Development and Functional Analysis of Lithocarpus polystachyus (wall.) Rehd Black Tea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of LPRBT
2.3. Physicochemical Indices of LPRBT
2.4. Preparation of LPRBT Extracts
2.5. Determination of Anti-Oxidation Function of LPRBT
2.5.1. Measurement of 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid) Diammonium Salt (ABTS) Radical Clearance Activity
2.5.2. Measurement of 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Clearance Activity
2.5.3. Measurement of Hydroxyl Radical Clearance Activity
2.5.4. Measurement of Superoxide Anion Radical Clearance Activity
2.6. Determination of Hypoglycemic Function of LPRBT
2.6.1. α-Glucosidase Activity Inhibition Assay
2.6.2. α-Amylase Activity Inhibition Assay
2.7. Determination of Tumor Suppressor Function of LPRBT
2.8. Statistical Analysis
3. Results
3.1. Optimization of BT–LPR Ratio
3.2. Orthogonal Test of LPRBT
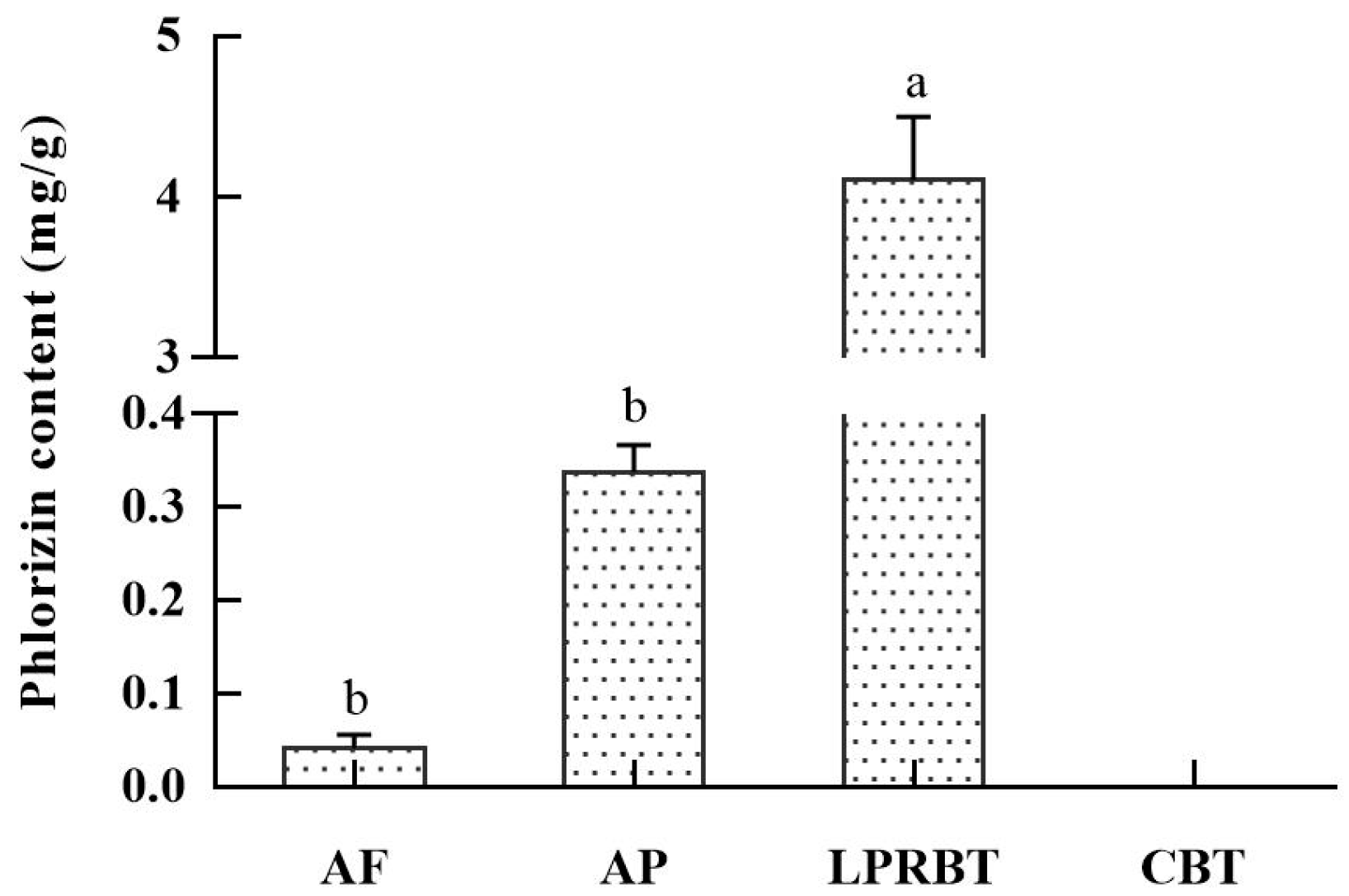
3.3. Physiochemical Indices
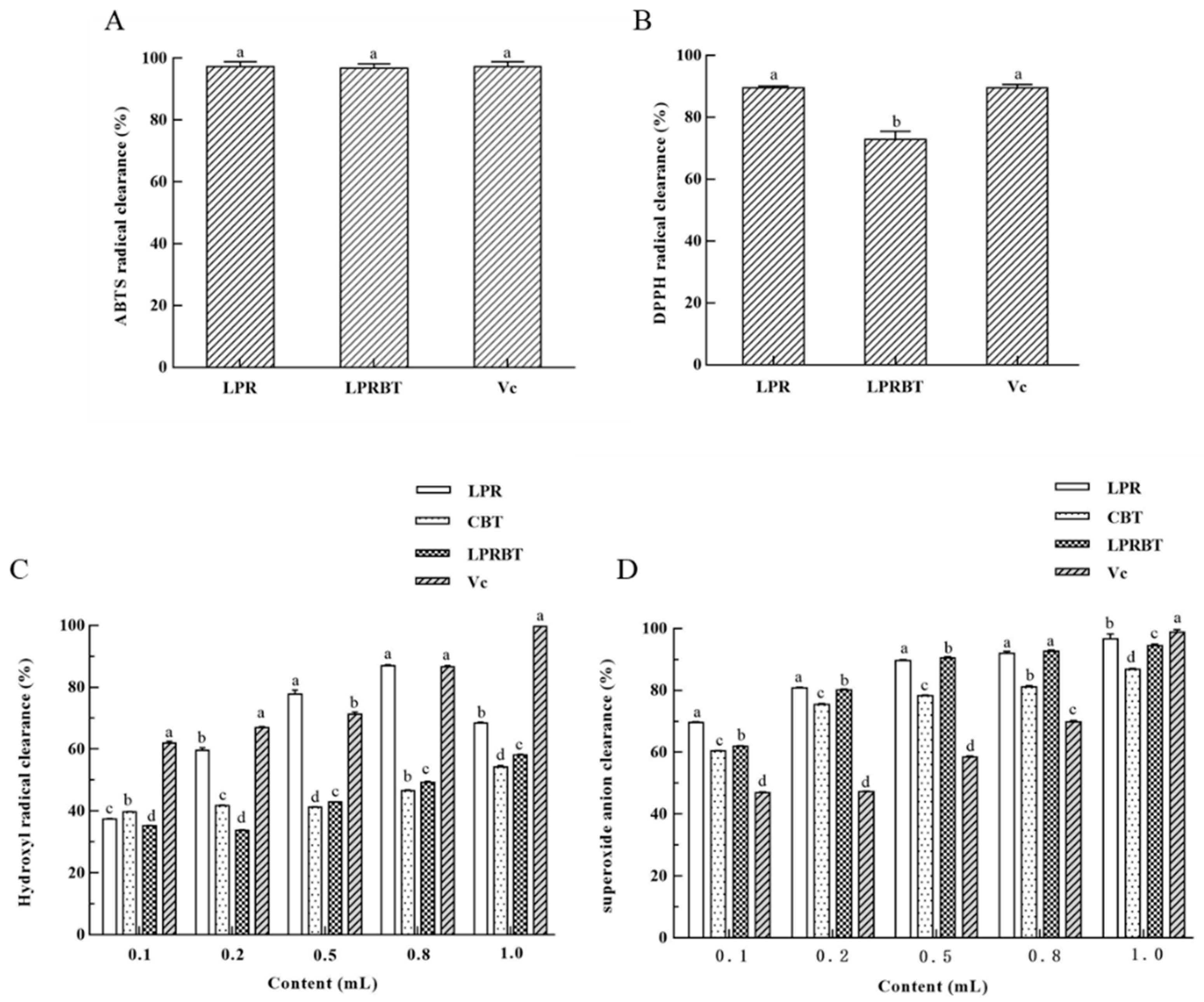
3.4. Anti-Oxidation Function of LPRBT
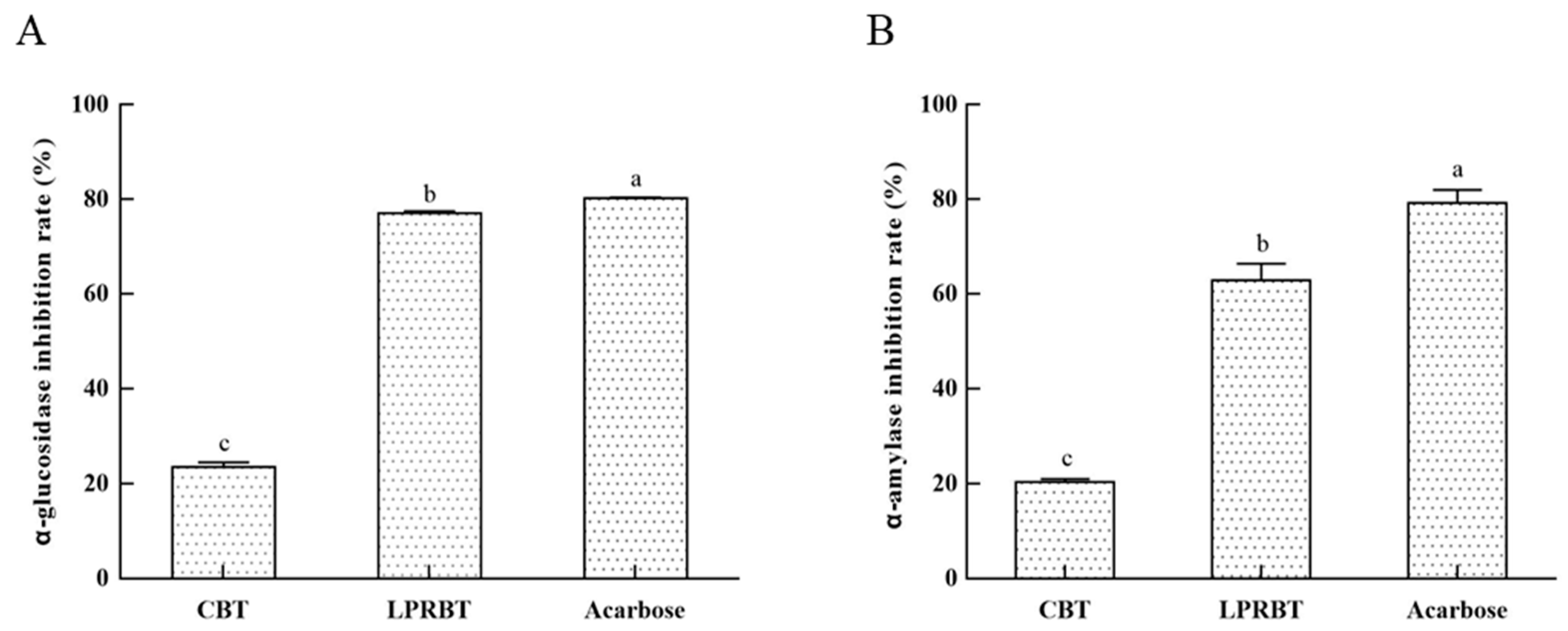
3.5. Hypoglycemic Function of LPRBT
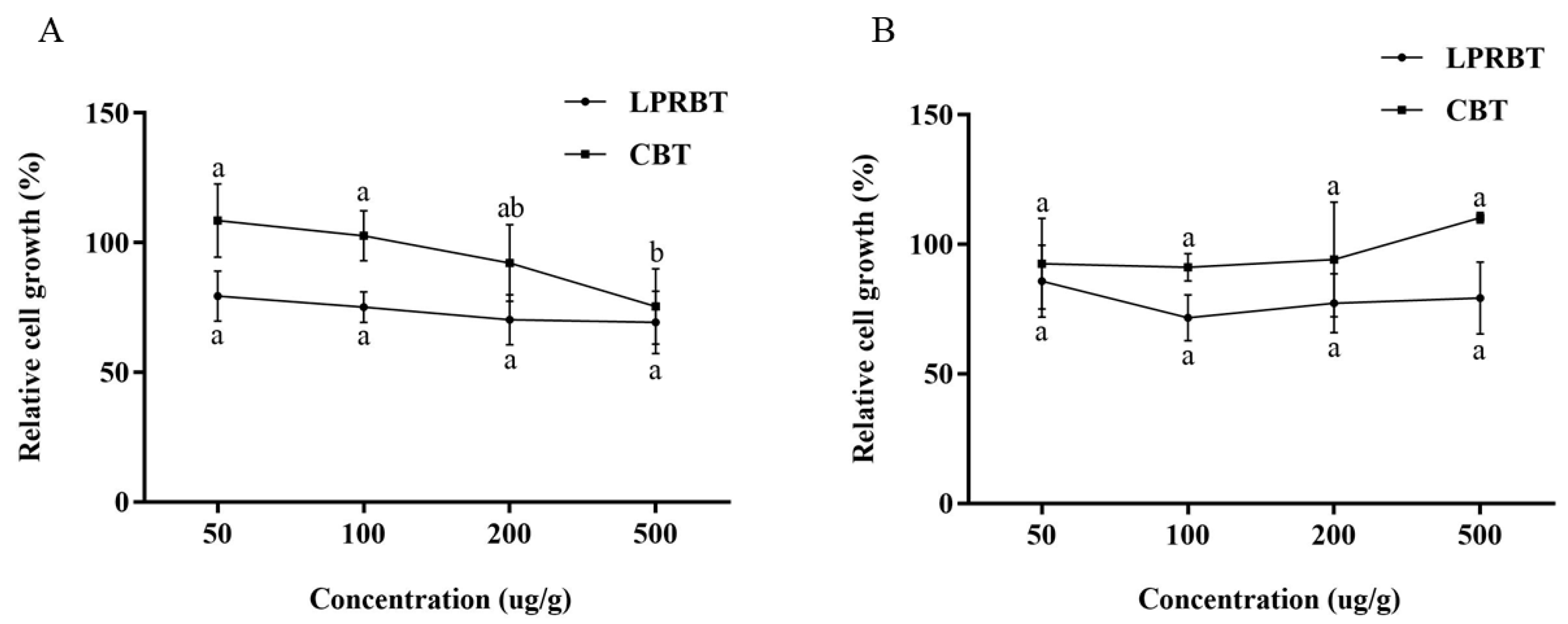
3.6. Tumor Suppressor Function of LPRBT
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meng, Y.; Ding, L.; Wang, Y.; Nie, Q.; Xing, Y.; Ren, Q. Phytochemical identification of lithocarpus polystachyus extracts by ultra-high-performance liquid chromatography-quadrupole time-of-flight-ms and their protein tyrosine phosphatase 1b and alpha-glucosidase activities. Biomed. Chromatogr. 2020, 34, e4705. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, X.; Zeng, X.; Huang, S.; Hou, S.; Lai, X. Characterization of phenolic constituents in lithocarpus polystachyus. Anal. Methods 2014, 6, 1359–1363. