Zinc(II) Sulfanyltribenzoporphyrazines with Bulky Peripheral Substituents—Synthesis, Photophysical Characterization, and Potential Photocytotoxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. General Procedures
2.2. Synthetic Procedures
2.2.1. 22,23-Bis[4-(3,5-dibutoxycarbonylphenoxy)butylthio]-tribenzo[b,g,l]porphyrazinato Zinc(II) (6)
2.2.2. 22,23-Bis{4-[3,5-di(hydroxymethyl)phenoxy]butylthio}tribenzo[b,g,l]porphyrazinato Zinc(II) (7)
2.3. UV/Vis Measurements
2.4. Electrochemical Measurements
2.5. Singlet Oxygen Generation Study
2.6. Acute Toxicity Assessment
3. Results and Discussion
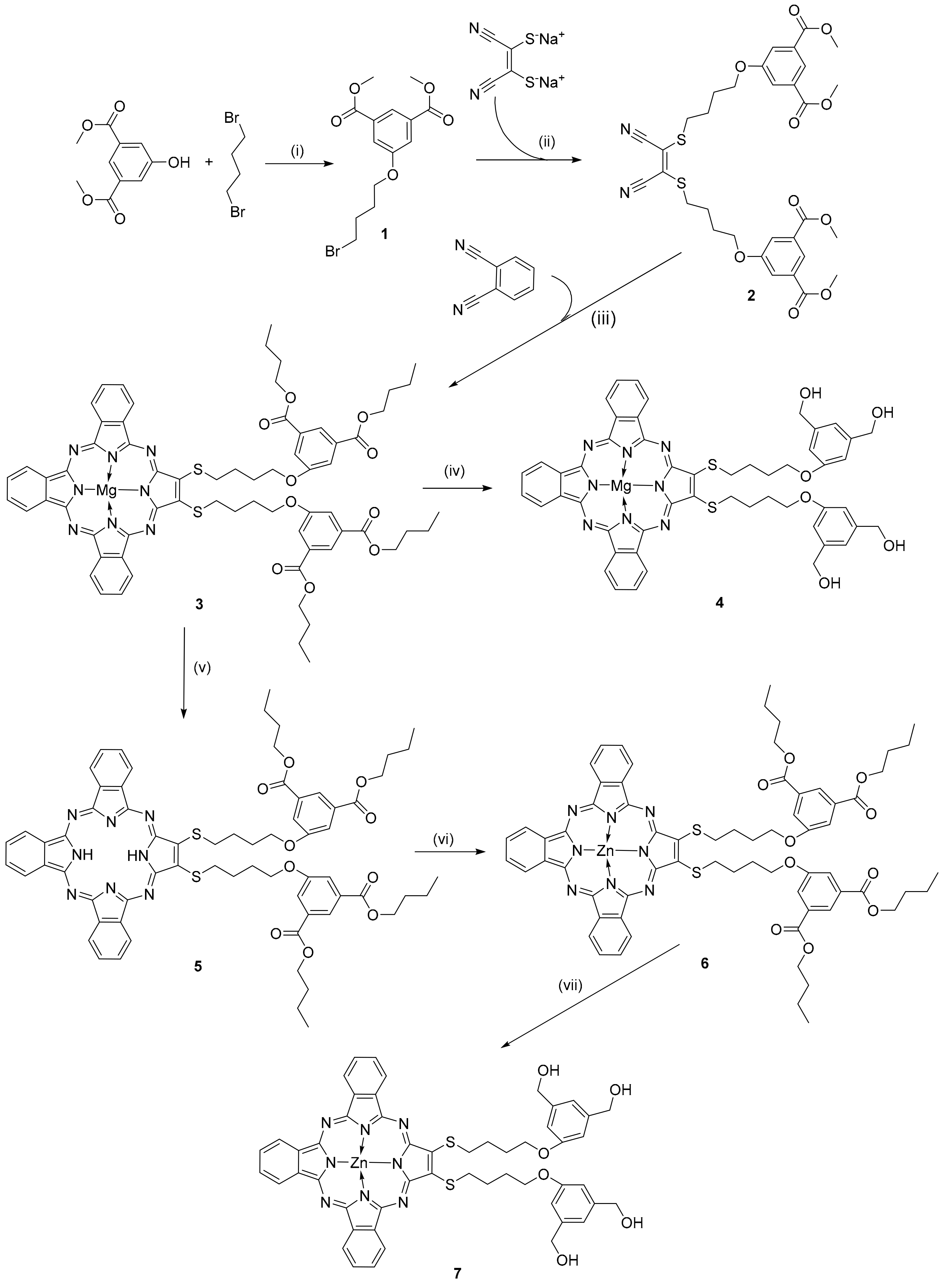
3.1. Synthesis and Characterization
3.2. Absorption Properties of New Porphyrazines
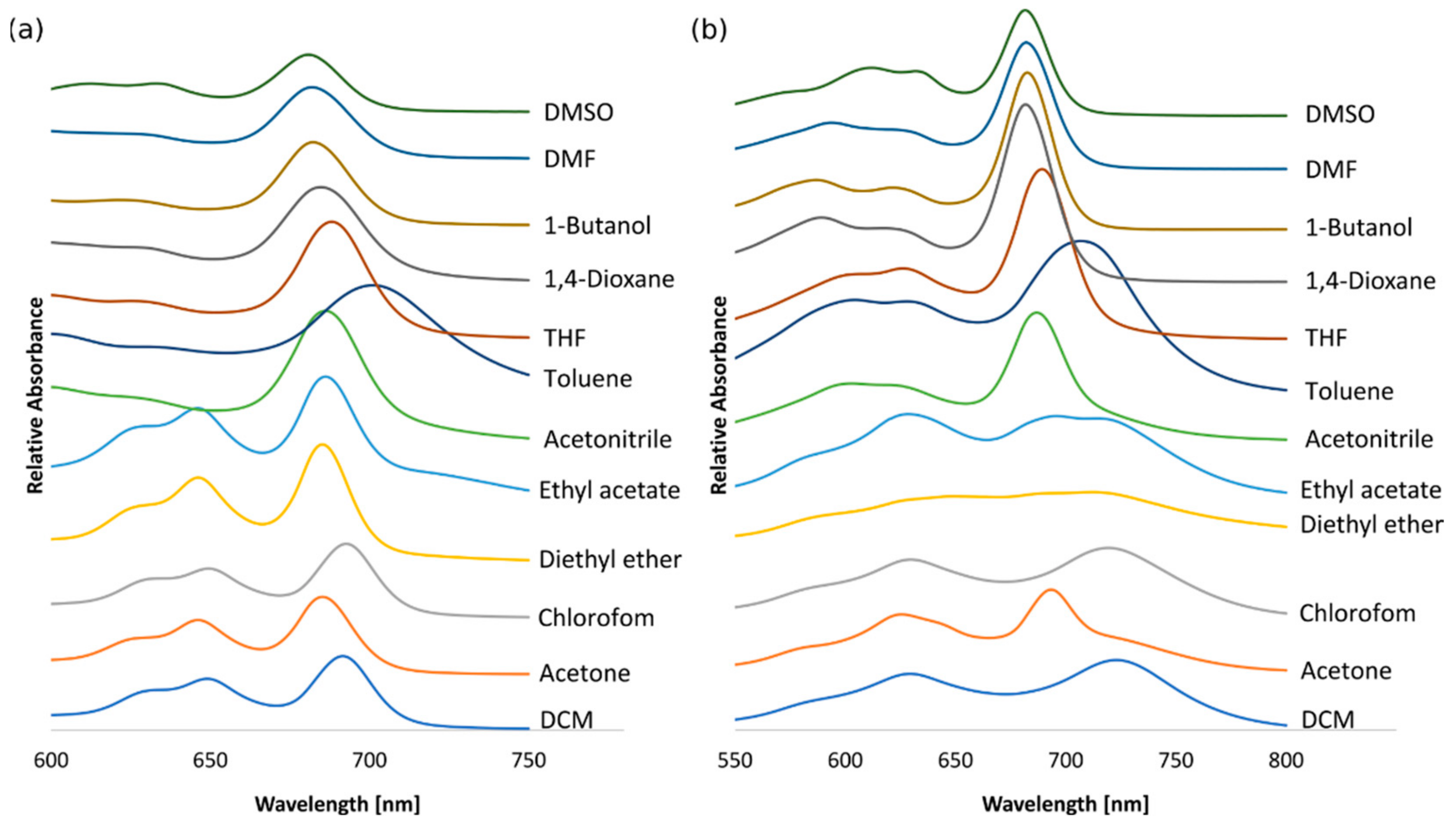
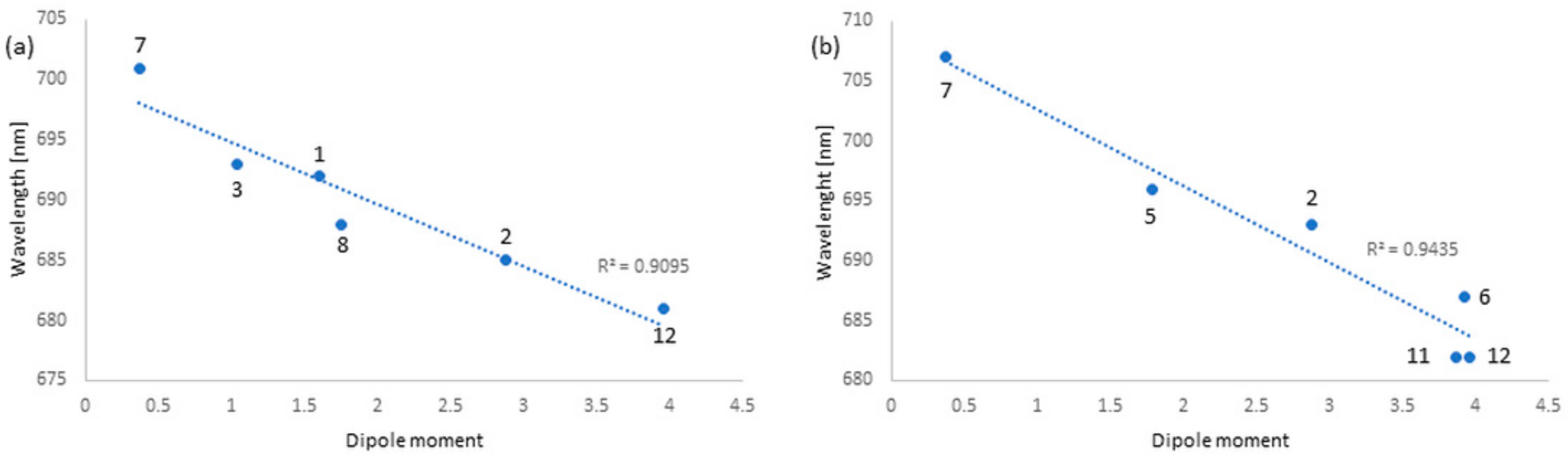
3.3. Solvatochromic Studies
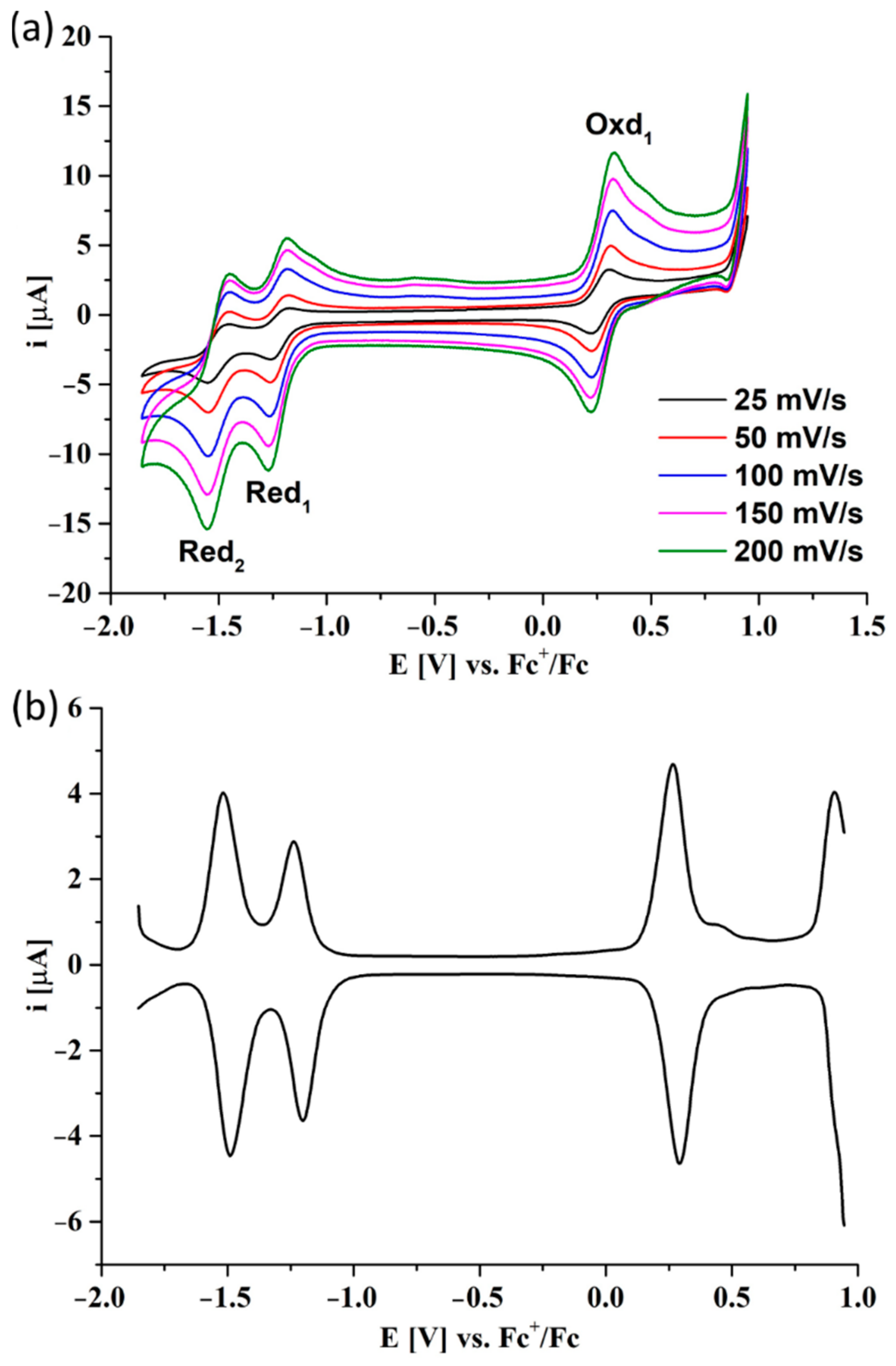
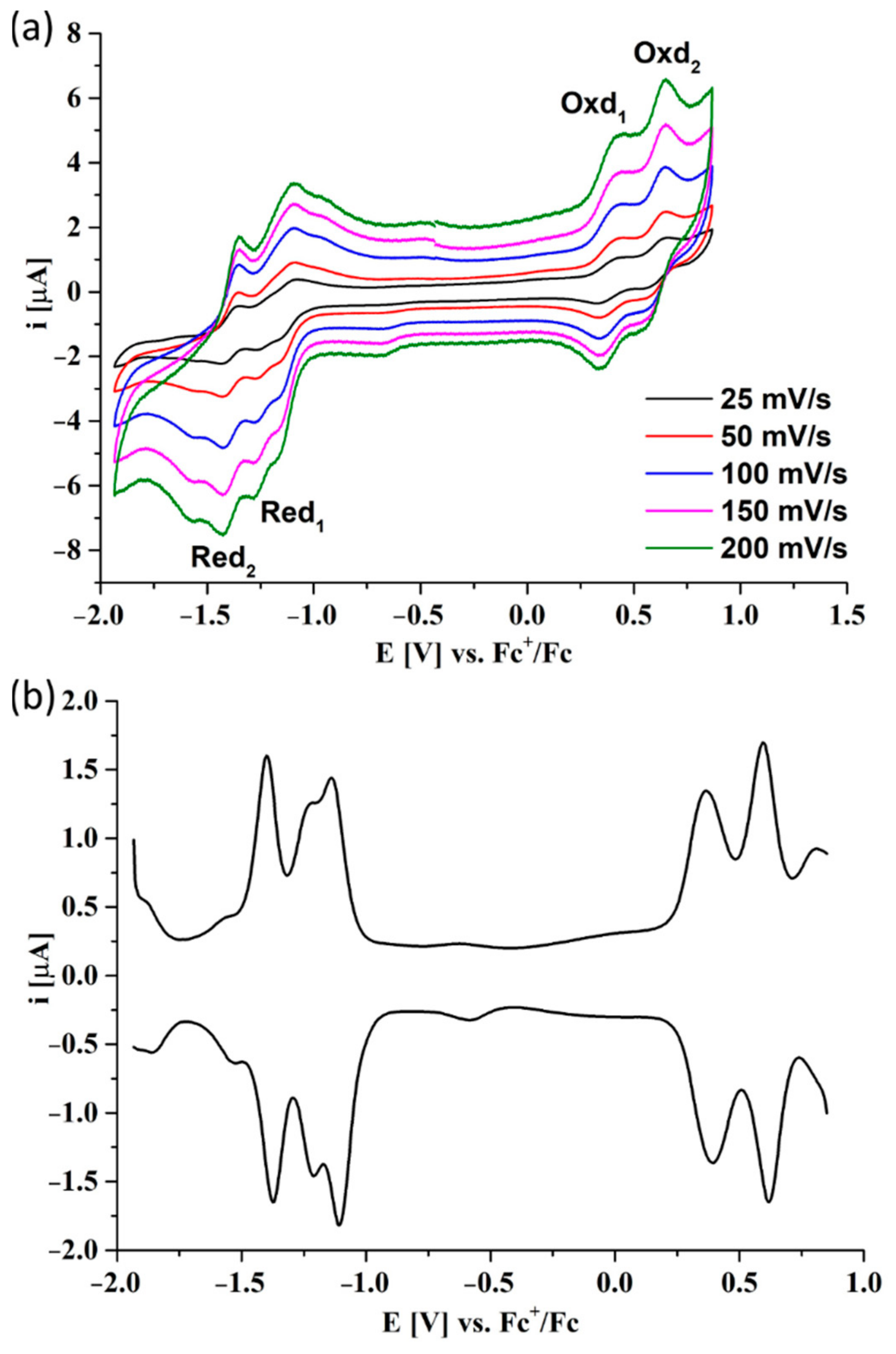
3.4. Electrochemical Studies
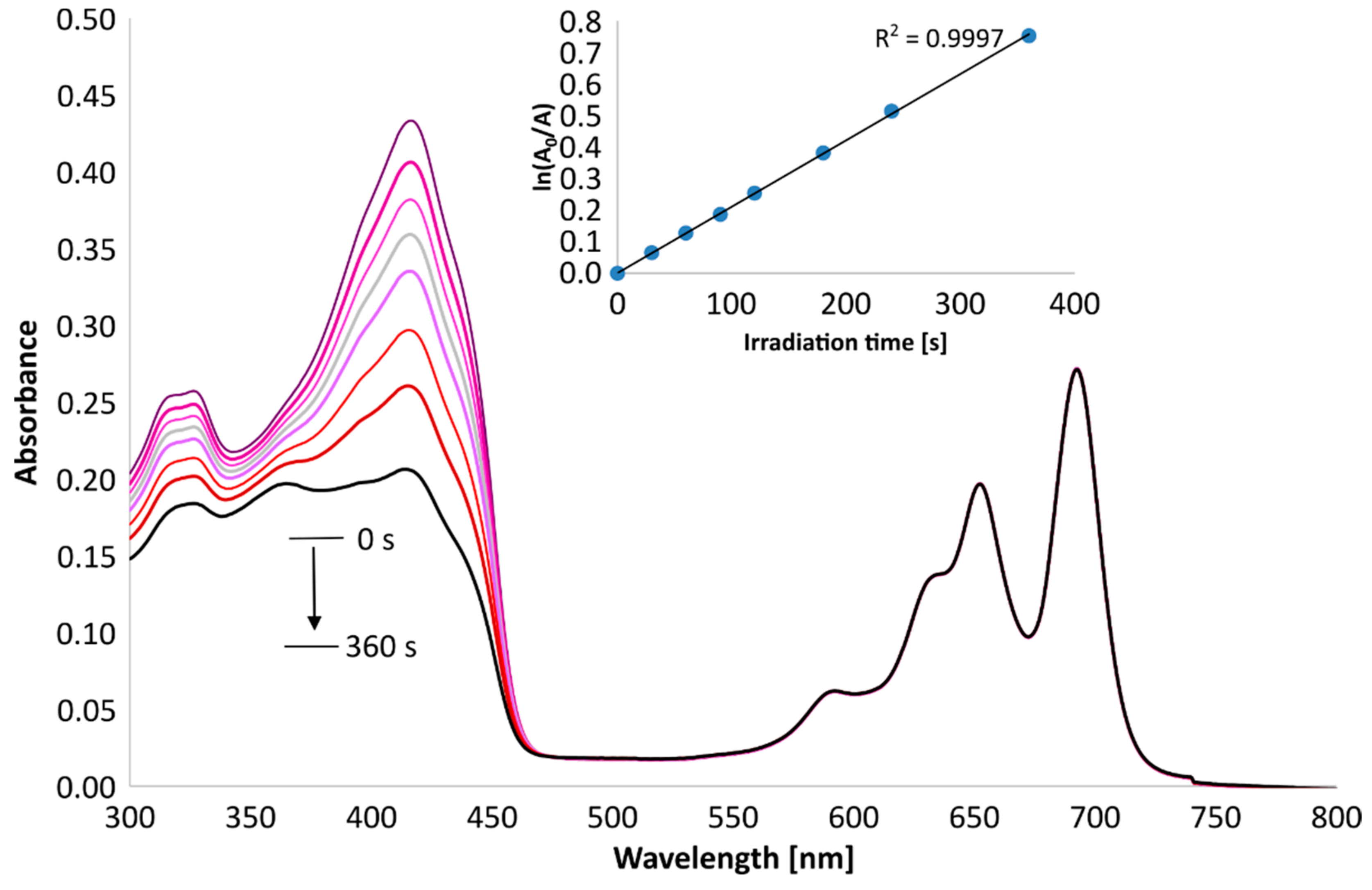
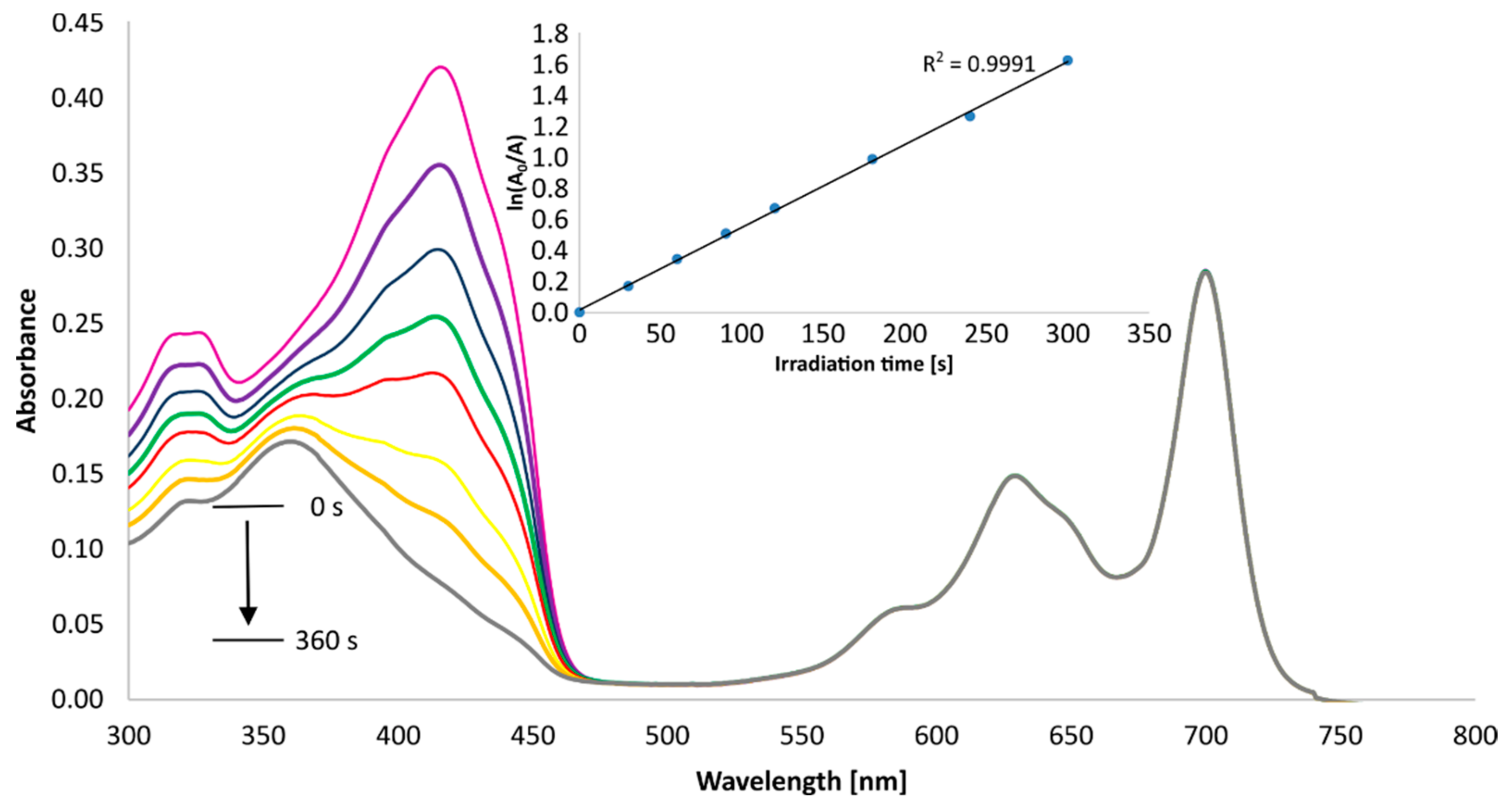
3.5. Singlet Oxygen Generation Study
3.6. Ecotoxicity Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodríguez-Morgade, M.S.; Stuzhin, P.A. The Chemistry of Porphyrazines: An Overview. J. Porphyr. Phthalocyanines 2004, 8, 1129–1165. [Google Scholar] [CrossRef]
- Zong, H.; Sun, P.; Mirkin, C.A.; Barrett, A.G.M.; Hoffman, B.M. Varying the Electrochemical Potential and Thickness of Porphyrazine SAMs by Molecular Design. J. Phys. Chem. B 2009, 113, 14892–14903. [Google Scholar] [CrossRef] [PubMed]
- Motyka, M.; Steer, R.P.; Williams, C.C.; Lee, S.; Ghiggino, K.P. Concerning the Dual Emission of Porphyrazines Employed in Biomedical Imaging. Photochem. Photobiol. Sci. 2013, 12, 1086. [Google Scholar] [CrossRef] [PubMed]
