The Use of Hanseniaspora occidentalis in a Sequential Must Inoculation to Reduce the Malic Acid Content of Wine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains Used
2.2. Must Fermentation
2.3. Analysis of the Wines
2.4. Statistical Analysis
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Drumonde-Neves, J.; Fernandes, T.; Lima, T.; Pais, C.; Franco-Duarte, R. Learning from 80 years of studies: A comprehensive catalogue of non-Saccharomyces yeasts associated with viticulture and winemaking. FEMS Yeast Res. 2021, 21, foab017. [Google Scholar] [CrossRef] [PubMed]
- Malfeito-Ferreira, M. Yeasts and wine off-flavours: A technological perspective. Ann. Microbiol. 2011, 61, 95–102. [Google Scholar] [CrossRef]
- Mendoza, L.M.; De Nadra, M.C.M.; Farías, M.E. Kinetics and metabolic behavior of a composite culture of Kloeckera apiculata and Saccharomyces cerevisiae wine related strains. Biotechnol. Lett. 2007, 29, 1057–1063. [Google Scholar] [CrossRef]
- Egli, C.M.; Edinger, W.D.; Mitrakul, C.M.; Henick-Kling, T. Dynamics of indigenous and inoculated yeast populations and their effect on the sensory character of Riesling and Chardonnay wines. J. Appl. Microbiol. 1998, 85, 779–789. [Google Scholar] [CrossRef]
- Wang, C.; Mas, A.; Esteve-Zarzoso, B. The interaction between Saccharomyces cerevisiae and non-Saccharomyces yeast during alcoholic fermentation is species and strain specific. Front. Microbiol. 2016, 7, 502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Hu, K.; Xu, Y.; Mei, W.; Tao, Y. Biomass suppression of Hanseniaspora uvarum by killer Saccharomyces cerevisiae highly increased fruity esters in mixed culture fermentation. LWT 2020, 132, 109839. [Google Scholar] [CrossRef]
- van Wyk, N.; Grossmann, M.; Wendland, J.; von Wallbrunn, C.; Pretorius, I.S. The whiff of wine yeast innovation: Strategies for enhancing aroma production by yeast during wine fermentation. J. Agric. Food Chem. 2019, 67, 13496–13505. [Google Scholar] [CrossRef]
- van Wyk, N.; von Wallbrunn, C.; Swiegers, J.H.; Pretorius, I.S. Biotechnology of wine yeasts. In Encyclopedia of Mycology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 34–44. ISBN 9781498740814. [Google Scholar]