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Y.; Yang, Z.; Zhou, C.; Hu, X. Identification and quantitative evaluation of major sweet ingredients in sweet tea (lithocarpus polystachyus rehd.) Based upon location, harvesting time, leaf age. J. Chem. Soc. Pak. 2018, 40, 158–164. [Google Scholar]
- Sun, Y.; Li, W.; Liu, Z. Preparative isolation, quantification and antioxidant activity of dihydrochalcones from sweet tea (lithocarpus polystachyus rehd.). J. Chromatogr. B 2015, 1002, 372–378. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.; Dong, S.; Hong, Y.; Zhou, X.; Zheng, W.; Zheng, C. Phlorizin exerts direct protective effects on palmitic acid (pa)-induced endothelial dysfunction by activating the pi3k/akt/enos signaling pathway and increasing the levels of nitric oxide (no). Med. Sci. Monit. Basic Res. 2018, 24, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Najafian, M.; Jahromi, M.Z.; Nowroznejhad, M.J.; Khajeaian, P.; Kargar, M.M.; Sadeghi, M.; Arasteh, A. Phloridzin reduces blood glucose levels and improves lipids metabolism in streptozotocin-induced diabetic rats. Mol. Biol. Rep. 2012, 39, 5299–5306. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, X.; Gan, R.; Sun, Q.; Meng, J.; Shang, A.; Mao, Q.; Li, H. Targeting gut microbiota for the prevention and management of diabetes mellitus by dietary natural products. Foods 2019, 8, 440. [Google Scholar] [CrossRef] [Green Version]
- Nagata, T.; Suzuki, M.; Fukazawa, M.; Honda, K.; Yamane, M.; Yoshida, A.; Azabu, H.; Kitamura, H.; Toyota, N.; Suzuki, Y.; et al. Competitive inhibition of sglt2 by tofogliflozin or phlorizin induces urinary glucose excretion through extending splay in cynomolgus monkeys. Am. J. Physiol. Renal 2014, 306, 1520–1533. [Google Scholar] [CrossRef]