- Miletin, M.; Zimcik, P.; Novakova, V. Photodynamic Properties of Aza-Analogues of Phthalocyanines. Photochem. Photobiol. Sci. 2018, 17, 1749–1766. [Google Scholar] [CrossRef] [PubMed]
- Novakova, V.; Donzello, M.P.; Ercolani, C.; Zimcik, P.; Stuzhin, P.A. Tetrapyrazinoporphyrazines and Their Metal Derivatives. Part II: Electronic Structure, Electrochemical, Spectral, Photophysical and Other Application Related Properties. Coord. Chem. Rev. 2018, 361, 1–73. [Google Scholar] [CrossRef]
- Dąbrowski, J.M.; Pucelik, B.; Regiel-Futyra, A.; Brindell, M.; Mazuryk, O.; Kyzioł, A.; Stochel, G.; Macyk, W.; Arnaut, L.G. Engineering of Relevant Photodynamic Processes through Structural Modifications of Metallotetrapyrrolic Photosensitizers. Coord. Chem. Rev. 2016, 325, 67–101. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, L.; Zhang, Z.; Liu, Z. Advances in Photosensitizer-Related Design for Photodynamic Therapy. Asian J. Pharm. Sci. 2021, 16, 668–686. [Google Scholar] [CrossRef]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and Nonporphyrin Photosensitizers in Oncology: Preclinical and Clinical Advances in Photodynamic Therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Stolarska, M.; Glowacka-Sobotta, A.; Ziental, D.; Dlugaszewska, J.; Falkowski, M.; Goslinski, T.; Sobotta, L. Photochemical Properties and Promising Activity against Staphylococci of Sulfanyl Porphyrazines with Dendrimeric Moieties. Inorg. Chim. Acta 2021, 521, 120321. [Google Scholar] [CrossRef]
