar] [CrossRef]
- Hekimoğlu, A.P.; Çalış, M.; Ayata, G. Effect of Strontium and Magnesium Additions on the Microstructure and Mechanical Properties of Al–12Si Alloys. Met. Mater. Int. 2019, 25, 1488–1499. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, Q.; Zhai, C.; Zhu, Y. Effects of Strontium and Titanium on the Microstructure, Tensile Properties and Creep Behavior of AM50 Alloys. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2007, 444, 318–326. [Google Scholar] [CrossRef]
- Gungor, A.A.; Nadaroglu, H.; Gultekin, D.D. Synthesis and Characterization of Nano-Strontium Oxide (SrO) Using Erzincan Cimin Grape (Vitis Vinifera, Cimin). Chem. Sci. Int. J. 2019, 26, 1–7. [Google Scholar] [CrossRef]
- Cardenas, D.; Turyanskaya, A.; Rauwolf, M.; Panahifar, A.; Cooper, D.; Wohl, G.R.; Streli, C.; Wobrauschek, P.; Pejović-Milić, A. Determining Elemental Strontium Distribution in Rat Bones Treated with Strontium Ranelate and Strontium Citrate Using 2D Micro-XRF and 3D Dual Energy K-edge Subtraction Synchrotron Imaging. X-ray Spectrom. 2020, 49, 424–433. [Google Scholar] [CrossRef]
- Zhao, F.; Lei, B.; Li, X.; Mo, Y.; Wang, R.; Chen, D.; Chen, X. Promoting in Vivo Early Angiogenesis with Sub-Micrometer Strontium-Contained Bioactive Microspheres through Modulating Macrophage Phenotypes. Biomaterials 2018, 178, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Leite, Á.J.; Gonçalves, A.I.; Rodrigues, M.T.; Gomes, M.E.; Mano, J.F. Strontium-Doped Bioactive Glass Nanoparticles in Osteogenic Commitment. ACS Appl. Mater. Interfaces 2018, 10, 23311–23320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayalakshmi, S.; Anupriya, P.; Surya, P.; Vijayalakshmi, C. Synthesis and Characterization of Strontia Nanoparticles. Int. J. Pure Appl. Math. 2018, 119, 1299–1305. [Google Scholar]
- Fekri, H.S.; Ranjbar, M.; Noudeh, G.D.; Ziasistani, N. Green Synthesis of Strontium Nanoparticles Self-assembled in the Presence of Carboxymethyl Cellulose: An in Vivo Imaging Study. Luminescence 2019, 34, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Hirata, E. Carbon Nanomaterials for Bone Tissue Regeneration. Hokkaido J. Dent. Sci. 2017, 38, 99–103. [Google Scholar]
- Venkatesan, J.; Pallela, R.; Kim, S.-K. Applications of Carbon Nanomaterials in Bone Tissue Engineering. J. Biomed. Nanotechnol. 2014, 10, 3105–3123. [Google Scholar] [CrossRef]
- Schrand, A.M.; Dai, L.; Schlager, J.J.; Hussain, S.M.; Osawa, E. Differential Biocompatibility of Carbon Nanotubes and Nanodiamonds. Diam. Relat. Mat. 2007, 16, 2118–2123. [Google Scholar] [CrossRef]
- Alhaddad, A.; Adam, M.-P.; Botsoa, J.; Dantelle, G.; Perruchas, S.; Gacoin, T.; Mansuy, C.; Lavielle, S.; Malvy, C.; Treussart, F.; et al. Nanodiamond as a Vector for SiRNA Delivery to Ewing Sarcoma Cells. Small 2011, 7, 3087–3095. [Google Scholar] [CrossRef] [Green Version]
- Grausova, L.; Bacakova, L.; Kromka, A.; Potocky, S.; Vanecek, M.; Nesladek, M.; Lisa, V. Nanodiamond as Promising Material for Bone Tissue Engineering. J. Nanosci. Nanotech. 2009, 9, 3524–3534. [Google Scholar] [CrossRef]
- Moore, L.; Gatica, M.; Kim, H.; Osawa, E.; Ho, D. Multi-Protein Delivery by Nanodiamonds Promotes Bone Formation. J. Dent. Res. 2013, 92, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Noh, S.H.; Lim, C.O.; Kim, H.-J.; Jo, H.-S.; Min, J.S.; Park, K.; Kim, S.E. Icariin-Functionalized Nanodiamonds to Enhance Osteogenic Capacity In Vitro. Nanomaterials 2020, 10, 2071. [Google Scholar] [CrossRef] [PubMed]
