Combining Androgen Deprivation and Immunotherapy in Prostate Cancer Treatment: A Mechanistic Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Model Development
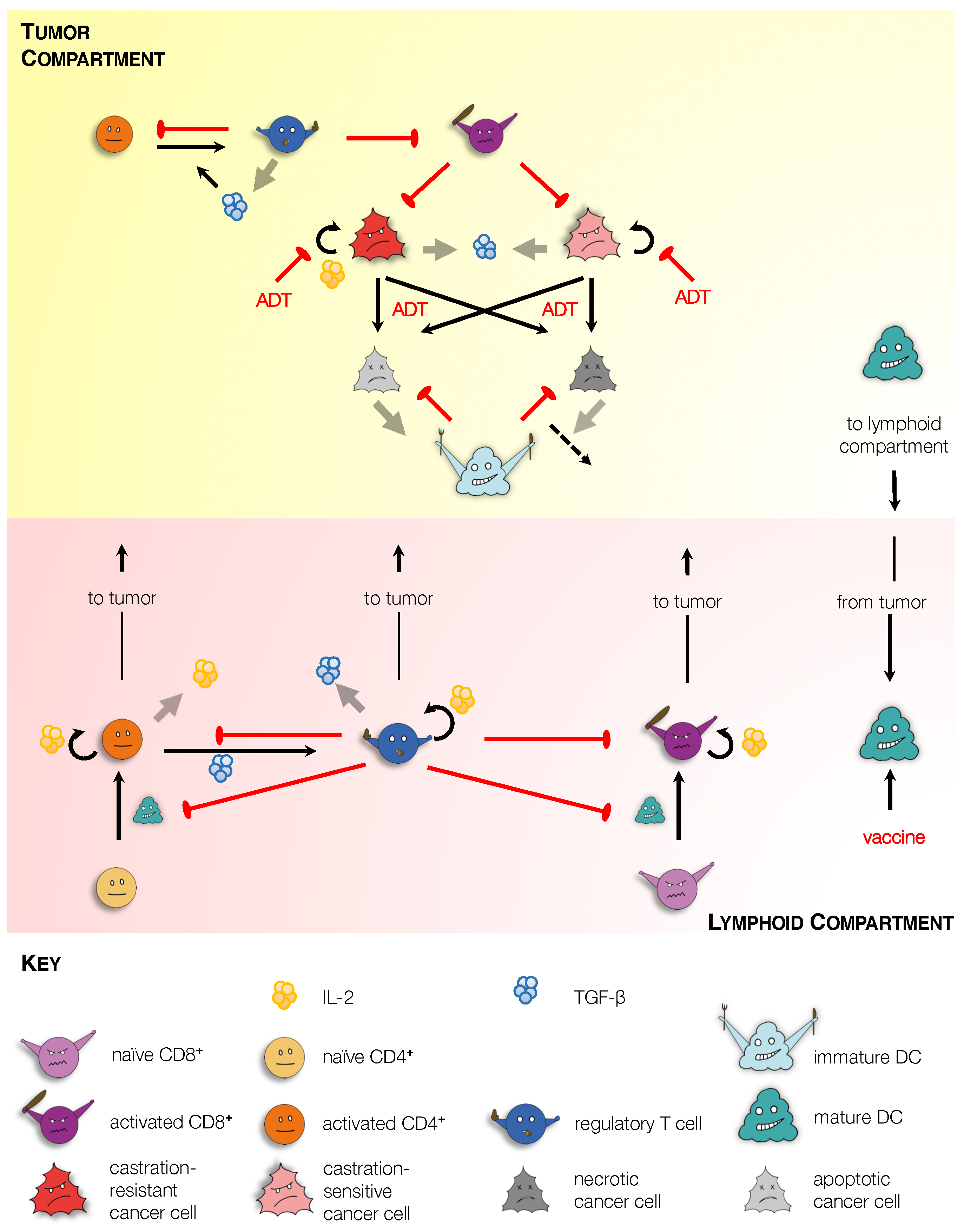
2.1.1. Tumor Compartment
- Tumor Cells: Within the tumor, our model includes androgen-sensitive (N) and castration-resistant (M) cancer cells. In the absence of treatment with ADT, N and M cells proliferate at rates and , respectively. In the absence of treatment, N and M cells undergo death via necrosis (at rates and , respectively), and apoptosis (at rates and , respectively) [16]. The effect of ADT on cell proliferation and death is accounted for via the dimensionless parameters , , , and . Specifically, in the absence of ADT, , while under ADT, and . We remark that ADT induces cell death primarily via apoptosis [17]. Tumor cell death may also occur due to the presence of activated CD8 T cells (), which induce apoptosis via direct contact in cells expressing their cognate antigen [18]. For simplicity, both N and M cells are assumed to be equally sensitive to CD8 T cells. Their immune-mediated death rate is taken to be an increasing and saturating function of , with a maximum rate, , and with half-saturation constant, . These assumptions yield the following equations governing the dynamics of proliferating tumor cells.
- Dendritic Cells: Immature dendritic cells () are recruited to the tumor due to the release of antigens and signaling molecules such as LysoPC and S1P, by dying cells [20]. For simplicity we do not explicitly model these chemokines; rather we assume that the rate of cell localization to the tumor is an increasing and saturating function of total dead cell number, with a maximum value of , and half-saturation constant, .