- Skupin-Mrugalska, P.; Piskorz, J.; Goslinski, T.; Mielcarek, J.; Konopka, K.; Düzgüneş, N. Current Status of Liposomal Porphyrinoid Photosensitizers. Drug Discov. Today 2013, 18, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Piskorz, J.; Mlynarczyk, D.T.; Szczolko, W.; Konopka, K.; Düzgüneş, N.; Mielcarek, J. Liposomal Formulations of Magnesium Sulfanyl Tribenzoporphyrazines for the Photodynamic Therapy of Cancer. J. Inorg. Biochem. 2018, 184, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Sobotta, L.; Dlugaszewska, J.; Kasprzycki, P.; Lijewski, S.; Teubert, A.; Mielcarek, J.; Gdaniec, M.; Goslinski, T.; Fita, P.; Tykarska, E. In Vitro Photodynamic Activity of Lipid Vesicles with Zinc Phthalocyanine Derivative against Enterococcus Faecalis. J. Photochem. Photobiol. B Biol. 2018, 183, 111–118. [Google Scholar] [CrossRef]
- Darwish, W. Polymers for Enhanced Photodynamic Cancer Therapy: Phthalocyanines as a Photosensitzer Model. Polym. Adv. Technol. 2020, 32, 919–930. [Google Scholar] [CrossRef]
- Shilyagina, N.Y.; Peskova, N.N.; Lermontova, S.A.; Brilkina, A.A.; Vodeneev, V.A.; Yakimansky, A.V.; Klapshina, L.G.; Balalaeva, I.V. Effective Delivery of Porphyrazine Photosensitizers to Cancer Cells by Polymer Brush Nanocontainers. J. Biophoton. 2017, 10, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.S.; Mandal, N.; Patel, G.; Kirar, S.; Reddy, Y.N.; Kushwah, V.; Jain, S.; Kalia, Y.N.; Bhaumik, J.; Banerjee, U.C. Co-Administration of Zinc Phthalocyanine and Quercetin via Hybrid Nanoparticles for Augmented Photodynamic Therapy. Nanomed. Nanotechnol. Biol. Med. 2021, 33, 102368. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Lijewski, S.; Gierszewski, M.; Sobotta, L.; Piskorz, J.; Kordas, P.; Kucinska, M.; Baranowski, D.; Gdaniec, Z.; Murias, M.; Karolczak, J.; et al. Photophysical Properties and Photochemistry of a Sulfanyl Porphyrazine Bearing Isophthaloxybutyl Substituents. Dye. Pigment. 2015, 113, 702–708. [Google Scholar] [CrossRef]
