Cold Storage Media versus Optisol-GS in the Preservation of Corneal Quality for Keratoplasty: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Research Question and Eligibility Criteria
2.3. Information Sources
2.4. Search Strategy
2.5. Selection Process
2.6. Data Collection Process and Data Items
2.7. Study Risk of Bias Assessment
2.8. Effect Measures and Synthesis Methods
3. Results
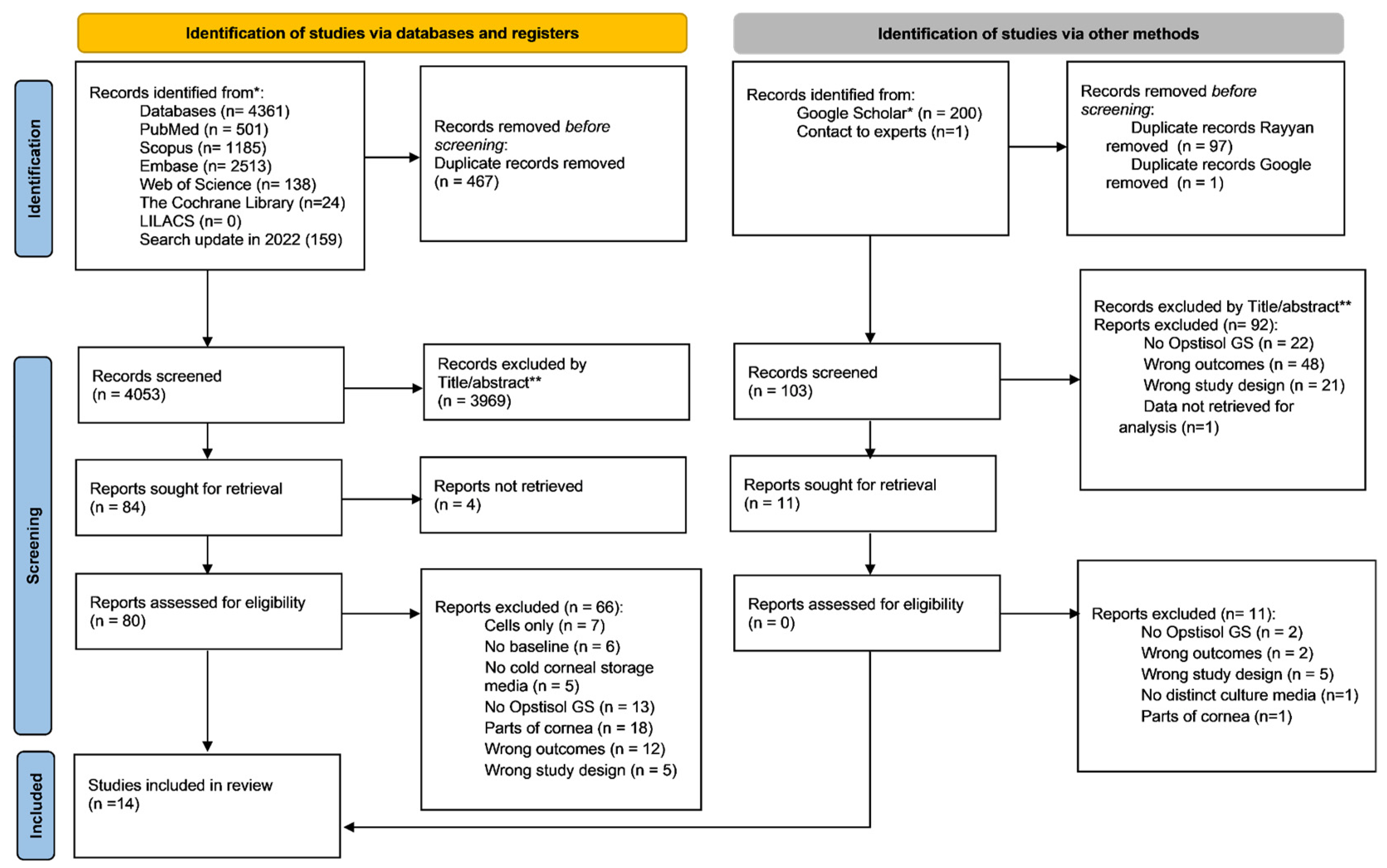
3.1. Study Selection
3.2. Quality Assessment
3.3. Study Characteristics
3.4. Results of Synthesis
3.4.1. Cornea Preservation Quantitative Parameters
3.4.2. Cornea Preservation Qualitative Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adán, C.B.D.; Diniz, A.R.; Perlatto, D.; Hirai, F.E.; Sato, E.H. Ten years of corneal donation to the Hospital São Paulo Eye Bank: Characteristics of cornea donors from 1996 to 2005. Arq. Bras. Oftalmol. 2008, 71, 176–181. [Google Scholar] [PubMed] [Green Version]
- Singh, R.; Gupta, N.; Vanathi, M.; Tandon, R. Corneal transplantation in the modern era. Indian J. Med. Res. 2019, 150, 7–22. [Google Scholar]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global survey of corneal transplantation and eye banking. JAMA ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koulouri, I.; Hellwinkel, O.J.C. A brief review of corneal transplantation: Techniques, indications and methods of corneal preservation. J. Transl. Sci. 2021, 7, 1–8. [Google Scholar]
- Lindstrom, R.L.; Kaufman, H.E.; Skelnik, D.L.; Laing, R.A.; Lass, J.H.; Musch, D.C.; Gordon, J.F. Optisol corneal storage medium., Optisol corneal storage medium. Am. J. Ophthalmol. 1992, 114, 345–356. [Google Scholar] [CrossRef]
- Mannis, M.J.; Holland, E.J. Cornea, E-Book, 5th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021; pp. 256–257. [Google Scholar]
- Sharma, N.; Arora, T.; Singhal, D.; Maharana, P.K.; Garg, P.; Nagpal, R.; Basak, S. Procurement, storage and utilization trends of eye banks in India. Ind. J. Ophthalmol. 2019, 67, 1056. [Google Scholar] [CrossRef]
- Smith, T.M.; Popplewell, J.; Nakamura, T.; Trousdale, M.D. Efficacy and safety of gentamicin and streptomycin in Optisol-GS, a preservation medium for donor corneas. Cornea 1995, 14, 49–55. [Google Scholar] [CrossRef]
- Lass, J.H.; Gordon, J.F.; Sugar, A.; Norden, R.A.; Reinhart, W.J.; Meyer, R.F.; Soong, H.K. Optisol containing streptomycin. Am. J. Ophthalmol. 1993, 116, 503–504. [Google Scholar] [CrossRef]
- Hwang, D.G.; Nakamura, T.; Trousdale, M.D.; Smith, T.M. Combination antibiotic supplementation of corneal storage medium. American journal of ophthalmology. Am. J. Ophthalmol. 1993, 115, 299–308. [Google Scholar] [CrossRef]
