Incorporation of Torsion Springs in a Knee Exoskeleton for Stance Phase Correction of Crouch Gait
Abstract
1. Introduction
2. Design and Methods
2.1. Mathematic Model of Human Gait
2.2. OpenSim 4.3: Gait Cycle Simulation
2.3. SolidWorks: Modeling and Motion Analysis
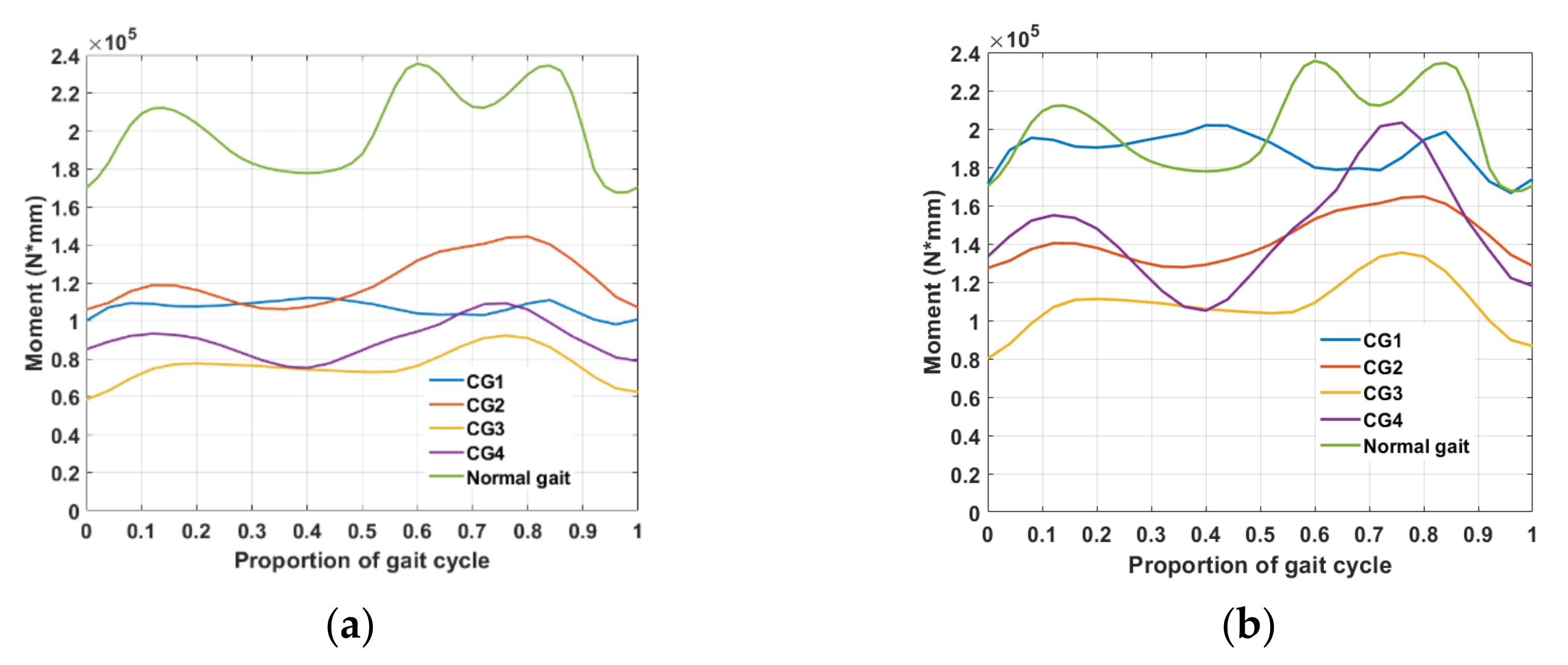
3. Results
3.1. Passive Correction (Torsion Spring Only)
3.2. Passive Elements with Motorized Angular Correction
3.3. Gap Analysis of the Data
4. Discussion
5. Conclusions
6. Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Delp, S.L.; Arnold, A.S.; Piazza, S.J. Graphics-based modeling and analysis of gait abnormalities. Biomed. Mater. Eng. 1998, 8, 227–240. [Google Scholar] [PubMed]
- Steele, K.M.; DeMers, M.S.; Schwartz, M.H.; Delp, S.L. Compressive tibiofemoral force during crouch gait. Gait Posture 2012, 35, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Steele, K.M.; van der Krogt, M.M.; Schwartz, M.H.; Delp, S.L. How much muscle strength is required to walk in a crouch gait? J. Biomech. 2012, 45, 2564–2569. [Google Scholar] [CrossRef] [PubMed]
- Lerner, Z.F.; Damiano, D.L.; Bulea, T.C. A robotic exoskeleton to treat crouch gait from cerebral palsy: Initial kinematic and neuromuscular evaluation. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; IEEE: Washington, DC, USA, 2016; pp. 2214–2217. [Google Scholar]
- Ganjwala, D.; Shah, H. Management of the knee problems in spastic cerebral palsy. Indian J. Orthop. 2019, 53, 53–62. [Google Scholar] [CrossRef]
- Hilberink, S.R.; Roebroeck, M.E.; Nieuwstraten, W.; Jalink, L.; Verheijden, J.; Stam, H.J. Health issues in young adults with cerebral palsy: Towards a life-span perspective. J. Rehabil. Med. 2007, 39, 605–611. [Google Scholar] [CrossRef]
- Shah, K.; Solan, M.; Dawe, E. The gait cycle and its variations with disease and injury. Orthop. Trauma 2020, 34, 153–160. [Google Scholar] [CrossRef]
- Arnold, A.S.; Liu, M.Q.; Schwartz, M.H.; Ounpuu, S.; Delp, S.L. The role of estimating muscle-tendon lengths and velocities of the hamstrings in the evaluation and treatment of crouch gait. Gait Posture 2006, 23, 273–281. [Google Scholar] [CrossRef]
- Rose, J.; Gamble, J.G.; Medeiros, J.; Burgos, A.; Haskell, W.L. Energy cost of walking in normal children and in those with cerebral palsy: Comparison of heart rate and oxygen uptake. J. Pediatr. Orthop. 1989, 9, 276–279. [Google Scholar] [CrossRef]