- Klein, T.; Ziegler, T. First Example of an Octa-Glycoconjugated Magnesium(II)Porphyrazine. Tetrahedron Lett. 2016, 57, 495–497. [Google Scholar] [CrossRef]
- Mlynarczyk, D.T.; Lijewski, S.; Falkowski, M.; Piskorz, J.; Szczolko, W.; Sobotta, L.; Stolarska, M.; Popenda, L.; Jurga, S.; Konopka, K.; et al. Dendrimeric Sulfanyl Porphyrazines: Synthesis, Physico-Chemical Characterization, and Biological Activity for Potential Applications in Photodynamic Therapy. ChemPlusChem 2016, 81, 460–470. [Google Scholar] [CrossRef]
- Mlynarczyk, D.T.; Piskorz, J.; Popenda, L.; Stolarska, M.; Szczolko, W.; Konopka, K.; Jurga, S.; Sobotta, L.; Mielcarek, J.; Düzgüneş, N.; et al. S-Seco-Porphyrazine as a New Member of the Seco-Porphyrazine Family-Synthesis, Characterization and Photocytotoxicity against Cancer Cells. Bioorg. Chem. 2020, 96, 103634. [Google Scholar] [CrossRef] [PubMed]
- Sobotta, L.; Fita, P.; Szczolko, W.; Wrotynski, M.; Wierzchowski, M.; Goslinski, T.; Mielcarek, J. Functional Singlet Oxygen Generators Based on Porphyrazines with Peripheral 2,5-Dimethylpyrrol-1-Yl and Dimethylamino Groups. J. Photochem. Photobiol. A Chem. 2013, 269, 9–16. [Google Scholar] [CrossRef]
- Seotsanyana-Mokhosi, I.; Kuznetsova, N.; Nyokong, T. Photochemical Studies of Tetra-2,3-Pyridinoporphyrazines. J. Photochem. Photobiol. A Chem. 2001, 140, 215–222. [Google Scholar] [CrossRef]
- Ogunsipe, A.; Maree, D.; Nyokong, T. Solvent Effects on the Photochemical and Fluorescence Properties of Zinc Phthalocyanine Derivatives. J. Mol. Struct. 2003, 650, 131–140. [Google Scholar] [CrossRef]