- Jeng, B.H. Preserving the cornea: Corneal storage media. Curr. Opin. Ophthalmol. 2006, 17, 332–337. [Google Scholar] [CrossRef]
- Sundaresan, Y.; Gaikwad, G.G.; Prajapati, K.A.; Prajna, N.V.; Chidambaranathan, G.P. Comparison of structural integrity and functional status of corneal endothelium stored in Cornisol and Optisol-GS. Indian J. Ophthalmol. 2019, 67, 579–1584. [Google Scholar]
- Parekh, M.; Salvalaio, G.; Ferrari, S.; Amoureux, M.C.; Albrecht, C.; Fortier, D.; Ponzin, D. A quantitative method to evaluate the donor corneal tissue quality used in a comparative study between two hypothermic preservation media. Cell Tissue Bank 2014, 15, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 202110, 1–11. [Google Scholar]
- Schneider, K.; Schwarz, M.; Burkholder, I.; Kopp-Schneider, A.; Edler, L.; Kinsner-Ovaskainen, A.; Hoffmann, S. “ToxRTool”, a new tool to assess the reliability of toxicological data. Toxicol. Lett. 2009, 189, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.K.; Cavanagh, H.D.; Jester, J.V.; Ma, L.; Petroll, W.M. Changes in corneal endothelial apical junctional protein organization after corneal cold storage. Cornea 1999, 18, 712–720. [Google Scholar] [CrossRef]
- Ogino, H.; Yukari, K.; Terada, H.; Sawa, M. Effect of a newly developed corneal storage medium on corneal endothelium--morphological study by scanning electron microscopy. Nihon. Ganka Gakkai Zasshi 1995, 99, 387–391. [Google Scholar]
- Maffrand, R.A.; de Fabro, S.P. Significative lengthening of the time of cornea conservation supporting its natural tension and curvature. Rev. Fac. Cien. Med. Univ. Nac. Cordoba. 2000, 57, 163–180. [Google Scholar]
- Vila, J.; Hasany, S.M.; Parker, J.A.; Rootman, D.S. In-vitro comparative study of a locally prepared corneal storage medium and Optisol. Can. J. Ophthalmol. 1996, 31, 221–227. [Google Scholar]
- Tachibana, A.; Sawa, M.A. Development of novel corneal storage medium: First report. Examinations of rabbit cornea. Jpn. J. Ophthalmol. 2002, 46, 377–383. [Google Scholar] [CrossRef]
- Sharma, N.; Shaikh, F.; Nagpal, R.; Maharana, P.K.; Agarwal, T.; Sinha, R.; Titiyal, J.S. Evaluation of various preservation media for storage of donor corneas. Indian J. Ophthalmol. 2021, 69, 2452. [Google Scholar] [CrossRef]
- Basak, S.; Prajna, N.V. A Prospective, In Vitro, Randomized Study to Compare Two Media for Donor Corneal Storage. Cornea 2016, 35, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Cid, D.A.C.; Araújo, M.C.; Passos, M.V.S.; Brasil, I.R.C.; Nunes, J.F.; Salgueiro, C.C.D.M.; Costa, D.C. Human cornea conservation in coconut water solution. Arq. Bras. Oftalmol. 2021, 84, 163–169. [Google Scholar] [CrossRef]