- Osorio, H.; Bautista, R.; Rios, A.; Franco, M.; Arellano, A.; Vargas-Robles, H.; Romo, E.; Escalante, B. Effect of phlorizin on sglt2 expression in the kidney of diabetic rats. J. Nephrol. 2010, 23, 541–546. [Google Scholar]
- Wang, Z.; Gao, Z.; Wang, A.; Jia, L.; Zhang, X.; Fang, M.; Yi, K.; Li, Q.; Hu, H. Comparative oral and intravenous pharmacokinetics of phlorizin in rats having type 2 diabetes and in normal rats based on phase ii metabolism. Food Funct. 2019, 10, 1582–1594. [Google Scholar] [CrossRef]
- Lu, M.; Kong, Q.; Xu, X.; Lu, H.; Lu, Z.; Yu, W.; Zuo, B.; Su, J.; Guo, R. Evaluation of apoptotic and growth inhibitory activity of phloretin in bgc823 gastric cancer cell. Trop. J. Pharm. Res. 2015, 14, 27–31. [Google Scholar] [CrossRef]
- Xu, M.; Gu, W.; Shen, Z.; Wang, F. Anticancer activity of phloretin against human gastric cancer cell lines involves apoptosis, cell cycle arrest, and inhibition of cell invasion and ink signalling pathway. Med. Sci. Monit. 2018, 24, 6551–6558. [Google Scholar] [CrossRef] [PubMed]
- Kirmizikaya, G.; Karakaya, M.; Babaoglu, A.S. Black, green, and white tea infusions and powder forms improve oxidative stability of minced beef throughout refrigerated storage. J. Food Process. Preserv. 2021, 45, e15359. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, J.; Yu, J.; Jiang, H.; Ao, C.; Huang, H. Processing technology of tea bakery foods—A review. Czech J. Food Sci. 2019, 37, 391–402. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, H.; Zhu, B.; Li, J.; Yang, T.; Zhang, Z.; Deng, W. Molecular and biochemical characterization of jasmonic acid carboxyl methyltransferase involved in aroma compound production of methyl jasmonate during black tea processing. J. Agric. Food Chem. 2021, 69, 3154–3164. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Cheng, L. Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in ‘honeycrisp’ apple flesh. Food Chem. 2010, 123, 1013–1018. [Google Scholar] [CrossRef]