- Prylutska, S.; Bilyy, R.; Shkandina, T.; Rotko, D.; Bychko, A.; Cherepanov, V.; Stoika, R.; Rybalchenko, V.; Prylutskyy, Y.; Tsierkezos, N.; et al. Comparative Study of Membranotropic Action of Single- and Multi-Walled Carbon Nanotubes. J. Biosci. Bioeng. 2013, 115, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Hu, H.; Mandal, S.K.; Haddon, R.C. A Bone Mimic Based on the Self-Assembly of Hydroxyapatite on Chemically Functionalized Single-Walled Carbon Nanotubes. Chem. Mater. 2005, 17, 3235–3241. [Google Scholar] [CrossRef]
- Meng, D.; Ioannou, J.; Boccaccini, A.R. Bioglass®-Based Scaffolds with Carbon Nanotube Coating for Bone Tissue Engineering. J. Mater. Sci.-Mater. Med. 2009, 20, 2139–2144. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.K. Stimulation of Minerals by Carbon Nanotube Grafted Glucosamine in Mouse Mesenchymal Stem Cells for Bone Tissue Engineering. J. Biomed. Nanotechnol. 2012, 8, 676–685. [Google Scholar] [CrossRef]
- Fonseca-García, A.; Mota-Morales, J.D.; Quintero-Ortega, I.A.; García-Carvajal, Z.Y.; Martínez-López, V.; Ruvalcaba, E.; Landa-Solís, C.; Solis, L.; Ibarra, C.; Gutiérrez, M.C.; et al. Effect of Doping in Carbon Nanotubes on the Viability of Biomimetic Chitosan-Carbon Nanotubes-Hydroxyapatite Scaffolds: Effect of Doping in Carbon Nanotubes. J. Biomed. Mater. Res. Part A 2014, 102, 3341–3351. [Google Scholar] [CrossRef]
- Jamilpour, N.; Fereidoon, A.; Rouhi, G. The Effects of Replacing Collagen Fibers with Carbon Nanotubes on the Rate of Bone Remodeling Process. J. Biomed. Nanotechnol. 2011, 7, 542–548. [Google Scholar] [CrossRef]
- Flores-Cedillo, M.L.; Alvarado-Estrada, K.N.; Pozos-Guillén, A.J.; Murguía-Ibarra, J.S.; Vidal, M.A.; Cervantes-Uc, J.M.; Rosales-Ibáñez, R.; Cauich-Rodríguez, J.V. Multiwall Carbon Nanotubes/Polycaprolactone Scaffolds Seeded with Human Dental Pulp Stem Cells for Bone Tissue Regeneration. J. Mater. Sci.-Mater. Med. 2016, 27, 35. [Google Scholar] [CrossRef]
- Mei, F.; Zhong, J.; Yang, X.; Ouyang, X.; Zhang, S.; Hu, X.; Ma, Q.; Lu, J.; Ryu, S.; Deng, X. Improved Biological Characteristics of Poly(L-Lactic Acid) Electrospun Membrane by Incorporation of Multiwalled Carbon Nanotubes/Hydroxyapatite Nanoparticles. Biomacromolecules 2007, 8, 3729–3735. [Google Scholar] [CrossRef]
- Zhang, H. Electrospun Poly (Lactic-Co-Glycolic Acid)/Multiwalled Carbon Nanotubes Composite Scaffolds for Guided Bone Tissue Regeneration. J. Bioact. Compat. Polym. 2011, 26, 347–362. [Google Scholar] [CrossRef]
- Crowder, S.W.; Prasai, D.; Rath, R.; Balikov, D.A.; Bae, H.; Bolotin, K.I.; Sung, H.-J. Three-Dimensional Graphene Foams Promote Osteogenic Differentiation of Human Mesenchymal Stem Cells. Nanoscale 2013, 5, 4171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Qi, Y.; Tai, Z.; Yan, X.; Zhu, F.; Xue, Q. Preparation, Mechanical Properties and Biocompatibility of Graphene Oxide/Ultrahigh Molecular Weight Polyethylene Composites. Eur. Polym. J. 2012, 48, 1026–1033. [Google Scholar] [CrossRef]
- Das, S.; Wajid, A.S.; Bhattacharia, S.K.; Wilting, M.D.; Rivero, I.V.; Green, M.J. Electrospinning of Polymer Nanofibers Loaded with Noncovalently Functionalized Graphene. J. Appl. Polym. Sci. 2013, 128, 4040–4046. [Google Scholar] [CrossRef]