- Waters, R.L.; Mulroy, S. The energy expenditure of normal and pathologic gait. Gait Posture 1999, 9, 207–231. [Google Scholar] [CrossRef]
- Jahnsen, R.; Villien, L.; Aamodt, G.; Stanghelle, J.; Holm, I. Musculoskeletal pain in adults with cerebral palsy compared with the general population. J. Rehabil. Med. 2004, 36, 78–84. [Google Scholar] [CrossRef]
- Bottos, M.; Gericke, C. Ambulatory capacity in cerebral palsy: Prognostic criteria and consequences for intervention. Dev. Med. Child Neurol. 2003, 45, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Brandon, S.C.; Thelen, D.G.; Smith, C.R.; Novacheck, T.F.; Schwartz, M.H.; Lenhart, R.L. The coupled effects of crouch gait and patella alta on tibiofemoral and patellofemoral cartilage loading in children. Gait Posture 2018, 60, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, B.K.; Park, K.B.; Abdel-Baki, S.W.; Rhee, I.; Kim, C.W.; Kim, H.W. Distal femoral shortening osteotomy for severe knee flexion contracture and crouch gait in cerebral palsy. J. Clin. Med. 2019, 8, 1354. [Google Scholar] [CrossRef] [PubMed]
- Pelrine, E.R.; Novacheck, T.F.; Boyer, E.R. Knee pain and crouch gait in individuals with cerebral palsy: What impact does crouch-related surgery have? Dev. Med. Child Neurol. 2020, 62, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Graham, H.K.; Selber, P. Musculoskeletal aspects of cerebral palsy. J. Bone Jt. Surg. Br. Vol. 2003, 85, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Mullaji, A.; Shetty, G.M. Persistent hindfoot valgus causes lateral deviation of weightbearing axis after total knee arthroplasty. Clin. Orthop. Relat. Res. 2011, 469, 1154–1160. [Google Scholar] [CrossRef]
- Patthanacharoenphon, C.; Maples, D.L.; Saad, C.; Forness, M.J.; Halanski, M.A. The effects of patellar tendon advancement on the immature proximal tibia. J. Child. Orthop. 2013, 7, 139–146. [Google Scholar] [CrossRef]
- Li, G.; DeFrate, L.; Zayontz, S.; Park, S.; Gill, T. The effect of tibiofemoral joint kinematics on patellofemoral contact pressures under simulated muscle loads. J. Orthop. Res. 2004, 22, 801–806. [Google Scholar] [CrossRef]
- Classification of Gait Patterns in Cerebral Palsy. Physiopedia. 13 September 2019. Available online: https://www.physio-pedia.com/index.php?title=Classification_of_Gait_Patterns_in_Cerebral_Palsy&oldid=222810 (accessed on 29 November 2021).