- Dumitriu, D.; Peinado, R.A.; Peinado, J.; Lopez De Lerma, N. Grape pomace extract improves the in vitro and in vivo antioxidant properties of wines from sun light dried pedro ximenez grapes. J. Funct. Foods 2015, 17, 380–387. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, X.; Ahmad, H.; Zhang, H.; Zhang, L.; Wang, T. Assessment of free radicals scavenging activity of seven natural pigments and protective effects in aaph-challenged chicken erythrocytes. Food Chem. 2014, 145, 57–65. [Google Scholar] [CrossRef]
- Yang, W. Preparation of konjac oligoglucomannans with different molecular weights and their in vitro and in vivo antioxidant activities. Open Life Sci. 2020, 15, 799–807. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, T.; Jin, Z.; Xu, X.; Wang, J.; Zha, X.; Chen, H. Structural characterisation, physicochemical properties and antioxidant activity of polysaccharide from lilium lancifolium thunb. Food Chem. 2015, 169, 430–438. [Google Scholar] [CrossRef]
- Liao, H.; Banbury, L. Different proportions of huangqi (radix astragali mongolici) and honghua (flos carthami) injection on alpha-glucosidase and alpha-amylase activities. Evid.-Based Complement. Altern. Med. 2015, 2015, 785193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores, F.P.; Singh, R.K.; Kerr, W.L.; Pegg, R.B.; Kong, F. Antioxidant and enzyme inhibitory activities of blueberry anthocyanins prepared using different solvents. J. Agric. Food Chem. 2013, 61, 4441–4447. [Google Scholar] [CrossRef]