- Kalbacova, M.; Broz, A.; Kalbac, M. Influence of the Fetal Bovine Serum Proteins on the Growth of Human Osteoblast Cells on Graphene. J. Biomed. Mater. Res. Part A 2012, 100A, 3001–3007. [Google Scholar] [CrossRef]
- Kim, S.; Ku, S.H.; Lim, S.Y.; Kim, J.H.; Park, C.B. Graphene-Biomineral Hybrid Materials. Adv. Mater. 2011, 23, 2009–2014. [Google Scholar] [CrossRef]
- Bosi, S.; Da Ros, T.; Castellano, S.; Banfi, E.; Prato, M. Antimycobacterial Activity of Ionic Fullerene Derivatives. Bioorg. Med. Chem. Lett. 2000, 10, 1043–1045. [Google Scholar] [CrossRef]
- Chaudhuri, P.; Paraskar, A.; Soni, S.; Mashelkar, R.A.; Sengupta, S. Fullerenol-Cytotoxic Conjugates for Cancer Chemotherapy. ACS Nano 2009, 3, 2505–2514. [Google Scholar] [CrossRef]
- Bacakova, L.; Grausova, L.; Vacik, J.; Fraczek, A.; Blazewicz, S.; Kromka, A.; Vanecek, M.; Svorcik, V. Improved Adhesion and Growth of Human Osteoblast-like MG 63 Cells on Biomaterials Modified with Carbon Nanoparticles. Diam. Relat. Mat. 2007, 16, 2133–2140. [Google Scholar] [CrossRef]
- Kopova, I.; Bacakova, L.; Lavrentiev, V.; Vacik, J. Growth and Potential Damage of Human Bone-Derived Cells on Fresh and Aged Fullerene C60 Films. Int. J. Mol. Sci. 2013, 14, 9182–9204. [Google Scholar] [CrossRef] [Green Version]
- Piotrowski, P.; Klimek, K.; Ginalska, G.; Kaim, A. Beneficial Influence of Water-Soluble PEG-Functionalized C60 Fullerene on Human Osteoblast Growth In Vitro. Materials 2021, 14, 1566. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon Quantum Dots and Their Applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Hao, X.; Wang, L.; Hu, Y.; Meng, L.; Zheng, S.; Ren, F.; Bu, W.; Wang, H.; Li, D.; et al. Metformin Carbon Dots for Promoting Periodontal Bone Regeneration via Activation of ERK/AMPK Pathway. Adv. Healthc. Mater. 2021, 10, 2100196. [Google Scholar] [CrossRef]
- Jin, N.; Jin, N.; Wang, Z.; Liu, L.; Meng, L.; Li, D.; Li, X.; Zhou, D.; Liu, J.; Bu, W.; et al. Osteopromotive Carbon Dots Promote Bone Regeneration through the PERK-EIF2α-ATF4 Pathway. Biomater. Sci. 2020, 8, 2840–2852. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Shi, Y.; Fan, W.; Liu, T. Ditungsten Carbide Nanoparticles Embedded in Electrospun Carbon Nanofiber Membranes as Flexible and High-Performance Supercapacitor Electrodes. Compos. Commun. 2019, 12, 21–25. [Google Scholar] [CrossRef]
- Aoki, K.; Haniu, H.; Kim, Y.A.; Saito, N. The Use of Electrospun Organic and Carbon Nanofibers in Bone Regeneration. Nanomaterials 2020, 10, 562. [Google Scholar] [CrossRef] [Green Version]
- Nekounam, H.; Kandi, M.R.; Shaterabadi, D.; Samadian, H.; Mahmoodi, N.; Hasanzadeh, E.; Faridi-Majidi, R. Silica Nanoparticles-Incorporated Carbon Nanofibers as Bioactive Biomaterial for Bone Tissue Engineering. Diam. Relat. Mat. 2021, 115, 108320. [Google Scholar] [CrossRef]
- Shadjou, N.; Hasanzadeh, M. Silica-Based Mesoporous Nanobiomaterials as Promoter of Bone Regeneration Process: Bone Regeneration Process Using Silica-Based Mesoporous Nanobiomaterials. J. Biomed. Mater. Res. Part A 2015, 103, 3703–3716. [Google Scholar] [CrossRef]