- Chelminiak-Dudkiewicz, D.; Rybczynski, P.; Smolarkiewicz-Wyczachowski, A.; Mlynarczyk, D.T.; Wegrzynowska-Drzymalska, K.; Ilnicka, A.; Goslinski, T.; Marszałł, M.P.; Ziegler-Borowska, M. Photosensitizing Potential of Tailored Magnetite Hybrid Nanoparticles Functionalized with Levan and Zinc(II) Phthalocyanine. Appl. Surf. Sci. 2020, 524, 146602. [Google Scholar] [CrossRef]
- Linstead, R.P.; Whalley, M. 944. Conjugated Macrocylces. Part XXII. Tetrazaporphin and Its Metallic Derivatives. J. Chem. Soc. 1952, 4839–4846. [Google Scholar] [CrossRef]
- Montalban, A.G.; Lange, S.J.; Beall, L.S.; Mani, N.S.; Williams, D.J.; White, A.J.P.; Barrett, A.G.M.; Hoffman, B.M. Seco-Porphyrazines: Synthetic, Structural, and Spectroscopic Investigations. J. Org. Chem. 1997, 62, 9284–9289. [Google Scholar] [CrossRef]
- Höger, S. Methoxycarbonyl-Terminated Dendrons via the Mitsunobu Reaction: An Easy Way to Functionalized Hyperbranched Building Blocks. Synthesis 1997, 1997, 20–22. [Google Scholar] [CrossRef]
- Nostrum van, C.F.; Benneker, F.B.G.; Brussaard, H.; Kooijman, H.; Veldman, N.; Spek, A.L.; Schoonman, J.; Feiters, M.C.; Nolte, R.J.M. Dithiacrown Ether Substituted Porphyrazines: Synthesis, Single-Crystal Structure, and Control of Aggregation in Solution by Complexation of Transition-Metal Ions. Inorg. Chem. 1996, 35, 959–969. [Google Scholar] [CrossRef][Green Version]
- Sibert, J.W.; Baumann, T.F.; Williams, D.J.; White, A.J.P.; Barrett, A.G.M.; Hoffman, B.M. Gemini-Porphyrazines: The Synthesis and Characterization of Metal-Capped Cis-and Trans -Porphyrazine Tetrathiolates. J. Am. Chem. Soc. 1996, 118, 10487–10493. [Google Scholar] [CrossRef]