- Hruba, M.; Baxant, J.; Cizkova, H.; Smutna, V.; Kovarik, F.; Sevcik, R.; Hanusova, K.; Rajchl, A. Phloridzin as a marker for evaluation of fruit product’s authenticity. Czech J. Food Sci. 2021, 39, 49–57. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Q.; Granato, D.; Xu, Y.; Ho, C. Association between chemistry and taste of tea: A review. Trends Food Sci. Tech. 2020, 101, 139–149. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Zhang, Y.; Zhai, Y.; Xu, W.; Zhao, B.; Liu, D.; Yu, H. Biotransformation of phlorizin by human intestinal flora and inhibition of biotransformation products on tyrosinase activity. Food Chem. 2012, 132, 936–942. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, Y.; Cao, S. Antioxidant capacity of caffeic acid, phloretin and glutathione mixtures and formula optimization. Asian J. Chem. 2013, 25, 3971–3978. [Google Scholar] [CrossRef]
- Rubio-Perez, J.M.; Vidal-Guevara, M.L.; Zafrilla, P.; Morillas-Ruiz, J.M. A new antioxidant beverage produced with green tea and apple. Int. J. Food Sci. Nutr. 2014, 65, 552–557. [Google Scholar] [CrossRef]
- Lu, W.; Li, B.; Yu, F.; Cai, Q.; Zhang, Z.; Yin, M.; Gao, H. Quantitative proteomics study on the protective mechanism of phlorizin on hepatic damage in diabetic db/db mice. Mol. Med. Rep. 2012, 5, 1285–1294. [Google Scholar]
- Malatiali, S.; Francis, I.; Barac-Nieto, M. Phlorizin prevents glomerular hyperfiltration but not hypertrophy in diabetic rats. Exp. Diabetes Res. 2008, 2008, 305403. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Li, M.; Zhu, F.; Liu, F.; Huang, J. Inhibitory potential of trilobatin from lithocarpus polystachyus rehd against alpha-glucosidase and alpha-amylase linked to type 2 diabetes. Food Chem. 2012, 130, 261–266. [Google Scholar] [CrossRef]
- Han, L.; Fang, C.; Zhu, R.; Peng, Q.; Li, D.; Wang, M. Inhibitory effect of phloretin on alpha-glucosidase: Kinetics, interaction mechanism and molecular docking. Int. J. Biol. Macromol. 