- Bregman, D.J.; Van der Krogt, M.; De Groot, V.; Harlaar, J.; Wisse, M.; Collins, S. The effect of ankle foot orthosis stiffness on the energy cost of walking: A simulation study. Clin. Biomech. 2011, 26, 955–961. [Google Scholar] [CrossRef]
- Kerkum, Y.L.; Buizer, A.I.; Van Den Noort, J.C.; Becher, J.G.; Harlaar, J.; Brehm, M.A. The effects of varying ankle foot orthosis stiffness on gait in children with spastic cerebral palsy who walk with excessive knee flexion. PLoS ONE 2015, 10, e0142878. [Google Scholar] [CrossRef]
- Damiano, D.L.; Arnold, A.S.; Steele, K.M.; Delp, S.L. Can strength training predictably improve gait kinematics? a pilot study on the effects of hip and knee extensor strengthening on lower-extremity alignment in cerebral palsy. Phys. Ther. 2010, 90, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.H.; Wiggin, M.B.; Sawicki, G.S. Reducing the energy cost of human walking using an unpowered exoskeleton. Nature 2015, 522, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Naf, M.B.; Junius, K.; Rossini, M.; Rodriguez-Guerrero, C.; Vanderborght, B.; Lefeber, D. Misalignment compensation for full human-exoskeleton kinematic compatibility: State of the art and evaluation. Appl. Mech. Rev. 2018, 70, 050802. [Google Scholar] [CrossRef]