- T Cells: In our formulation, we consider three T cell populations: CD8 T cells or cytotoxic T lymphocytes (CTLs); CD4 T cells or helper T cells (Th cells); and regulatory T cells (Tregs). Briefly, mature APCs migrate to lymphoid organs where they activate resident naïve T cell populations [23], which subsequently migrate back to the tumor site [24]. This process is explained further when we discuss cellular dynamics within the Lymphoid Compartment. Within the tumor, activated CTLs (), Th cells () and Tregs () localize from their respective lymphoid populations at rates , . Activated T cells are assumed to have the same average life-span, and undergo natural death at a rate . In addition, Tregs induce contact mediated apoptosis of activated CTLs and Th cells [25], at an assumed rate . Finally, activated Th cells transform into Tregs in the presence of the immunosuppressive cytokine TGF- [26], whose tumoral concentration is represented by the variable G. This transformation rate is taken to be a Michaelis–Menten-like function with maximum transition rate and half saturation constant . Taken together, these assumptions yield the following equations governing the dynamics of activated T cells within the tumor. In the equations below, the superscript L refers to the number of that cellular species in the Lymphoid Compartment.
- TGF-: In our model, TGF- (G) is taken to be a representative immunosuppressive cytokine. TGF- is expressed by T cells at a rate , and by tumor cells at a rate [26]. It undergoes natural degradation at a rate . These assumptions lead to the following equation governing TGF- dynamics.
2.1.2. Lymphoid Compartment
- Dendritic Cells: Mature APCs in the lymphoid compartment () arrive via migration from the tumor at a rate and undergo natural death at a rate , yielding the following equation.
- T Cells: Naïve CTLs () and Th cells () localize to the lymphoid compartment at assumed constant rates and , respectively. and cells are activated when they come in contact with mature APCs [22], with maximum rates of activation and , respectively. This activation is inhibited by activated Tregs in the lymphoid compartment (), most likely through competition for T cell binding sites on APCs [25]. A functional form similar to that in Robertson-Tessi et al. [14] is used to capture this inhibitory effect of Tregs, with half-saturation constant . Both naïve T cells are undergo natural death at a rate . These assumptions lead to the following equations governing naïve T cell dynamics.