- Duncan, K.; Parker, J.; Hoover, C.; Jeng, B.H. The effect of light exposure on the efficacy and safety of amphotericin B in corneal storage media. JAMA Ophthalmol. 2016, 134, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, A.; Hasany, S.M.; Rootman, D. Optisol vs. Dexsol as storage media for preservation of human corneal epithelium. Eye 2004, 18, 519–524. [Google Scholar] [PubMed] [Green Version]
- Javadi, M.A.; Rezaeian Akbarzadeh, A.; Chamani, T.; Rezaei Kanavi, M. Sinasol versus Optisol-GS for cold preservation of human cornea: A prospective ex vivo and clinical study. Cell Tissue Bank 2021, 22, 563–574. [Google Scholar] [CrossRef]
- Kanavi, M.R.; Javadi, M.A.; Chamani, T.; Fahim, P.; Javadi, F. Comparing quantitative and qualitative indices of the donated corneas maintained in Optisol-GS with those kept in Eusol-C. Cell Tissue Bank 2015, 16, 243–247. [Google Scholar] [CrossRef]
- Layer, N.; Cevallos, V.; Maxwell, A.J.; Hoover, C.; Keenan, J.D.; Jeng, B.H. Efficacy and safety of antifungal additives in Optisol-GS corneal storage medium. JAMA Ophthalmol. 2014, 132, 832–837. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Feiz, V.; Vieira, A.C.; Davis-Boozer, D.; Polage, C. The safety and efficacy of linezolid and daptomycin as an additive in optisol-GS against methicillin-resistant Staphylococcus aureus. Cornea 2012, 31, 551–558. [Google Scholar] [CrossRef]
- Mistò, R.; Giurgola, L.; Pateri, F.; Limongelli, A.; Ragazzi, E.; Tóthová, J.D.A. A new storage medium containing amphotericin B versus Optisol-GS for preservation of human donor corneas. Br. J. Ophthalmol. 2022, 06, 184–189. [Google Scholar] [CrossRef]
- Nelson, L.R.; Hodge, D.O.; Bourne, W.M. In vitro comparison of Chen medium and Optisol-GS medium for human corneal storage. Cornea 2000, 19, 782–787. [Google Scholar] [CrossRef]
- Perry, I.; Peterson, K.; Tóthová, J.D.A.; Tramber, M.; Botsay, S.; Tremblay, D. Performance of new hypothermic corneal storage media with an antimycotic tablet in comparison to traditional hypothermic media during simulated eye bank processing. Cornea 2020, 39, 1031–1039. [Google Scholar] [CrossRef]
- Tachibana, A.; Sawa, M.; Hwang, D.G. [Development of corneal storage medium—Second report. Examination of human cornea]. Nihon. Ganka Gakkai Zasshi. 2001, 105, 295–300. [Google Scholar] [CrossRef]
- Rezaei Kanavi, M.; Ghalenoee, M.; Chamani, T.; Javadi, M.A.; Fazili, N. Comparison of Donor Cornea Storage in Optisol-GS and the Central Eye Bank of Iran (CEBI) Medium. Bina J. Ophthalmol. 2015, 20, 374–379. [Google Scholar]
- Sugar, J.; Glasser, D.; Mannis, M. EBAA Medical Standards. Int. J. Eye Bank. 2016, 4, 1–39. [Google Scholar]