- Scherer, M.J. Outcomes of Assistive Technology Use on Quality of Life. Disabil. Rehabil. 1996, 18, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.J.; Kimm, H.; Yun, J.E.; Jee, S.H. Thigh circumference and diabetes: Obesity as a potential effect modifier. J. Epidemiol. 2013, 23, 329–336. [Google Scholar] [CrossRef]
- Hicks, J.L.; Delp, S.L.; Schwartz, M.H. Can biomechanical variables predict improvement in crouch gait? Gait Posture 2011, 34, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Royer, T.D.; Martin, P.E. Manipulations of leg mass and moment of inertia: Effects on energy cost of walking. Med. Sci. Sports Exer. 2005, 37, 649–656. [Google Scholar] [CrossRef]
- Browning, R.C.; Modica, J.R.; Kram, R.; Goswami, A. The effects of adding mass to the legs on the energetics and biomechanics of walking. Med. Sci. Sports Exerc. 2007, 39, 515–525. [Google Scholar] [CrossRef]
- Chaichaowarat, R.; Granados, D.F.P.; Kinugawa, J.; Kosuge, K. Passive knee exoskeleton using torsion spring for cycling assistance. In Proceedings of the 2017 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Vancouver, BC, Canada, 24–28 September 2017; IEEE: Washington, DC, USA, 2017; pp. 3069–3074. [Google Scholar]