- IL-2 and TGF-: Lymphoid IL-2 () is produced by activated Th cells [22], at an assumed rate , and undergoes natural degradation at a rate . Lymphoid TGF- () is produced by Tregs [26], at an assumed rate , and undergoes natural degradation at a rate . These assumptions lead to the following equations governing cytokine dynamics in the lymphoid compartment.
2.2. Parameter Estimation
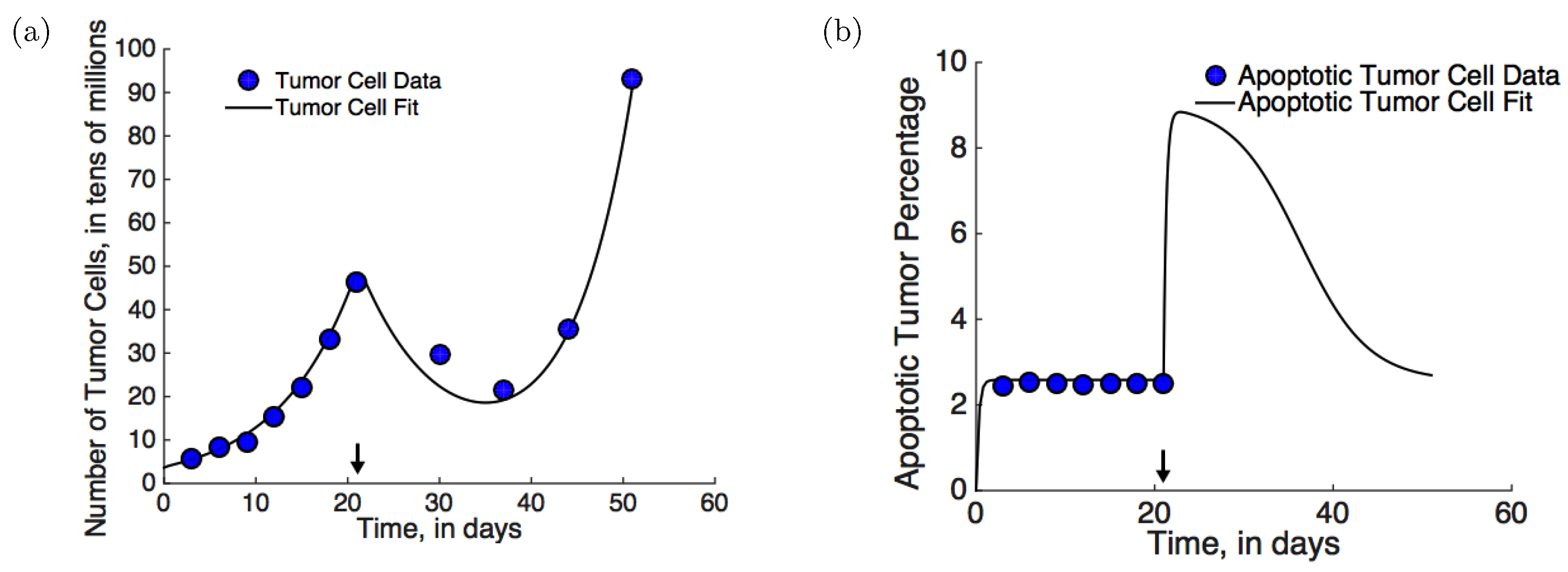
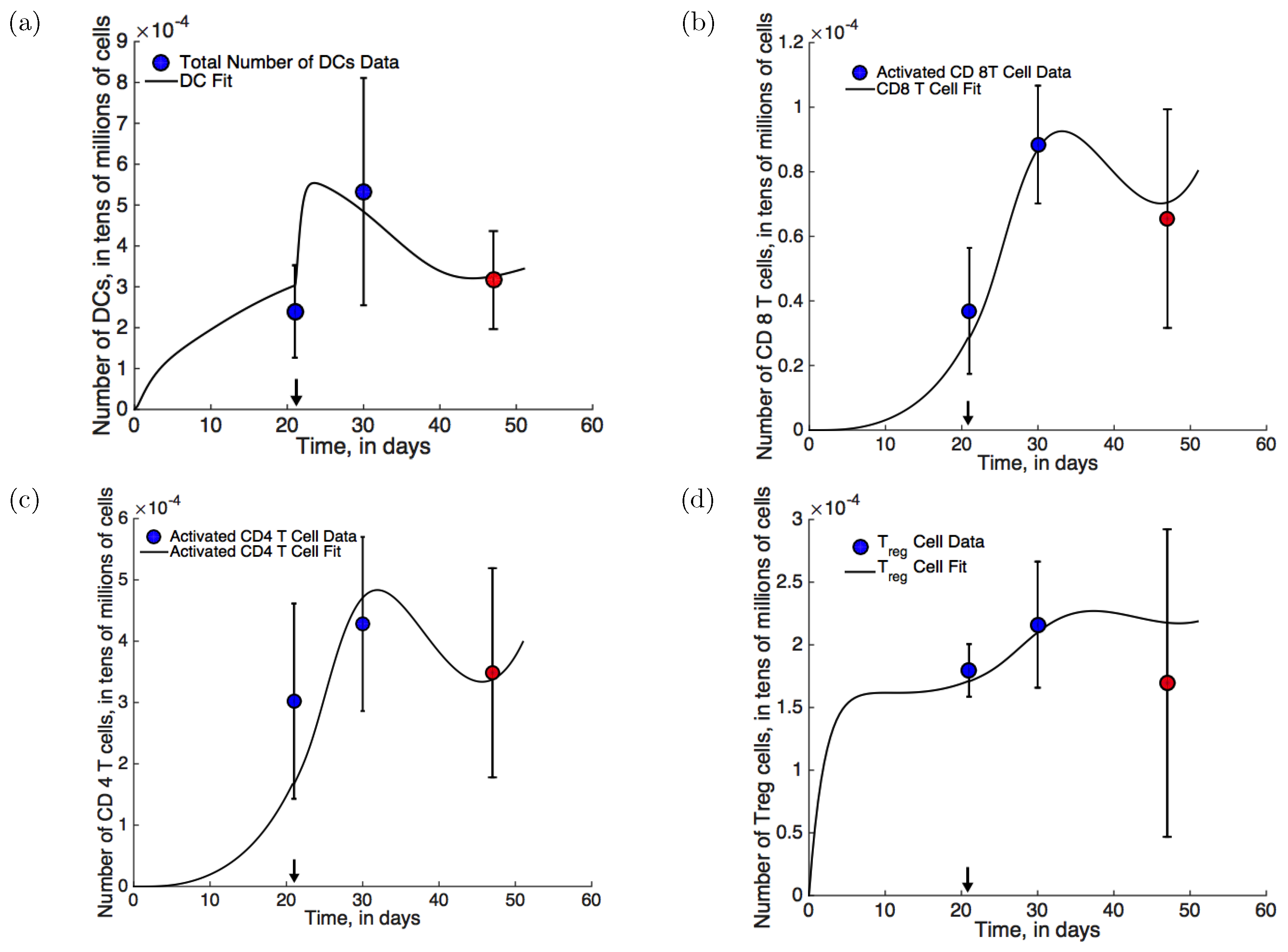
- Mouse xenograft experiments: In mouse xenograft experiments described in [28], eight to ten week old male FVB/NJ mice were injected subcutaneously with Myc-CaP cells. Myc-CaP cells are androgen-sensitive; however after treatment with ADT, castration resistance emerges spontaneously. ADT with degarelix acetate, a GnRH (gonadotropin releasing hormone) receptor antagonist that inhibits androgen production, was initiated on day 21. Tumor volume was recorded periodically (see Figure 2a, blue circles). Additionally, the numbers of dendritic cells, Th cells, CTLs, and Tregs were measured before treatment, post-treatment, and once castration resistance had emerged (see Figure 3, blue and red circles). The emergence of castration resistance was defined as a re-growth of tumor volume to ∼420 mm after an initial decline post-treatment. Best fits to this data-set are shown in Figure 2a and Figure 3 (solid lines).
- Cell death measurements under pre-treatment: In [29], experiments were performed to quantify the degree of apoptosis in PCa xenografts. Briefly, 4–5 week old nude/athymic mice were implanted subcutaneously with CWR22 tumors. CWR22 is an androgen-dependent human PCa cell line. The percentage of apoptotic cells was recorded periodically. These data (blue circles), together with best fit (solid line) are shown in Figure 2b.
- Initial conditions for model simulations: Initial conditions used in model simulations are: cells; cells; cells; cells; cells. All other variables were initiated at 0.