2017, 95, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Sampath, C.; Sang, S.; Ahmedna, M. In vitro and in vivo inhibition of aldose reductase and advanced glycation end products by phloretin, epigallocatechin 3-gallate and [6]-gingerol. Biomed. Pharmacother. 2016, 84, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sinha, K.; Sharma, R.; Purohit, R.; Padwad, Y. Phloretin and phloridzin improve insulin sensitivity and enhance glucose uptake by subverting ppar gamma/cdk5 interaction in differentiated adipocytes. Exp. Cell Res. 2019, 383, 111480. [Google Scholar] [CrossRef]
- Shen, X.; Zhou, N.; Mi, L.; Hu, Z.; Wang, L.; Liu, X.; Zhang, S. Phloretin exerts hypoglycemic effect in streptozotocin-induced diabetic rats and improves insulin resistance in vitro. Drug Des. Dev. Ther. 2017, 11, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Ning, Z.; Yu, L.; Li, L.; Lin, L.; Huang, J. Preparative separation and identification of the flavonoid phlorhizin from the crude extract of lithocarpus polystachyus rehd. Molecules 2007, 12, 552–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Wang, R.; Nan, Y.; Li, W.; Wang, Q.; Jin, F. Phloretin exhibits an anticancer effect and enhances the anticancer ability of cisplatin on non-small cell lung cancer cell lines by regulating expression of apoptotic pathways and matrix metalloproteinases. Int. J. Oncol. 2016, 48, 843–853. [Google Scholar] [CrossRef] [Green Version]
- Abu-Azzam, O.; Aldalaen, S.; Nasr, M. Potentiation of the cytotoxic activity of nutraceutical phloretin against cervical cancer by formulation into microemulsion. Pharm. Chem. J. 2022, 55, 1215–1218. [Google Scholar] [CrossRef]
| Level | Factors | |
|---|---|---|
| Withered Leaves: LPR | Fermentation Time (h) | |
| 1 | 9:1 | 5 |
| 2 | 8:1 | 6 |
| 3 | 7:1 | 7 |
| Sample Number | Appearance | Soup Color | Fragrance | Taste | Leaf Base | Overall Rating |