- Spring, A.N.; Kofman, J.; Lemaire, E.D. Design and evaluation of an orthotic knee-extension assist. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 678–687. [Google Scholar] [CrossRef]
- Winters, T.F.; Gage, J.R.; Hicks, R. Gait patterns in spastic hemiplegia in children and young adults. J. Bone Jt. Surg. Am. 1987, 69, 437–441. [Google Scholar]
- Rome, L.C.; Flynn, L.; Yoo, T.D. Rubber bands reduce the cost of carrying loads. Nature 2006, 444, 1023–1024. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, S.H.; Park, H.S. A fully soft and passive assistive device to lower the metabolic cost of sit-to-stand. Front. Bioeng. Biotechnol. 2020, 8, 966. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, W.; Van der Kooij, H.; Hekman, E. A Passive Exoskeleton with Artificial Tendons: Design and Experimental Evaluation. In Proceedings of the IEEE International Conference on Rehabilitation Robotics, Zurich, Switzerland, 29 June–1 July 2011; pp. 1–6. [Google Scholar]
- Panizzolo, F.A.; Bolgiani, C.; Di Liddo, L.; Annese, E.; Marcolin, G. Reducing the energy cost of walking in older adults using a passive hip flexion device. J. Neuroeng. Rehab. 2019, 16, 117. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Neptune, R.R. Differences in muscle function during walking and running at the same speed. J. Biomech. 2006, 39, 2005–2013. [Google Scholar] [CrossRef]