3. Results
3.1. Model Validation and the Emergence of Castration-Resistance
3.2. Treatment with Provenge Alone
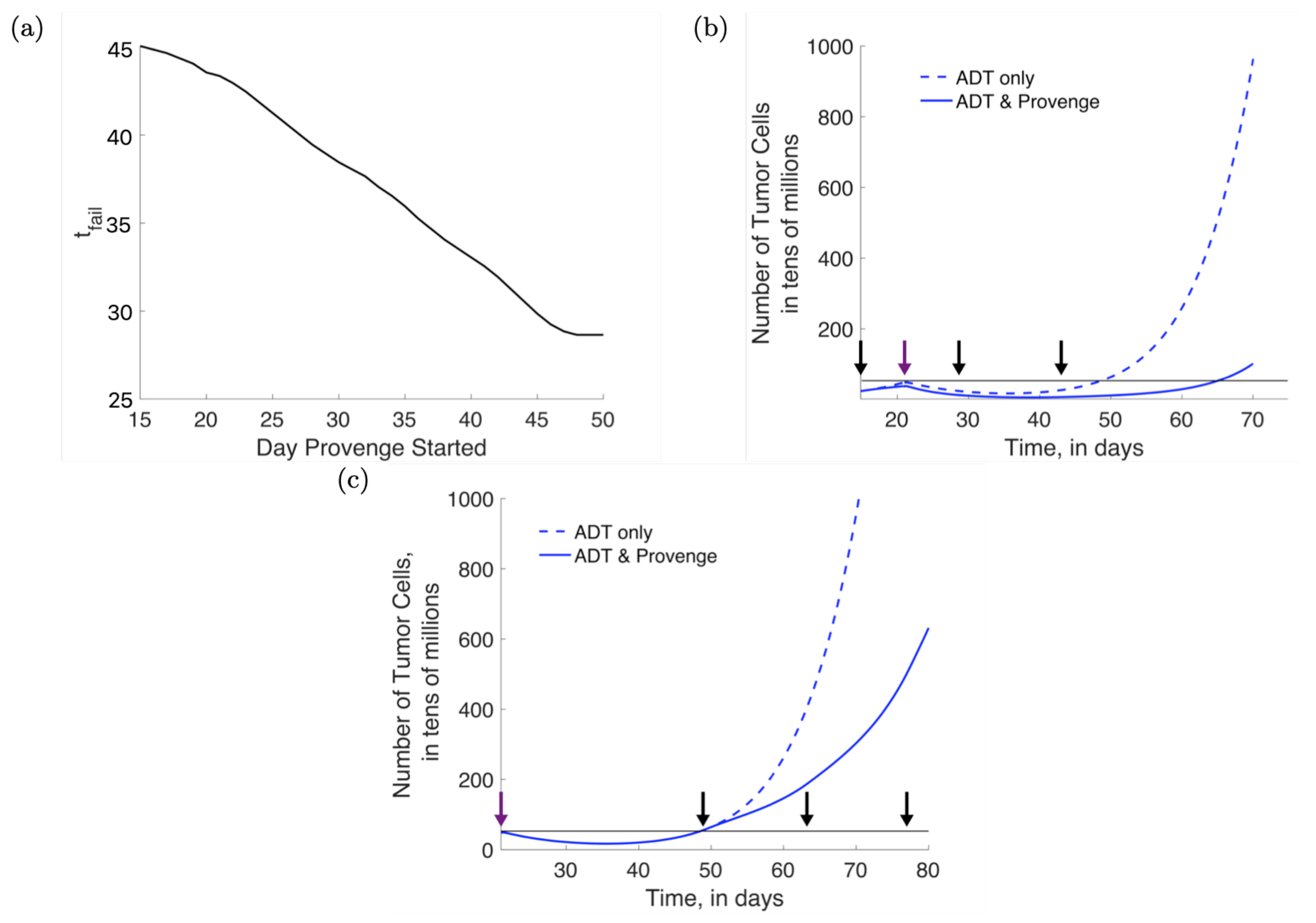
3.3. Combination Therapy: Concurrent Administration of ADT and Provenge
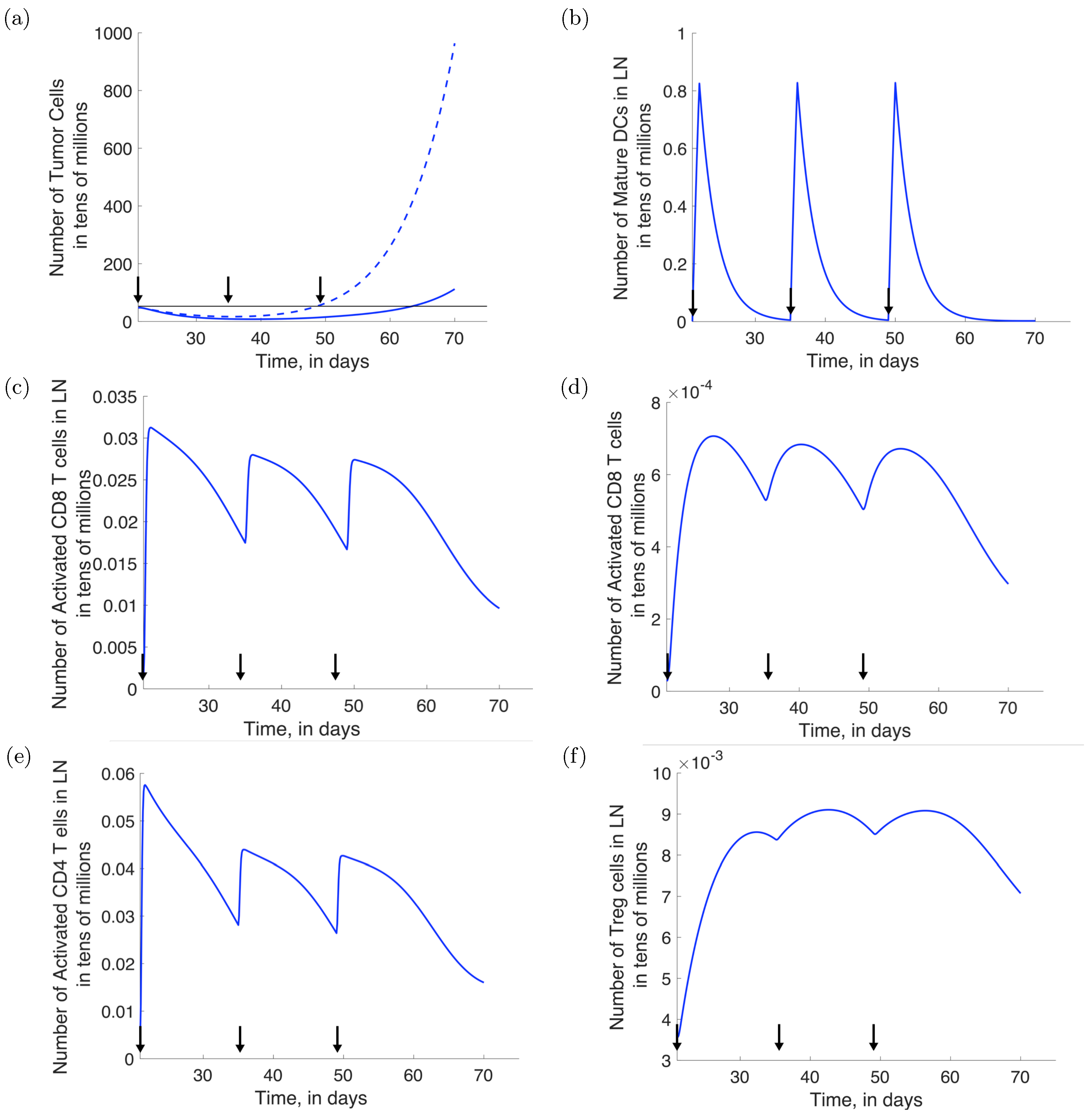
3.4. Combination Therapy: Effect of Varying Provenge Administration Schedules
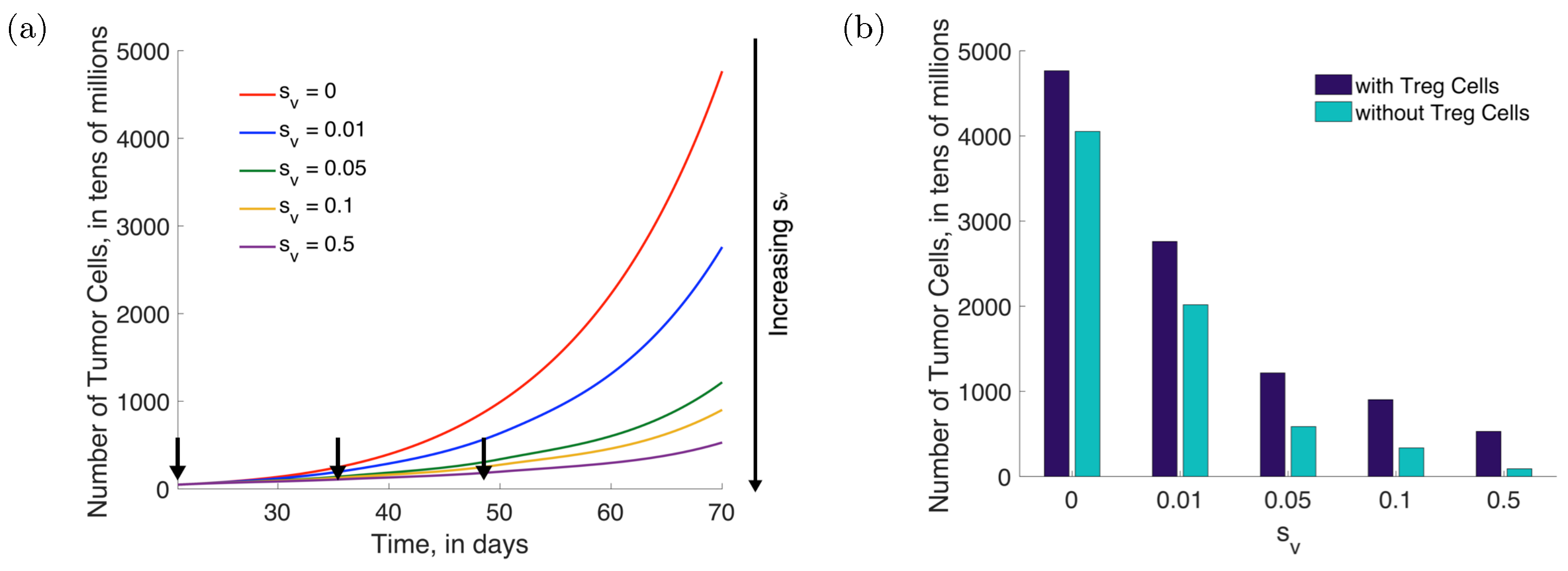
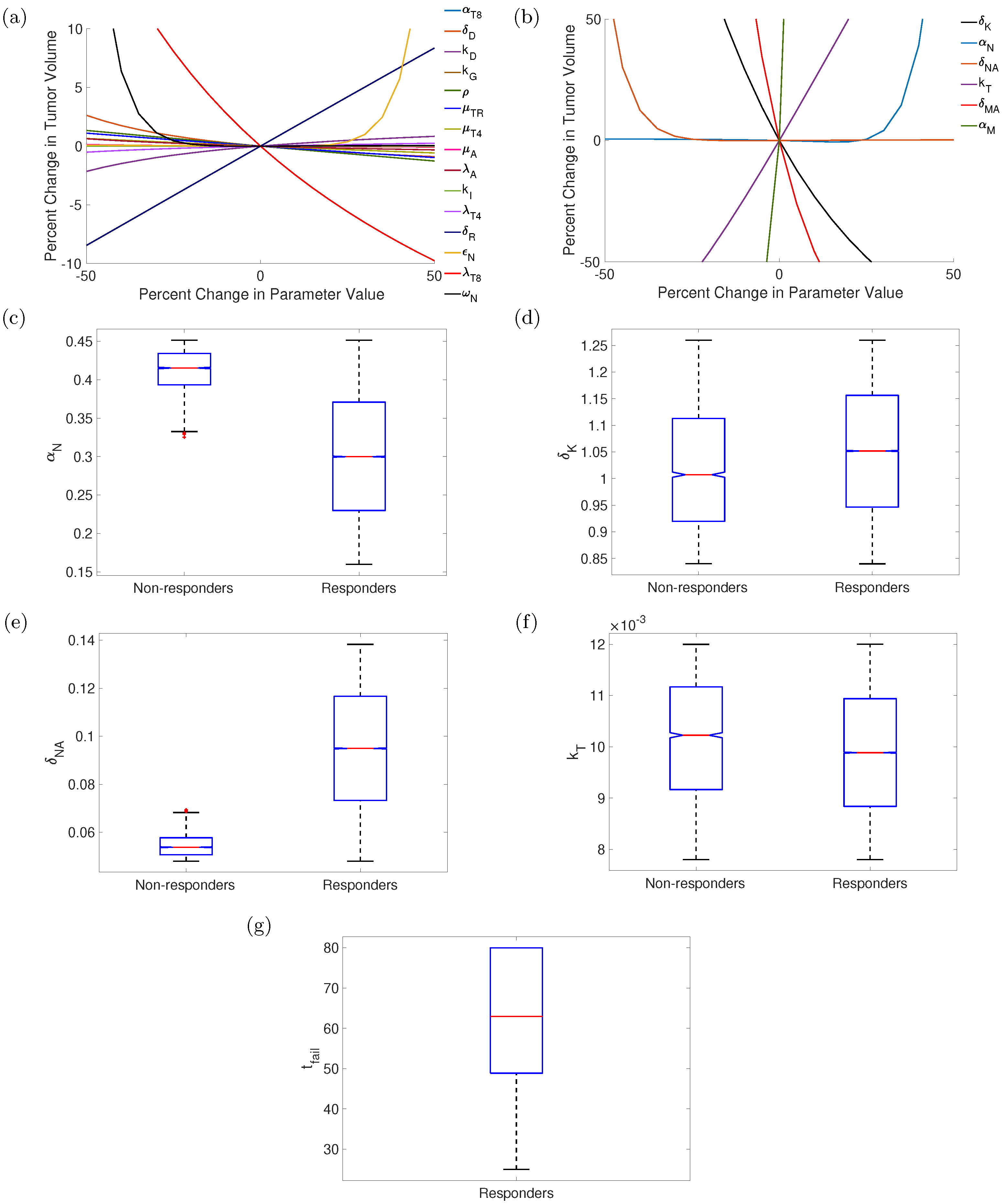
3.5. Sensitivity Analysis and Uncertainty Propagation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADT | Androgen deprivation therapy |
| APC | Antigen-presenting cell |
| CTL | CD8 Cytotoxic T lymphocyte |
| mCRPC | Metastatic castration-resistant prostate cancer |
| ODE | Ordinary differential Equation |
| PCa | Prostate cancer |
| Th cell | CD4 T helper cell |
| Treg | CD4 regulatory T cell |
References
- Ries, L.A.; Harkins, D.; Krapcho, M.; Mariotto, A.; Miller, B.; Feuer, E.J.; Clegg, L.X.; Eisner, M.; Horner, M.J.; Howlader, N.; et al. SEER Cancer Statistics Review, 1975–2003; National Cancer Institute: Bethesda, MD, USA, 2006. [Google Scholar]
- Feldman, B.J.; Feldman, D. The development of androgen-independent prostate cancer. Nat. Rev. Cancer 2001, 1, 34. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.G. Prostate cancer as a model for tumour immunotherapy. Nat. Rev. Immunol. 2010, 10, 580. [Google Scholar] [CrossRef] [Green Version]
- Perlmutter, M.A.; Lepor, H. Androgen deprivation therapy in the treatment of advanced prostate cancer. Rev. Urol. 2007, 9, S3–S8. [Google Scholar]
- Bilusic, M.; Madan, R.A.; Gulley, J.L. Immunotherapy of prostate cancer: Facts and hopes. Clin. Cancer Res. 2017, 23, 6764–6770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Lorenzo, G.; Ferro, M.; Buonerba, C. Sipuleucel-T (Provenge®) for castration-resistant prostate cancer. BJU Int. 2012, 110, E99–E104. [Google Scholar] [CrossRef]
- Han, P.; Hanlon, D.; Sobolev, O.; Chaudhury, R.; Edelson, R.L. Ex vivo dendritic cell generation—A critical comparison of current approaches. In International Review of Cell and Molecular Biology; Lhuillier, C., Galluzzi, L., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 349, pp. 251–307. [Google Scholar]
- Gamat, M.; McNeel, D.G. Androgen deprivation and immunotherapy for the treatment of prostate cancer. Endocr.-Relat. Cancer 2017, 24, T297–T310. [Google Scholar] [CrossRef]