- Viana, F.; Belloch, C.; Vallés, S.; Manzanares, P. Monitoring a mixed starter of Hanseniaspora vineae-Saccharomyces cerevisiae in natural must: Impact on 2-phenylethyl acetate production. Int. J. Food Microbiol. 2011, 151, 235–240. [Google Scholar] [CrossRef]
- Viana, F.; Gil, J.V.; Vallés, S.; Manzanares, P. Increasing the levels of 2-phenylethyl acetate in wine through the use of a mixed culture of Hanseniaspora osmophila and Saccharomyces cerevisiae. Int. J. Food Microbiol. 2009, 135, 68–74. [Google Scholar] [CrossRef]
- Granchi, L.; Ganucci, D.; Messini, A.; Vincenzini, M. Oenological properties of Hanseniaspora osmophila and Kloeckera corticis from wines produced by spontaneous fermentations of normal and dried grapes. FEMS Yeast Res. 2002, 2, 403–407. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Zhang, Y.; Qiu, Y.; Guo, H.; Ju, H.; Wang, Y.; Yuan, Y.; Yue, T. Chemical composition, sensorial properties, and aroma-active compounds of ciders fermented with Hanseniaspora osmophila and Torulaspora quercuum in co- and sequential fermentations. Food Chem. 2020, 306, 125623. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, B.; Zambelli, P.; Vigentini, I.; Bauer, F.F.; Setati, M.E. Investigating the effect of selected non-Saccharomyces species on wine ecosystem function and major volatiles. Front. Bioeng. Biotechnol. 2018, 6, 169. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, L.M.; Vega-Lopez, G.A.; Fernández de Ullivarri, M.; Raya, R.R. Population and oenological characteristics of non-Saccharomyces yeasts associated with grapes of Northwestern Argentina. Arch. Microbiol. 2019, 201, 235–244. [Google Scholar] [CrossRef] [PubMed]
- González-Robles, I.W.; Estarrón-Espinosa, M.; Díaz-Montaño, D.M. Fermentative capabilities and volatile compounds produced by Kloeckera/Hanseniaspora and Saccharomyces yeast strains in pure and mixed cultures during Agave tequilana juice fermentation. Antonie Van Leeuwenhoek 2015, 108, 525–536. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Aponte, M.; Piombino, P.; Lisanti, M.T.; Moio, L.; Ercolini, D.; Blaiotta, G. Influence of microbial communities on the chemical and sensory features of Falanghina sweet passito wines. Food Res. Int. 2019, 120, 740–747. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, D.; Duan, C.; Yan, G. Synergistic effect enhances 2-phenylethyl acetate production in the mixed fermentation of Hanseniaspora vineae and Saccharomyces cerevisiae. Process Biochem. 2020, 90, 44–49. [Google Scholar] [CrossRef]
- Olivera, V.; Boido, E.; Dellacassa, E. Wine aroma characterization of the two main fermentation yeast species of the apiculate genus Hanseniaspora. Fermentation 2021, 7, 162. [Google Scholar] [CrossRef]
- Sternes, P.R.; Lee, D.; Kutyna, D.R.; Borneman, A.R. Genome sequences of three species of Hanseniaspora isolated from spontaneous wine fermentations. Genome Announc. 2016, 4, e01287-16. [Google Scholar] [CrossRef] [Green Version]
- Saubin, M.; Devillers, H.; Proust, L.; Brier, C.; Grondin, C.; Pradal, M.; Legras, J.L.; Neuvéglise, C. Investigation of genetic relationships between Hanseniaspora species found in grape musts revealed interspecific hybrids with dynamic genome structures. Front. Microbiol. 2020, 10, 2960. [Google Scholar] [CrossRef] [Green Version]
- Steenwyk, J.L.; Opulente, D.A.; Kominek, J.; Shen, X.; Zhou, X.; Labella, A.L.; Bradley, N.P.; Eichman, B.F.; Libkind, D.; Devirgilio, J.; et al. Extensive loss of cell-cycle and DNA repair genes in an ancient lineage of bipolar budding yeasts. PLoS Biol. 2019, 1, e3000255. [Google Scholar] [CrossRef] [Green Version]
- Hoang, D.; Kopp, A.; Chandler, J.A. Interactions between Drosophila and its natural yeast symbionts-Is Saccharomyces cerevisiae a good model for studying the fly-yeast relationship? PeerJ 2015, 3, e1116. [Google Scholar] [CrossRef] [Green Version]
- Grangeteau, C.; Gerhards, D.; Rousseaux, S.; von Wallbrunn, C.; Alexandre, H.; Guilloux-Benatier, M. Diversity of yeast strains of the genus Hanseniaspora in the winery environment: What is their involvement in grape must fermentation? Food Microbiol. 