- Haufe, F.L.; Wolf, P.; Riener, R.; Grimmer, M. Biomechanical effects of passive hip springs during walking. J. Biomech. 2020, 98, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, R.; Ahmadi, A.; Ahmadabadi, M.N. Reducing the energy cost of human running using an unpowered exoskeleton. IEEE Trans. Neural Syst. Rehab. Eng. 2018, 26, 2026–2032. [Google Scholar] [CrossRef]
- Lovrenovic, Z.; Doumit, M. Development and testing of a passive walking assist exoskeleton. Biocybern. Biomed. Eng. 2019, 39, 992–1004. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, G.; Diao, Y.; Feng, Y.U.; Li, G. Performance analysis of unpowered lower limb exoskeleton during sit down and stand up. Robotica 2022, 40, 1274–1292. [Google Scholar] [CrossRef]
- Lee, K.M.; Wang, D. Design analysis of a passive weight support lower-extremity-exoskeleton with compliant knee joint. In Proceedings of the 2015 IEEE International Conference on Robotics and Automation (ICRA), Seattle, WA, USA, USA, 26–30 May 2015; pp. 5572–5577. [Google Scholar]


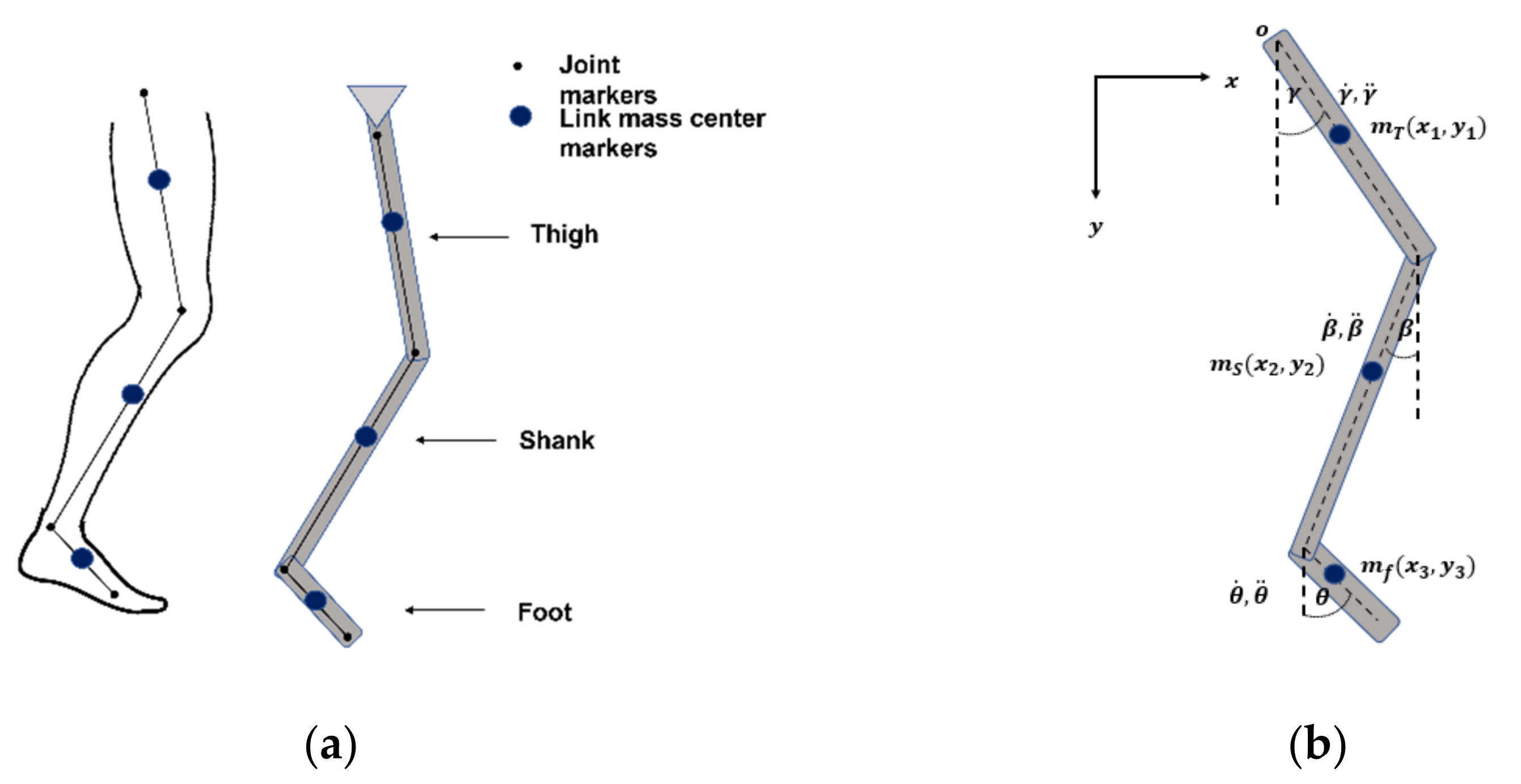

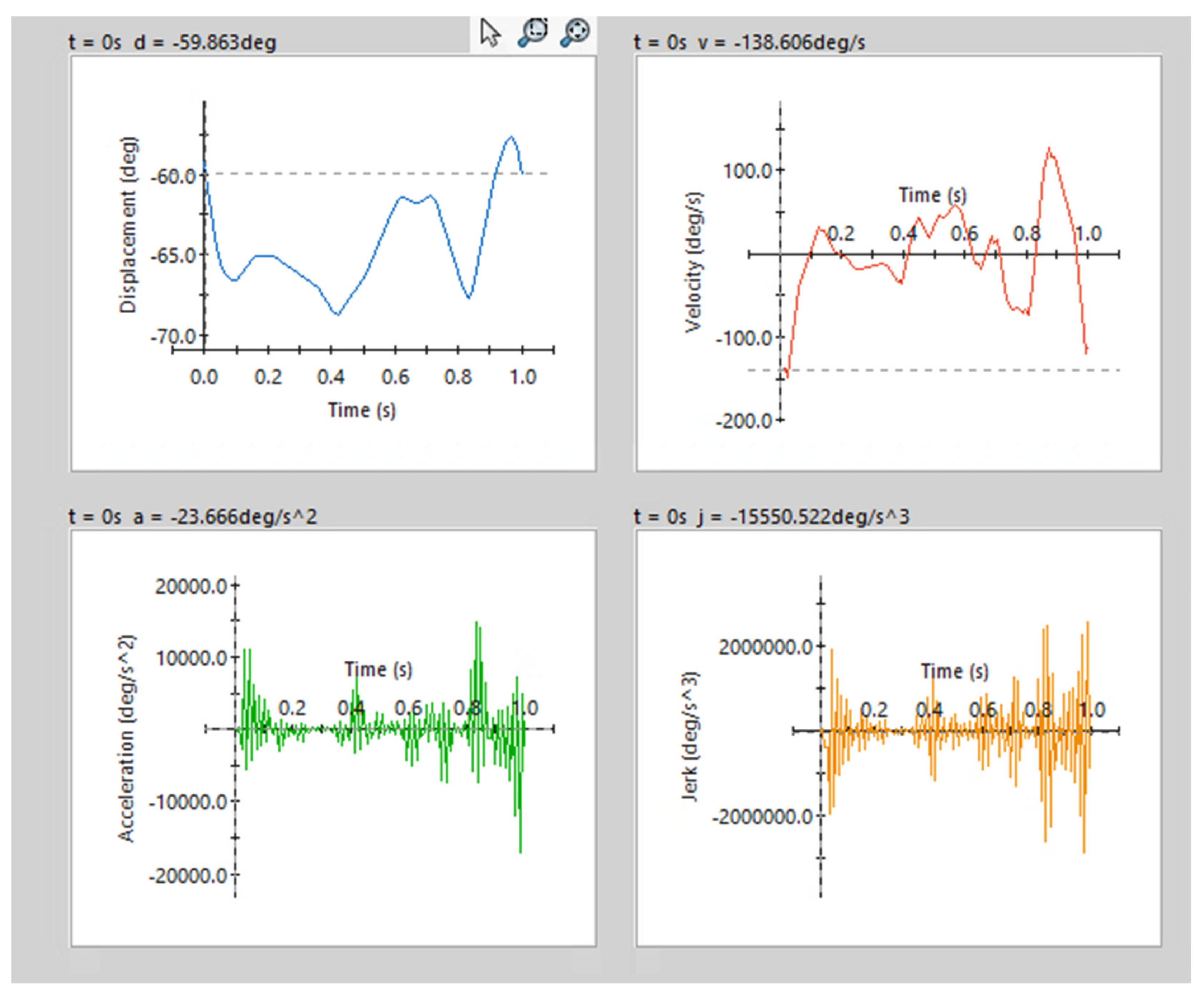