- Koh, Y.T.; Gray, A.; Higgins, S.A.; Hubby, B.; Kast, W.M. Androgen ablation augments prostate cancer vaccine immunogenicity only when applied after immunization. Prostate 2009, 69, 571–584. [Google Scholar] [CrossRef] [Green Version]
- Jain, H.V.; Sorribes, I.C.; Handelman, S.K.; Barnaby, J.; Jackson, T.L. Standing Variations Modeling Captures Inter-Individual Heterogeneity in a Deterministic Model of Prostate Cancer Response to Combination Therapy. Cancers 2021, 13, 1872. [Google Scholar] [CrossRef]
- Brady-Nicholls, R.; Nagy, J.D.; Gerke, T.A.; Zhang, T.; Wang, A.Z.; Zhang, J.; Gatenby, R.A.; Enderling, H. Prostate-specific antigen dynamics predict individual responses to intermittent androgen deprivation. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Cunningham, J.J.; Brown, J.S.; Gatenby, R.A. Integrating evolutionary dynamics into treatment of metastatic castrate-resistant prostate cancer. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Radunskaya, A.; Kim, R.; Woods, I. Mathematical modeling of tumor immune interactions: A closer look at the role of a PD-L1 inhibitor in cancer immunotherapy. Spora J. Biomath. 2018, 4, 25–41. [Google Scholar] [CrossRef]
- Robertson-Tessi, M.; El-Kareh, A.; Goriely, A. A mathematical model of tumor–immune interactions. J. Theor. Biol. 2012, 294, 56–73. [Google Scholar] [CrossRef]
- Rutter, E.M.; Kuang, Y. Global dynamics of a model of joint hormone treatment with dendritic cell vaccine for prostate cancer. Discret. Contin. Dyn. Syst. Ser. B 2017, 22, 1001–1021. [Google Scholar] [CrossRef] [Green Version]
- Søgaard, C.K.; Moestue, S.A.; Rye, M.B.; Kim, J.; Nepal, A.; Liabakk, N.B.; Bachke, S.; Bathen, T.F.; Otterlei, M.; Hill, D.K. APIM-peptide targeting PCNA improves the efficacy of docetaxel treatment in the TRAMP mouse model of prostate cancer. Oncotarget 2018, 9, 11752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.X.; Firus, J.; Prieur, B.; Jia, W.; Rennie, P.S. To die or to survive, a fatal question for the destiny of prostate cancer cells after androgen deprivation therapy. Cancers 2011, 3, 1498–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, K.; Doolan, K.; Hung, C.F.; Wu, T. Perspectives for preventive and therapeutic HPV vaccines. J. Formos. Med. Assoc. 2010, 109, 4–24. [Google Scholar] [CrossRef] [Green Version]