2015, 50, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Mecca, D.; Benito, S.; Beisert, B.; Brezina, S.; Fritsch, S.; Semmler, H.; Rauhut, D. Influence of nutrient supplementation on Torulaspora delbrueckii wine fermentation aroma. Fermentation 2020, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Brandt, M. The Influence of Abiotic Factors on the Composition of Berries, Juice and Wine in Vitis vinifera L. cv. Riesling. Ph.D. Thesis, Hochschule Geisenheim, Geisenheim, Germany, 2021. [Google Scholar]
- Jung, R.; Kumar, K.; Patz, C.; Rauhut, D.; Tarasov, A.; Schuessler, C. Influence of transport temperature profiles on wine quality. Food Packag. Shelf Life 2021, 29, 100706. [Google Scholar] [CrossRef]
- Scansani, S.; van Wyk, N.; Nader, K.B.; Beisert, B.; Brezina, S.; Fritsch, S.; Semmler, H.; Pasch, L.; Pretorius, I.S.; von Wallbrunn, C.; et al. The film-forming Pichia spp. in a winemaker’s toolbox: A simple isolation procedure and their performance in a mixed-culture fermentation of Vitis vinifera L. cv. Gewürztraminer must. Int. J. Food Microbiol. 2022, 365, 109549. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 12 May 2022).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Ciani, M.; Fatichenti, F. Selective sugar consumption by apiculate yeasts. Lett. Appl. Microbiol. 1999, 28, 203–206. [Google Scholar] [CrossRef]
- Rossouw, D.; Bauer, F.F. Exploring the phenotypic space of non-Saccharomyces wine yeast biodiversity. Food Microbiol. 2016, 55, 32–46. [Google Scholar] [CrossRef]
- Čadež, N.; Smith, M.T. Hanseniaspora Zikes (1912); Elsevier B.V.: Amsterdam, The Netherlands, 2011; Volume 2, ISBN 9780444521491. [Google Scholar]
- Koslitz, S.; Renaud, L.; Kohler, M.; Wüst, M. Stereoselective formation of the varietal aroma compound rose oxide during alcoholic fermentation. J. Agric. Food Chem. 2008, 56, 1371–1375. [Google Scholar] [CrossRef]
- Mira de Orduña, R. Climate change associated effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Satora, P.; Sroka, P.; Gojniczek, I. Chemical composition of cool-climate grapes and enological parameters of cool-climate wines. Fruits 2014, 69, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Vilela, A. Biological demalication and deacetification of musts and wines: Can wine yeasts make the wine taste better? Fermentation 2017, 3, 51. [Google Scholar] [CrossRef] [Green Version]