- Araújo, A.R.L.; Tomé, A.C.; Santos, C.I.M.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Simões, M.M.Q.; Moura, N.M.M.; Abu-Orabi, S.T.; Cavaleiro, J.A.S. Azides and Porphyrinoids: Synthetic Approaches and Applications. Part 2—Azides, Phthalocyanines, Subphthalocyanines and Porphyrazines. Molecules 2020, 25, 1745. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.F.M.; Tasso, T.T. From Cuvette to Cells: How the Central Metal Ion Modulates the Properties of Phthalocyanines and Porphyrazines as Photosensitizers. Inorg. Chim. Acta 2021, 519, 120271. [Google Scholar] [CrossRef]
- Salvati, A.; Ristori, S.; Pietrangeli, D.; Oberdisse, J.; Calamai, L.; Martini, G.; Ricciardi, G. Insertion of a Magnesium(II)-Octacarboranyl(Hexylsulfanyl) Porphyrazine into Liposomes: A Physico-Chemical Study. Biophys. Chem. 2007, 131, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, N.S. The Effect of the Electrostatic Polarization of the Solvent on Electronic Absorption Spectra in Solution. J. Chem. Phys. 1950, 18, 292–296. [Google Scholar] [CrossRef]
- Chemical Rubber Company. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data, 78th ed.; Lide, D.R., Ed.; Edition 1997–1998; CRC Press: Boca Raton, FL, USA, 1997; ISBN 978-0-8493-0478-1. [Google Scholar]
- Rebis, T.; Lijewski, S.; Nowicka, J.; Popenda, L.; Sobotta, L.; Jurga, S.; Mielcarek, J.; Milczarek, G.; Goslinski, T. Electrochemical Properties of Metallated Porphyrazines Possessing Isophthaloxybutylsulfanyl Substituents: Application in the Electrocatalytic Oxidation of Hydrazine. Electrochim. Acta 2015, 168, 216–224. [Google Scholar] [CrossRef]
- Koczorowski, T.; Szczolko, W.; Teubert, A.; Goslinski, T. Sulfanyl Porphyrazines with Morpholinylethyl Periphery—Synthesis, Electrochemistry, and Photocatalytic Studies after Deposition on Titanium(IV) Oxide P25 Nanoparticles. Molecules 2021, 26, 2280. [Google Scholar] [CrossRef]