- Green, D.; Oguin, T.H.; Martinez, J. The clearance of dying cells: Table for two. Cell Death Differ. 2016, 23, 915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochreiter-Hufford, A.; Ravichandran, K.S. Clearing the dead: Apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb. Perspect. Biol. 2013, 5, a008748. [Google Scholar] [CrossRef] [Green Version]
- Sauter, B.; Albert, M.L.; Francisco, L.; Larsson, M.; Somersan, S.; Bhardwaj, N. Consequences of cell death: Exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 2000, 191, 423–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, K.; Weaver, C. Janeway’s Immunobiology; Garland Science: New York, NY, USA, 2012. [Google Scholar]
- Gardner, A.; Ruffell, B. Dendritic cells and cancer immunity. Trends Immunol. 2016, 37, 855–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Vignali, D.A.; Collison, L.W.; Workman, C.J. How regulatory T cells work. Nat. Rev. Immunol. 2008, 8, 523. [Google Scholar] [CrossRef] [Green Version]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.C.; Ghasemzadeh, A.; Kochel, C.M.; Nirschl, T.R.; Francica, B.J.; Lopez-Bujanda, Z.A.; Haro, M.A.C.; Tam, A.; Anders, R.A.; Selby, M.J.; et al. Combining intratumoral Treg depletion with androgen deprivation therapy (ADT): Preclinical activity in the Myc-CaP model. Prostate Cancer Prostatic Dis. 2018, 21, 113. [Google Scholar] [CrossRef]
- Smitherman, A.B.; Gregory, C.W.; Mohler, J.L. Apoptosis levels increase after castration in the CWR22 human prostate cancer xenograft. Prostate 2003, 57, 24–31. [Google Scholar] [CrossRef]
- Kushwah, R.; Hu, J. Dendritic cell apoptosis: Regulation of tolerance versus immunity. J. Immunol. 2010, 185, 795–802. [Google Scholar] [CrossRef] [Green Version]
- Lanzavecchia, A.; Sallusto, F. Regulation of T cell immunity by dendritic cells. Cell 2001, 106, 263–266. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsov, V.A.; Makalkin, I.A.; Taylor, M.A.; Perelson, A.S. Nonlinear dynamics of immunogenic tumors: Parameter estimation and global bifurcation analysis. Bull. Math. Biol. 1994, 56, 295–321. [Google Scholar] [CrossRef]
- Wei, S.; Kryczek, I.; Zou, W. Regulatory T-cell compartmentalization and trafficking. Blood 2006, 108, 426–431. [Google Scholar] [CrossRef] [Green Version]