- Volschenk, H.; van Vuuren, H.J.J.; Viljoen-Bloom, M. Malic acid in wine: Origin, function and metabolism during vinification. S. Afr. J. Enol. Vitic. 2006, 27, 123–136. [Google Scholar] [CrossRef] [Green Version]
- Main, G.L.; Threlfall, R.T.; Morris, J.R. Reduction of malic acid in wine using natural and genetically enhanced microorganisms. Am. J. Enol. Vitic. 2007, 3, 341–345. [Google Scholar]
- Knoll, C.; Fritsch, S.; Schnell, S.; Grossmann, M.; Rauhut, D.; Du Toit, M. Influence of pH and ethanol on malolactic fermentation and volatile aroma compound composition in white wines. LWT Food Sci. Technol. 2011, 44, 2077–2086. [Google Scholar] [CrossRef]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic amine production by lactic acid bacteria: A review. Foods 2019, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Scansani, S.; Rauhut, D.; Brezina, S.; Semmler, H.; Benito, S. The impact of chitosan on the chemical composition of wines fermented with Schizosaccharomyces pombe and Saccharomyces cerevisiae. Foods 2020, 9, 1423. [Google Scholar] [CrossRef]
- Cioch-Skoneczny, M.; Grabowski, M.; Satora, P.; Skoneczny, S.; Klimczak, K. The use of yeast mixed cultures for deacidification and improvement of the composition of cold climate grape wines. Molecules 2021, 26, 2628. [Google Scholar] [CrossRef]
- Volschenk, H.; Viljoen-Bloom, M.; Subden, R.E.; Van Vuuren, H.J.J. Malo-ethanolic fermentation in grape must by recombinant strains of Saccharomyces cerevisiae. Yeast 2001, 18, 963–970. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Liu, H.; Liu, J.; Jiao, Z. Profiles of sugar and organic acid of fruit juices: A comparative study and implication for authentication. J. Food Qual. 2020, 2020, 7236534. [Google Scholar] [CrossRef]
- Hu, K.; Jin, G.J.; Xu, Y.H.; Tao, Y.S. Wine aroma response to different participation of selected Hanseniaspora uvarum in mixed fermentation with Saccharomyces cerevisiae. Food Res. Int. 2018, 108, 119–127. [Google Scholar] [CrossRef]