- Gierszewski, M.; Falkowski, M.; Sobotta, L.; Stolarska, M.; Popenda, L.; Lijewski, S.; Wicher, B.; Burdzinski, G.; Karolczak, J.; Jurga, S.; et al. Porphyrazines with Peripheral Isophthaloxyalkylsulfanyl Substituents and Their Optical Properties. J. Photochem. Photobiol. A Chem. 2015, 307, 54–67. [Google Scholar] [CrossRef]
- Mlynarczyk, D.T.; Dlugaszewska, J.; Falkowski, M.; Popenda, L.; Kryjewski, M.; Szczolko, W.; Jurga, S.; Mielcarek, J.; Goslinski, T. Tribenzoporphyrazines with Dendrimeric Peripheral Substituents and Their Promising Photocytotoxic Activity against Staphylococcus Aureus. J. Photochem. Photobiol. B Biol. 2020, 204, 111803. [Google Scholar] [CrossRef]



| 6 | 7 | |||
|---|---|---|---|---|
| SOLVENT | λ2/nm (Soret Band) | λ1/nm (Q-Band) | λ2/nm (Soret Band) | λ1/nm (Q-Band) |
| dichloromethane | 352 | 692 | 358 | 723 |
| acetone | 354 | 685 | 356 | 693 |
| chloroform | 353 | 693 | 357 | 719 |
| diethyl ether | 353 | 685 | 357 | 713 |
| ethyl acetate | 354 | 686 | 355 | 696 |
| acetonitrile | 356 | 686 | 356 | 687 |
| toluene | 361 | 701 | 360 | 707 |
| thf | 355 | 688 | 356 | 689 |
| 1,4-dioxane | 346 | 685 | 357 | 682 |
| 1-butanol | 347 | 682 | 354 | 683 |
| dmf | 364 | 682 | 366 | 682 |
| dmso | 337 | 681 | 338 | 682 |
| Solvent | Refractive Index (nD) | Dipole Moment (µ) | Q-Band (λmax, nm) 6 | Q-Band (λmax, nm) 7 |
|---|---|---|---|---|
| dichloromethane | 1.445 | 1.60 | 692 | 723 |
| acetone | 1.359 | 2.88 | 685 | 693 |
| chloroform | 1.446 | 1.04 | 693 | 719 |
| diethyl ether | 1.352 | 1.15 | 685 | 713 |
| ethyl acetate | 1.372 | 1.78 | 686 | 696 |
| acetonitrile | 1.344 | 3.92 | 686 | 687 |
| toluene | 1.497 | 0.37 | 701 | 707 |
| THF | 1.407 | 1.75 | 688 | 689 |