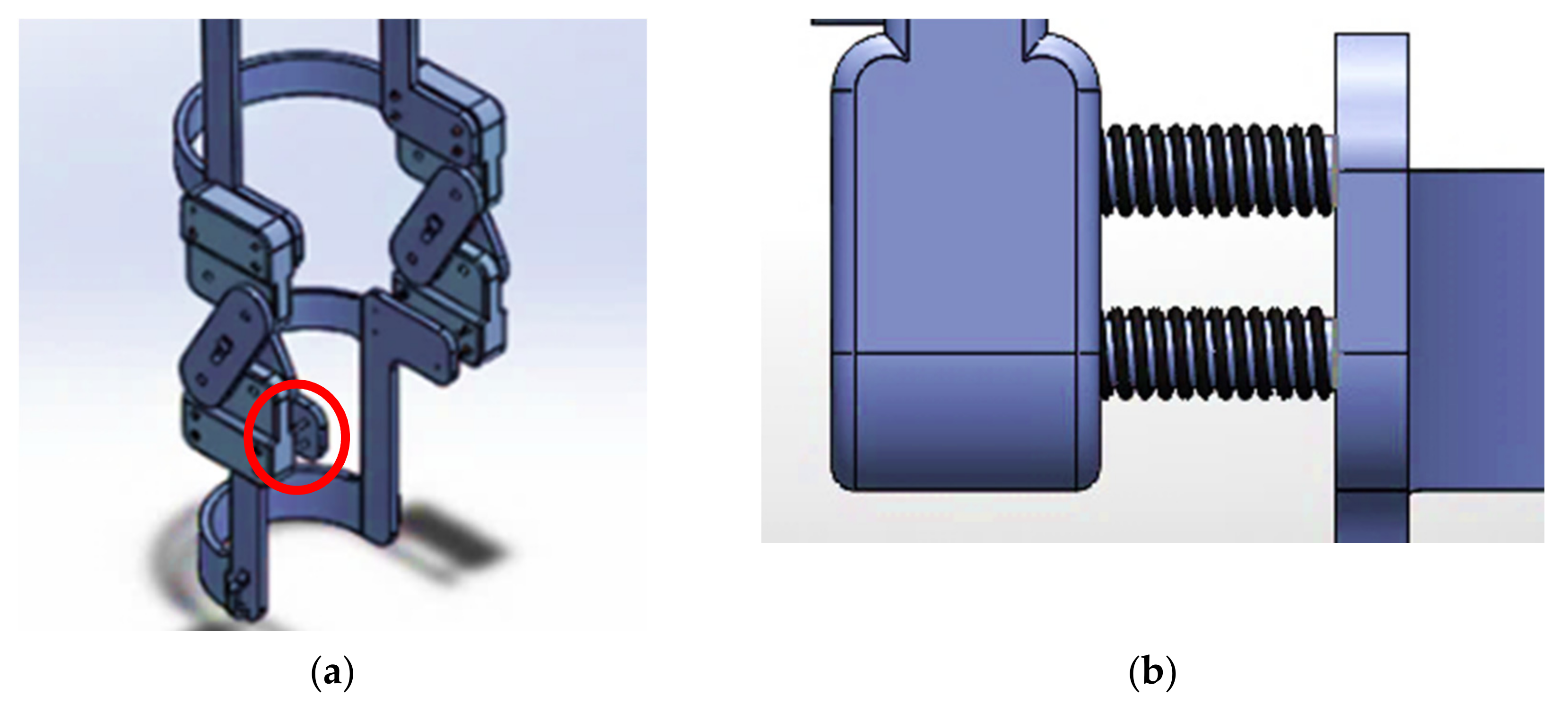
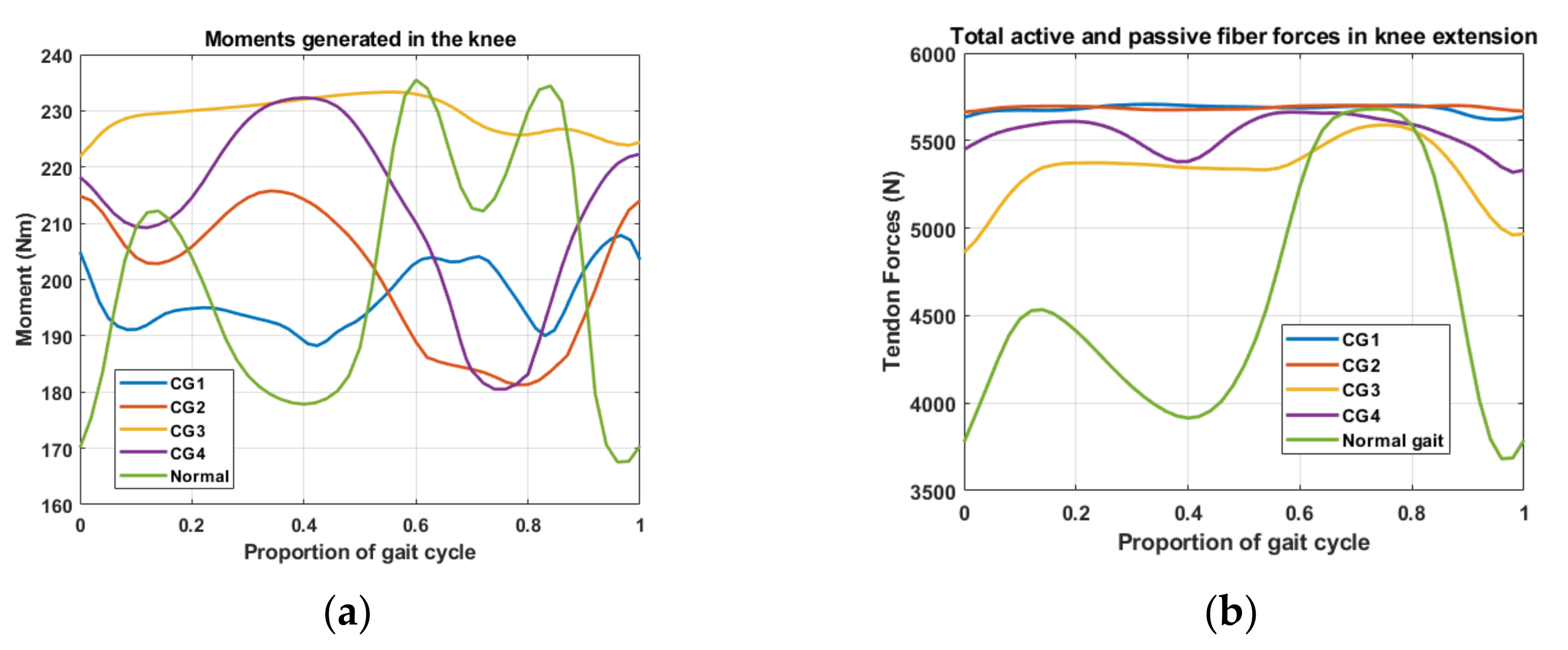

| Segment | Proximal Joint to Mass Center |
|---|---|
| Thigh | 0.430 |
| Shank | 0.443 |
| Foot | 0.436 |
| Segment | Circumference (cm) |
|---|---|
| Thigh | 39.5 |
| Shank | 52.0 |
| Maximum Moments | Torsion Springs 20 k Nmm/Deg | %Gait Cycle | Motorized Correction 20 k Nmm/Deg | %Gait Cycle | Torsion Springs 30 k Nmm/Deg | %Gait Cycle | Motorized Correction 30 k Nmm/Deg | %Gait Cycle |
|---|---|---|---|---|---|---|---|---|
| CG1 | 112,117.5 | 40 | 51,566.5 | 12 | 201,854.6 | 60 | 64,088.1 | 12 |
| CG2 | 118,782.2 | 12 | 51,730.6 | 12 | 140,307.0 | 12 | 64,240.4 | 12 |
| CG3 | 77,376.4 | 20 | 49,834.2 | 12 | 111,187.3 | 20 | 62,483.8 | 12 |
| CG4 | 93,155.36 | 12 | 51,238.6 | 12 | 154,915.8 | 12 | 63,784.0 | 12 |
| Minimum Moments | Torsion Springs 20 k Nmm/Deg | %Gait Cycle | Motorized Correction 20 k Nmm/Deg | %Gait Cycle | Torsion Springs 30 k Nmm/Deg | %Gait Cycle | Motorized Correction 30 k Nmm/Deg | %Gait Cycle |
|---|---|---|---|---|---|---|---|---|
| CG1 | 103,800.8 | 60 | 42,931.9 | 40 | 179,830.6 | 60 | 44,058.2 | 40 |
| CG2 | 106,115.8 | 36 | 42,701.4 | 40 | 127,834 | 36 | 43,828.5 | 40 |
| CG3 | 72,958.89 | 52 | 40,303.2 | 40 | 103,772.2 | 52 | 41,439.1 | 40 |