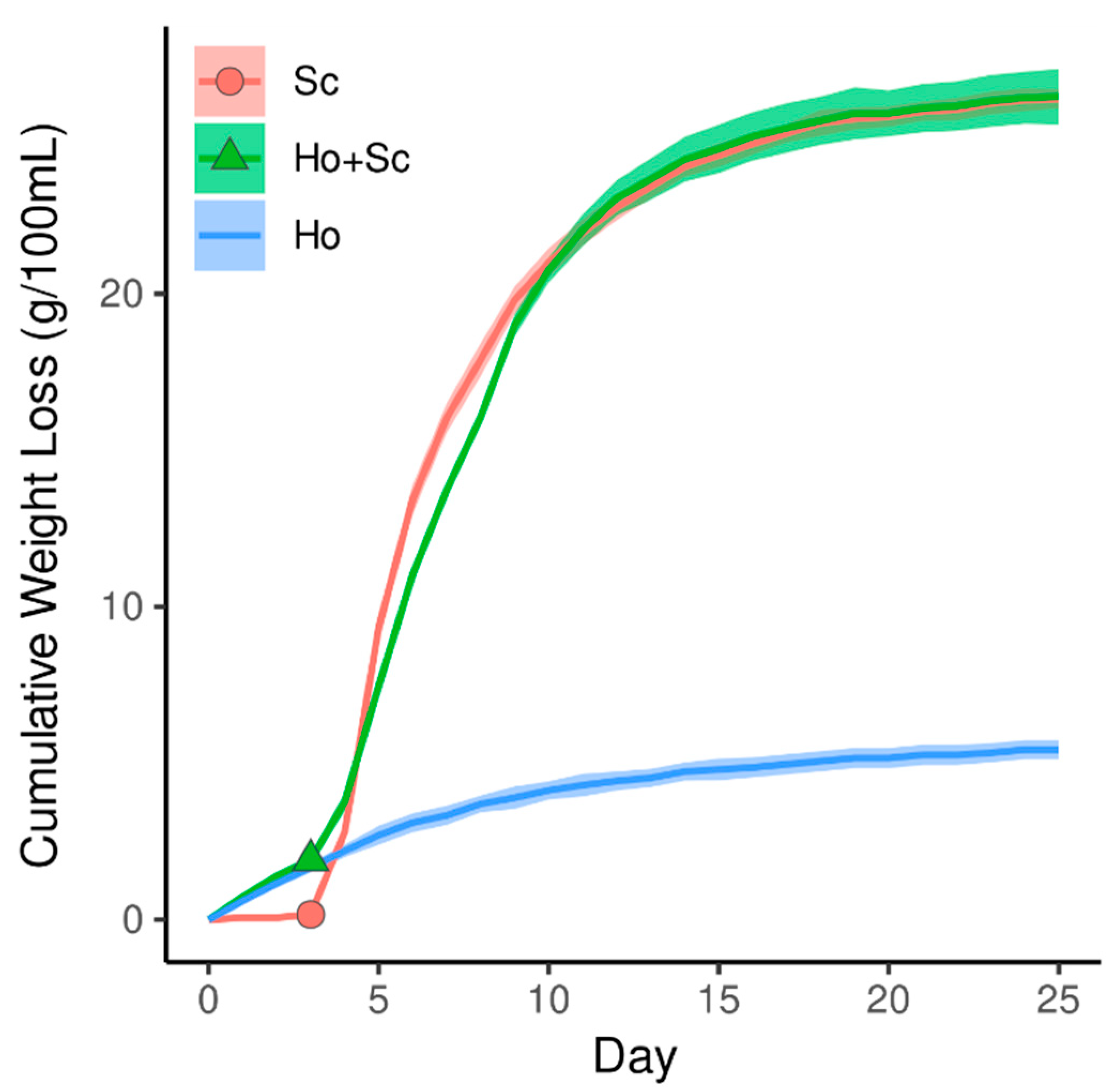
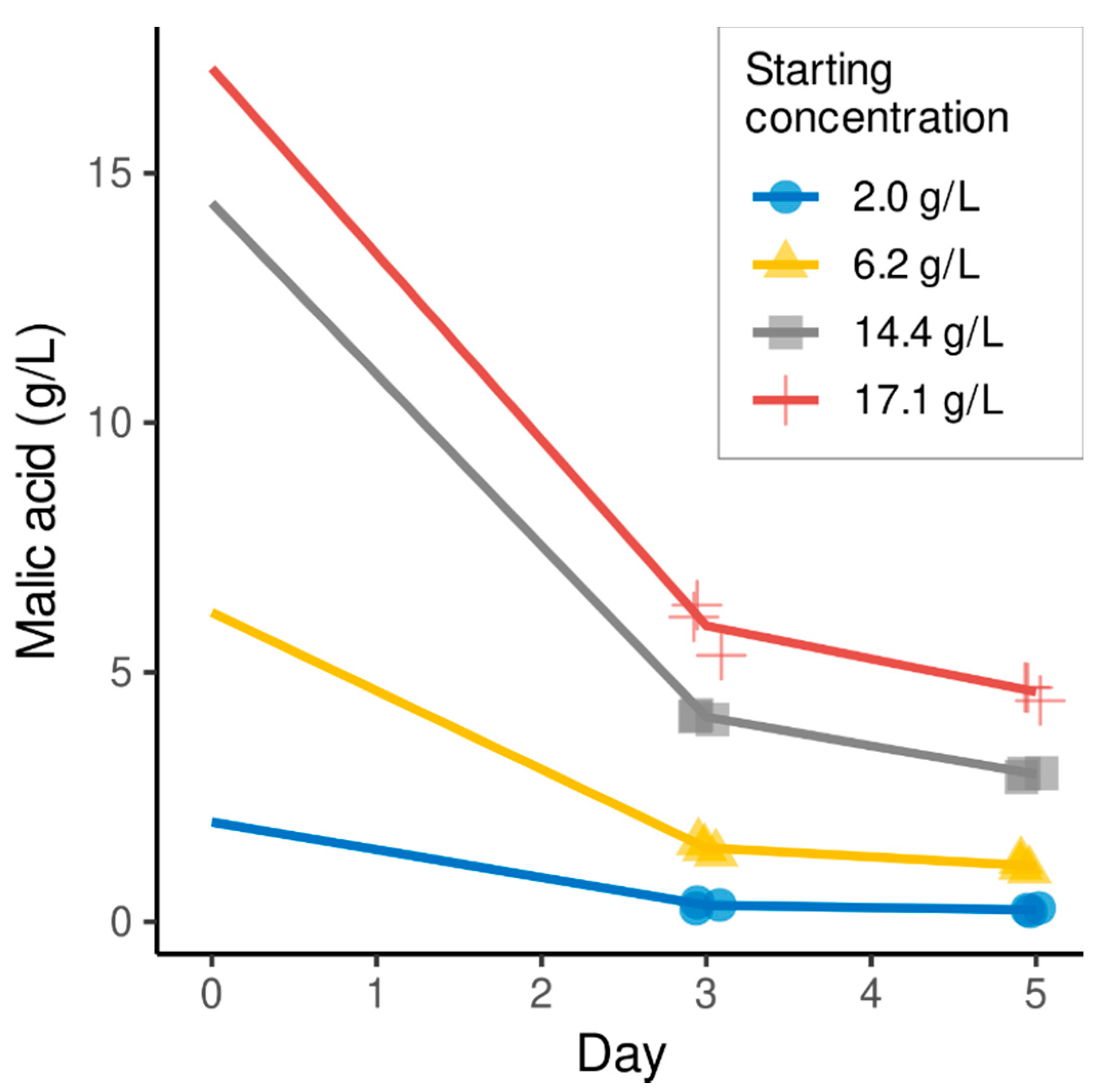
| Parameter | Sc | Ho + Sc | p-Value 1 | Ho | ||
|---|---|---|---|---|---|---|
| Glucose (g/L) | <1 | <1 | 65.4 ± 1.8 | |||
| Fructose (g/L) | 3.2 ± 1.0 | 2.4 ± 0.0 | 0.663 | 122.7 ± 2.0 | ||
| Ethanol (g/L) | 123.7 ± 0.3 | 128.7 ± 8.7 | 0.663 | 24.7 ± 0.8 | ||
| Glycerol (g/L) | 7.0 ± 0.1 | 7.8 ± 0.6 | 0.081 | . | 3.4 ± 0.2 | |
| Tartaric acid (g/L) | 4.0 ± 0.2 | 4.3 ± 0.3 | 0.188 | 5.6 ± 0.0 | ||
| Malic acid (g/L) | 1.5 ± 0.1 | 0.1 ± 0.0 | 9.6 × 10−5 | *** | 0.2 ± 0.0 | |
| Lactic acid (g/L) | <0.1 | 0.1 ± 0.0 | <0.1 | |||
| Acetic acid (g/L) | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.036 | * | 0.3 ± 0.0 | |
| Citric acid (g/L) | <0.1 | <0.1 | <0.1 | |||
| Acetaldehyde (mg/L) | 10.2 ± 0.3 | 10.8 ± 0.8 | 0.386 | - | ||
| pH | 3.19 ± 0.02 | 3.31 ± 0.02 | 0.002 | ** | - | |
| Class | Compound | Sc | Ho + Sc | p-Value 1 | |
|---|---|---|---|---|---|
| Higher alcohols | Isobutanol (mg/L) | 23.2 ± 2.0 | 35.1 ± 4.5 | 0.029 | * |
| Isoamyl alcohol (mg/L) | 124.9 ± 11.2 | 116.8 ± 15.4 | 0.508 | ||
| Active amyl alcohol (mg/L) | 25.2 ± 1.7 | 28.6 ± 3.3 | 0.218 | ||
| Hexanol (mg/L) | 297.0 ± 11.5 | 301.0 ± 11.9 | 0.696 | ||
| 2-Phenylethanol (mg/L) | 21.9 ± 2.2 | 18.3 ± 0.9 | 0.087 | ||
| Medium-chain fatty acids | Hexanoic acid (mg/L) | 6.6 ± 0.3 | 6.7 ± 0.4 | 0.760 | |
| Octanoic acid (mg/L) | 6.8 ± 0.7 | 7.0 ± 0.8 | 0.701 | ||
| Decanoic acid (mg/L) | 3.9 ± 1.1 | 4.0 ± 0.3 | 0.897 | ||
| Ethyl esters | Ethyl propionate (µg/L) | 102.3 ± 8.9 | 216.9 ± 14.1 | 7.2 × 10−4 | *** |
| Ethyl isobutanoate (µg/L) | 39.1 ± 1.7 | 38.9 ± 3.1 | 0.916 | ||
| Ethyl butanoate (µg/L) | 532.5 ± 24.3 | 694.1 ± 103.7 | 0.107 | ||
| Ethyl hexanoate (µg/L) | 1139.3 ± 77.5 | 1256.5 ± 208.5 | 0.439 | ||
| Ethyl octanoate (µg/L) | 3002.8 ± 148.1 | 2751.2 ± 401.8 | 0.396 | ||
| Ethyl decanoate (µg/L) | 1657.3 ± 45.1 | 1467.2 ± 207.7 | 0.190 | ||
| Diethyl succinate (µg/L) | 431.9 ± 3.7 | 387.0 ± 10.3 | 0.010 | * | |
| Acetate esters | Ethyl acetate (mg/L) | 98.9 ± 6.7 | 318.6 ± 26.5 | 0.003 | ** |
| Isoamyl acetate (µg/L) | 2029.3 ± 137.0 | 3189.2 ± 508.4 | 0.050 | . | |