| 1,4-dioxane | 1.422 | 0 | 685 | 682 |
| 1-butanol | 1.399 | 1.66 | 682 | 683 |
| DMF | 1.430 | 3.82 | 682 | 682 |
| DMSO | 1.478 | 3.96 | 681 | 682 |
| Pz | Red2 | Red1 | Oxd1 | Oxd2 | Egap | Ref. |
|---|---|---|---|---|---|---|
| 6 | −1.50 V | −1.22 V | 0.28 V | - | 1.50 eV | This work |
| 7 | −1.39 V | −1.12 V | 0.38 V | 0.61 V | 1.50 eV | This work |
| Sym ZnPz 1 | −1.24 V | −1.01 V | 0.67 V | - | 1.68 eV | [36] |
| Sym ZnPz 2 | −1.34 V | −0.74 V | 0.53 V | - | 1.27 eV | [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koza, P.; Koczorowski, T.; Mlynarczyk, D.T.; Goslinski, T. Zinc(II) Sulfanyltribenzoporphyrazines with Bulky Peripheral Substituents—Synthesis, Photophysical Characterization, and Potential Photocytotoxicity. Appl. Sci. 2022, 12, 6825. https://doi.org/10.3390/app12136825
Koza P, Koczorowski T, Mlynarczyk DT, Goslinski T. Zinc(II) Sulfanyltribenzoporphyrazines with Bulky Peripheral Substituents—Synthesis, Photophysical Characterization, and Potential Photocytotoxicity. Applied Sciences. 2022; 12(13):6825. https://doi.org/10.3390/app12136825
Chicago/Turabian StyleKoza, Patrycja, Tomasz Koczorowski, Dariusz T. Mlynarczyk, and Tomasz Goslinski. 2022. "Zinc(II) Sulfanyltribenzoporphyrazines with Bulky Peripheral Substituents—Synthesis, Photophysical Characterization, and Potential Photocytotoxicity" Applied Sciences 12, no. 13: 6825. https://doi.org/10.3390/app12136825
APA StyleKoza, P., Koczorowski, T., Mlynarczyk, D. T., & Goslinski, T. (2022). Zinc(II) Sulfanyltribenzoporphyrazines with Bulky Peripheral Substituents—Synthesis, Photophysical Characterization, and Potential Photocytotoxicity. Applied Sciences, 12(13), 6825. https://doi.org/10.3390/app12136825