- Ellis, L.; Lehet, K.; Ramakrishnan, S.; Adelaiye, R.; Pili, R. Development of a castrate resistant transplant tumor model of prostate cancer. Prostate 2012, 72, 587–591. [Google Scholar] [CrossRef] [Green Version]
- Rangarajan, A.; Weinberg, R.A. Comparative biology of mouse versus human cells: Modelling human cancer in mice. Nat. Rev. Cancer 2003, 3, 952. [Google Scholar] [CrossRef]
- Seaholm, S.K.; Ackerman, E.; Wu, S.C. Latin hypercube sampling and the sensitivity analysis of a Monte Carlo epidemic model. Int. J. Bio-Med. Comput. 1988, 23, 97–112. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef] [Green Version]
- Whiteside, T.L. FOXP3+ Treg as a therapeutic target for promoting anti-tumor immunity. Expert Opin. Ther. Targets 2018, 22, 353–363. [Google Scholar] [CrossRef]
- Tang, J.; Yu, J.X.; Hubbard-Lucey, V.M.; Neftelinov, S.T.; Hodge, J.P.; Lin, Y. Trial watch: The clinical trial landscape for PD1/PDL1 immune checkpoint inhibitors. Nat. Rev. Drug Discov. 2018, 17, 854–856. [Google Scholar] [CrossRef]
- Vale, C.L.; Burdett, S.; Rydzewska, L.H.; Albiges, L.; Clarke, N.W.; Fisher, D.; Fizazi, K.; Gravis, G.; James, N.D.; Mason, M.D.; et al. Addition of docetaxel or bisphosphonates to standard of care in men with localised or metastatic, hormone-sensitive prostate cancer: A systematic review and meta-analyses of aggregate data. Lancet Oncol. 2016, 17, 243–256. [Google Scholar] [CrossRef] [Green Version]

| Variable | Symbol | Units |
|---|---|---|
| Time | t | Days |
| Tumor Compartment | ||
| Androgen-Sensitive PCa Cells | tens of millions of cells | |
| Castration Resistant PCa Cells | tens of millions of cells | |
| Apoptotic PCa Cells | tens of millions of cells | |
| Necrotic PCa Cells | tens of millions of cells | |
| Immature Dendritic Cells | tens of millions of cells | |
| Mature Dendritic Cells | tens of millions of cells | |
| Activated CD8 T cells | tens of millions of cells | |
| Activated CD4 T cells | tens of millions of cells | |
| Regulatory T cells | tens of millions of cells | |
| TGF- | ng/mL | |
| Lymphoid Compartment | ||
| Mature Dendritic Cells | tens of millions of cells | |
| Naïve CD8 T cells | tens of millions of cells | |
| Activated CD8 T cells | tens of millions of cells | |
| Naïve CD4 T cells | tens of millions of cells | |
| Activated CD4 T cells | tens of millions of cells | |
| Regulatory T cells | tens of millions of cells | |
| TGF- | ng/mL | |