|---|---|---|---|---|---|---|
| 10:1 | Still tight and thin, yellowish-brown, moist, covered with gold cents | Bright yellow | Pure, slightly sweet, basically free of precipitation | Slightly astringent, slightly sweet | Tan, brighter | 91.8 |
| 6:1 | Tight and thin, curved, yellowish-brown, more moist, golden centimeters, lumps | Yellow red still bright | The sweet tea fragrance is obvious, lasting, and with slight precipitation | Mellow, sweet, refreshing | Tan, bright | 94.3 |
| 2:1 | Long block, brown, smooth, with gold cents | Yellow, brighter | Obvious sweet aroma, light tea aroma, with precipitation | Too sweet, obvious licorice flavor, weak tea flavor | Unexpanded, tan, bright | 85.25 |
| Test Serial Number | Factors and Levels | Index | |
|---|---|---|---|
| (A) Withered Leaves: LPR | (B) Fermentation Time (h) | Sensory Score | |
| 1 | 1 (7:1) | 1 (5) | 88.29 |
| 2 | 1 | 2 (6) | 87.83 |
| 3 | 1 | 3 (7) | 87.14 |
| 4 | 2 (8:1) | 1 | 87.62 |
| 5 | 2 | 2 | 87.61 |
| 6 | 2 | 3 | 87.28 |
| 7 | 3 (9:1) | 1 | 88.69 |
| 8 | 3 | 2 | 89.09 |
| 9 | 3 | 3 | 88.33 |
| K1 | 87.75 | 88.20 | |
| K2 | 87.50 | 88.18 | |
| K3 | 88.70 | 87.58 | |
| R | 1.2 | 0.62 | |
| Sample (Withered Leaves: LPR, Fermentation Time) | Content (%) | ||||
|---|---|---|---|---|---|
| Tea Polyphenols | Amino Acid | Caffeine | Flavone | Soluble Sugar | |
| 7:1, 5 h | 10.34 ± 0.39 d,e | 0.26 ± 0.02 d,e | 7.49 ± 0.57 b,c | 17.34 ± 0.32 b | 2.78 ± 0.06 b |
| 7:1, 6 h | 10.85 ± 0.54 c,d | 0.25 ± ND e | 6.33 ± 0.07 c,d | 21.07 ± 0.89 a | 2.40 ± 0.06 d,e |
| 7:1, 7 h | 12.05 ± 0.13 b,c | 0.19 ± 0.01 f | 6.86 ± 0.23 c,d,e | 12.75 ± 0.28 c | 2.60 ± 0.05 c |
| 8:1, 5 h | 9.42 ± 0.26 e | 0.25 ± 0.02 d,e | 7.26 ± 0.35 c,d | 12.96 ± 1.34 c | 2.15 ± 0.02 f |
| 8:1, 6 h | 11.3 ± 0.85 c,d | 0.31 ± 0.01 b | 8.19 ± 0.67 b | 20.73 ± 1.91 a | 2.45 ± 0.01 c,d,e |