| CG4 | 75,131.99 | 40 | 40,535.92 | 40 | 105,117.9 | 40 | 41,670.9 | 40 |
| Normal Gait | Maximum Moment | %Gait Cycle | Minimum Moment | %Gait Cycle |
|---|---|---|---|---|
| 212,194.5 | 14 | 177,850.8 | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bumbard, K.B.; Herrington, H.; Goh, C.-H.; Ibrahim, A. Incorporation of Torsion Springs in a Knee Exoskeleton for Stance Phase Correction of Crouch Gait. Appl. Sci. 2022, 12, 7034. https://doi.org/10.3390/app12147034
Bumbard KB, Herrington H, Goh C-H, Ibrahim A. Incorporation of Torsion Springs in a Knee Exoskeleton for Stance Phase Correction of Crouch Gait. Applied Sciences. 2022; 12(14):7034. https://doi.org/10.3390/app12147034
Chicago/Turabian StyleBumbard, Katy Baker, Harold Herrington, Chung-Hyun Goh, and Alwathiqbellah Ibrahim. 2022. "Incorporation of Torsion Springs in a Knee Exoskeleton for Stance Phase Correction of Crouch Gait" Applied Sciences 12, no. 14: 7034. https://doi.org/10.3390/app12147034
APA StyleBumbard, K. B., Herrington, H., Goh, C.-H., & Ibrahim, A. (2022). Incorporation of Torsion Springs in a Knee Exoskeleton for Stance Phase Correction of Crouch Gait. Applied Sciences, 12(14), 7034. https://doi.org/10.3390/app12147034