| IL-2 | ng/mL | |
| Parameter | Value | Dimension | Reference |
|---|---|---|---|
| 0.1066 | per day | [16] | |
| 0.1066 | per day | [16] | |
| 0.3151 | per day | [30] | |
| 0.4 | per day | [31] | |
| 0.012 | per day | [13] | |
| 0.0038 | cells per day | [14,32,33] | |
| 0.0072 | of cells per day | [14,32,33] | |
| 0.0005 | of cells per day | [14,32,33] | |
| 0.462 | per day | [13] | |
| 0.1199 | per day | [13] | |
| 16 | per day | [14] | |
| 1.9 | per day | [14] | |
| 2.1 | per day | [14] | |
| 0.022 | per day | [14] | |
| 1.1 | ng/mL per cell per day | [14] | |
| 1.8 | ng/mL per cell per day | [14] | |
| 14.2857 | per day | [14] | |
| 1.7 | ng/mL per cell per day | [14] | |
| 12.5 | per day | [14] | |
| 0.3 | ng/mL | [14] | |
| 2 | cells | [14] |
| Parameter | Value | Units |
|---|---|---|
| 0.3196 | per day | |
| [0.2788 , 1] | dimensionless | |
| 0.0922 | per day | |
| [1 , 3.5] | dimensionless | |
| 0.3516 | per day | |
| 1 | dimensionless | |
| 0.0922 | per day | |
| 1 | dimensionless | |
| 1 | per day | |
| 0.01 | tens of millions of cells | |
| 3.2486 | per day | |
| 0.0046 | tens of millions of cells per day | |
| 20.0926 | tens of millions of cells | |
| 1.2539 | per cell per day | |
| 9.1990 | per day | |
| 0.0258 | per day | |
| 16.9130 | per cell per day | |
| 0.3000 | per day | |
| 0.3 | tens of millions of cells | |
| 28.7035 | per cell per day | |
| 0.0218 | per day | |
| 1.5 | ng/mL | |
| 1.2907 | per day |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barnaby, J.; Jain, H.V. Combining Androgen Deprivation and Immunotherapy in Prostate Cancer Treatment: A Mechanistic Approach. Appl. Sci. 2022, 12, 6954. https://doi.org/10.3390/app12146954
Barnaby J, Jain HV. Combining Androgen Deprivation and Immunotherapy in Prostate Cancer Treatment: A Mechanistic Approach. Applied Sciences. 2022; 12(14):6954. https://doi.org/10.3390/app12146954
Chicago/Turabian StyleBarnaby, Johnna, and Harsh Vardhan Jain. 2022. "Combining Androgen Deprivation and Immunotherapy in Prostate Cancer Treatment: A Mechanistic Approach" Applied Sciences 12, no. 14: 6954. https://doi.org/10.3390/app12146954
APA StyleBarnaby, J., & Jain, H. V. (2022). Combining Androgen Deprivation and Immunotherapy in Prostate Cancer Treatment: A Mechanistic Approach. Applied Sciences, 12(14), 6954. https://doi.org/10.3390/app12146954