| 8:1, 7 h | 10.34 ± 0.74 d,e | 0.28 ± ND c,d | 7.49 ± 0.41 b,c | 12.20 ± 1.06 c | 2.62 ± 0.07 b,c |
| 9:1, 5 h | 11.44 ± 0.89 c,d | 0.30 ± ND b,c | 7.26 ± 0.35 c,d | 13.79 ± 1.09 c | 2.62 ± 0.02 b,c |
| 9:1, 6 h | 13.02 ± 0.29 a,b | 0.28 ± 0.01 b,c | 6.16 ± 0.18 e,f | 14.19 ± 0.99 c | 2.35 ± 0.01 e |
| 9:1, 7 h | 12.89 ± 0.59 a,b | 0.25 ± 0.01 e | 5.94 ± 0.40 f | 12.05 ± 1.57 c | 2.56 ± 0.28 c,d |
| CK, 5 h | 13.89 ± 1.60 a | 0.31 ± 0.04 a,b | 6.20 ± 0.50 e,f | 12.01 ± 0.16 c | 1.71 ± 0.07 g |
| CK, 6 h | 10.93 ± 0.45 c,d | 0.33 ± 0.02 a | 6.52 ± 0.18 d,e,f | 11.32 ± 0.89 c | 2.15 ± 0.03 f |
| CK, 7 h | 10.74 ± 0.30 c,d,e | 0.30 ± 0.01 b,c | 5.99 ± 0.12 f | 11.93 ± 0.51 c | 2.48 ± 0.06 c,d,e |
| LPR powder | 10.85 ± 1.16 c,d | 0.13 ± 0.01 g | 21.15 ± 0.81 a | 20.73 ± 4.16 a | 4.88 ± 0.09 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Teng, T.; Ye, K.; Zhou, G.; Yang, X.; Zhao, D.-G.; Zhao, Y. Development and Functional Analysis of Lithocarpus polystachyus (wall.) Rehd Black Tea. Appl. Sci. 2022, 12, 6991. https://doi.org/10.3390/app12146991
Liu Y, Teng T, Ye K, Zhou G, Yang X, Zhao D-G, Zhao Y. Development and Functional Analysis of Lithocarpus polystachyus (wall.) Rehd Black Tea. Applied Sciences. 2022; 12(14):6991. https://doi.org/10.3390/app12146991
Chicago/Turabian StyleLiu, Yuqian, Teng Teng, Kun Ye, Guolan Zhou, Xiulong Yang, De-Gang Zhao, and Yichen Zhao. 2022. "Development and Functional Analysis of Lithocarpus polystachyus (wall.) Rehd Black Tea" Applied Sciences 12, no. 14: 6991. https://doi.org/10.3390/app12146991
APA StyleLiu, Y., Teng, T., Ye, K., Zhou, G., Yang, X., Zhao, D.-G., & Zhao, Y. (2022). Development and Functional Analysis of Lithocarpus polystachyus (wall.) Rehd Black Tea. Applied Sciences, 12(14), 6991. https://doi.org/10.3390/app12146991