- Zhao, Y.; Cui, Z.; Liu, B.; Xiang, J.; Qiu, D.; Tian, Y.; Qu, X.; Yang, Z. An Injectable Strong Hydrogel for Bone Reconstruction. Adv. Healthc. Mater. 2019, 8, 1900709. [Google Scholar] [CrossRef]
- Gaihre, B.; Lecka-Czernik, B.; Jayasuriya, A.C. Injectable Nanosilica–Chitosan Microparticles for Bone Regeneration Applications. J. Biomater. Appl. 2018, 32, 813–825. [Google Scholar] [CrossRef]
- Shi, Z.; Xu, Y.; Mulatibieke, R.; Zhong, Q.; Pan, X.; Chen, Y.; Lian, Q.; Luo, X.; Shi, Z.; Zhu, Q. Nano-Silicate-Reinforced and SDF-1α-Loaded Gelatin-Methacryloyl Hydrogel for Bone Tissue Engineering. Int. J. Nanomed. 2020, 15, 9337–9353. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Shahbazi, M.-A.; Montes, S.; Hosseini, S.H.; Eskandari, M.R.; Zaunschirm, S.; Verwanger, T.; Mathur, S.; Milow, B.; Krammer, B.; et al. Mechanically Strong Silica-Silk Fibroin Bioaerogel: A Hybrid Scaffold with Ordered Honeycomb Micromorphology and Multiscale Porosity for Bone Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 17256–17269. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Cui, S.; Luo, D.; Liu, Y. Novel Inorganic Nanomaterial-Based Therapy for Bone Tissue Regeneration. Nanomaterials 2021, 11, 789. [Google Scholar] [CrossRef] [PubMed]
- Chocholata, P.; Kulda, V.; Dvorakova, J.; Supova, M.; Zaloudkova, M.; Babuska, V. In Situ Hydroxyapatite Synthesis Enhances Biocompatibility of PVA/HA Hydrogels. Int. J. Mol. Sci. 2021, 22, 9335. [Google Scholar] [CrossRef] [PubMed]
- Graziani, G.; Berni, M.; Gambardella, A.; De Carolis, M.; Maltarello, M.C.; Boi, M.; Carnevale, G.; Bianchi, M. Fabrication and Characterization of Biomimetic Hydroxyapatite Thin Films for Bone Implants by Direct Ablation of a Biogenic Source. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.P.S.L.; Mantilaka, M.M.M.G.P.G.; Chathuranga Senarathna, K.G.; Herath, H.M.T.U.; Premachandra, T.N.; Ranasinghe, C.S.K.; Rajapakse, R.P.V.J.; Rajapakse, R.M.G.; Edirisinghe, M.; Mahalingam, S.; et al. Preparation of Bone-Implants by Coating Hydroxyapatite Nanoparticles on Self-Formed Titanium Dioxide Thin-Layers on Titanium Metal Surfaces. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 63, 172–184. [Google Scholar] [CrossRef]
- Pighinelli, L.; Kucharska, M. Chitosan–Hydroxyapatite Composites. Carbohydr. Polym. 2013, 93, 256–262. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babuska, V.; Kasi, P.B.; Chocholata, P.; Wiesnerova, L.; Dvorakova, J.; Vrzakova, R.; Nekleionova, A.; Landsmann, L.; Kulda, V. Nanomaterials in Bone Regeneration. Appl. Sci. 2022, 12, 6793. https://doi.org/10.3390/app12136793
Babuska V, Kasi PB, Chocholata P, Wiesnerova L, Dvorakova J, Vrzakova R, Nekleionova A, Landsmann L, Kulda V. Nanomaterials in Bone Regeneration. Applied Sciences. 2022; 12(13):6793. https://doi.org/10.3390/app12136793
Chicago/Turabian StyleBabuska, Vaclav, Phanindra Babu Kasi, Petra Chocholata, Lucie Wiesnerova, Jana Dvorakova, Radana Vrzakova, Anna Nekleionova, Lukas Landsmann, and Vlastimil Kulda. 2022. "Nanomaterials in Bone Regeneration" Applied Sciences 12, no. 13: 6793. https://doi.org/10.3390/app12136793
APA StyleBabuska, V., Kasi, P. B., Chocholata, P., Wiesnerova, L., Dvorakova, J., Vrzakova, R., Nekleionova, A., Landsmann, L., & Kulda, V. (2022). Nanomaterials in Bone Regeneration. Applied Sciences, 12(13), 6793. https://doi.org/10.3390/app12136793