| Active amyl acetate (µg/L) | 166.0 ± 5.4 | 259.6 ± 40.3 | 0.081 | . | |
| Hexyl acetate (µg/L) | 32.5 ± 2.1 | 44.9 ± 6.7 | 0.075 | . | |
| 2-Phenylethyl acetate (µg/L) | 416.4 ± 26.5 | 609.4 ± 61.6 | 0.020 | * | |
| S compounds | Hydrogen sulfide (µg/L) | 5.9 ± 0.4 | 9.8 ± 0.1 | 0.077 | . |
| Class | Compound | Sc | Ho + Sc | p-Value 1 | |
|---|---|---|---|---|---|
| Terpenes (µg/L) | β-Myrcene | 5.06 ± 1.11 | 5.08 ± 0.86 | 0.973 | |
| Limonene | 1.14 ± 0.01 | 1.12 ± 0.04 | 0.362 | ||
| cis-Linalool oxide | 124.5 ± 3.7 | 126.9 ± 4.5 | 0.517 | ||
| Nerol oxide | 13.82 ± 0.52 | 13.3 ± 0.4 | 0.222 | ||
| trans-Linalool oxide | 86.2 ± 3.0 | 85.4 ± 1.9 | 0.728 | ||
| Linalool | 86.2 ± 2.9 | 91.8 ± 1.3 | 0.059 | . | |
| Hotrienol | 131.7 ± 1.5 | 130.6 ± 7.8 | 0.837 | ||
| Citronellol | 12.7 ± 1.4 | 13.1 ± 1.0 | 0.702 | ||
| cis-Rose oxide | 0.544 ± 0.02 | 0.884 ± 0.02 | 4.7 × 10−5 | *** | |
| trans-Rose oxide | 2.19 ± 0.07 | 3.37 ± 0.10 | 1.9 × 10−4 | *** | |
| α-Terpineol | 233.6 ± 5.3 | 233.0 ± 4.7 | 0.884 | ||
| Norisoprenoids (µg/L) | β-Damascenone | 4.49 ± 0.44 | 3.32 ± 0.20 | 0.029 | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Wyk, N.; Scansani, S.; Beisert, B.; Brezina, S.; Fritsch, S.; Semmler, H.; Pretorius, I.S.; Rauhut, D.; von Wallbrunn, C. The Use of Hanseniaspora occidentalis in a Sequential Must Inoculation to Reduce the Malic Acid Content of Wine. Appl. Sci. 2022, 12, 6919. https://doi.org/10.3390/app12146919
van Wyk N, Scansani S, Beisert B, Brezina S, Fritsch S, Semmler H, Pretorius IS, Rauhut D, von Wallbrunn C. The Use of Hanseniaspora occidentalis in a Sequential Must Inoculation to Reduce the Malic Acid Content of Wine. Applied Sciences. 2022; 12(14):6919. https://doi.org/10.3390/app12146919
Chicago/Turabian Stylevan Wyk, Niël, Stefano Scansani, Beata Beisert, Silvia Brezina, Stefanie Fritsch, Heike Semmler, Isak S. Pretorius, Doris Rauhut, and Christian von Wallbrunn. 2022. "The Use of Hanseniaspora occidentalis in a Sequential Must Inoculation to Reduce the Malic Acid Content of Wine" Applied Sciences 12, no. 14: 6919. https://doi.org/10.3390/app12146919