- Dubashynskaya, N.; Poshina, D.; Raik, S.; Urtti, A.; Skorik, Y.A. Polysaccharides in ocular drug delivery. Pharmaceutics 2019, 12, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Eye Bank Association (EEBA). Technical Guidelines for Ocular Tissue; European Eye Bank Association (EEBA): Venice, Italy, 2020. [Google Scholar]
- Yap, C.; Wong, A.M.; Naor, J.; Rootman, D.S. Corneal temperature reversal after storage in Chen medium compared with Optisol GS. Cornea 2001, 20, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Naor, J.; Slomovic, A.R.; Chipman, M.; Rootman, D.S. A randomized, double-masked clinical trial of Optisol-GS vs. Chen Medium for human corneal storage. Arch. Ophthalmol. 2002, 120, 1280–1285. [Google Scholar]
- Bourne, W.M.; Nelson, L.R.; Maguire, L.J.; Baratz, K.H.; Hodge, D.O. Comparison of Chen Medium and Optisol-GS for human corneal preservation at 4 °C: Results of transplantation. Cornea 2001, 20, 683–686. [Google Scholar] [CrossRef]
- Ritterband, D.C.; Shah, M.K.; Meskin, S.W.; Seedor, J.A.; Koplin, R.S.; Perez, W.; Dahl, P. Efficacy and safety of voriconazole as an additive in Optisol GS: A preservation medium for corneal donor tissue. Cornea 2007, 26, 343–347. [Google Scholar] [CrossRef]
- Camposampiero, D.; Tiso, R.; Zanetti, E.; Ruzza, A.; Bruni, A.; Ponzin, D. Improvement of human corneal endothelium in culture after prolonged hypothermic storage. European journal of ophthalmology. Eur. J. Ophthalmol. 2003, 13, 745–751. [Google Scholar] [CrossRef] [Green Version]
- Spolarics, Z.; Lang, C.H.; Bagby, G.J.; Spitzer, J.J. Glutamine and fatty acid oxidation are the main sources of energy for Kupffer and endothelial cells. Am. J. Physiol. 1991, 261, G185–G190. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.E. MLIS, American Academy of Ophtalmology. Imported EusolC to Fill the Gap Created by Optisol Shortage. 27 April 2022. Available online: https://www.aao.org/headline/imported-eusolc-to-fill-gap-created-by-optisol-sho (accessed on 28 June 2022).
| PECO Framework | |
|---|---|
| P Population | Human or animal corneas |
| E Exposure | Distinct cold storage corneal medium |
| C Comparator | Optisol-GS |
| O Outcomes | Evaluation of qualitative parameters: corneal transparency (CT) and endothelial cells (EC) morphology; quantitative parameters: endothelial cell density (ECD), central corneal thickness (CCT), and EC mortality |
| Database | Search Strategy |
|---|---|
| PUBMED | (Cornea[Mesh] OR Cornea*[tiab] OR cornea ex vivo[tiab]) AND (Culture Media[Mesh] OR HEPES[Mesh] OR Cryoprotective Agents[Mesh] OR Organ Preservation solutions[Mesh] OR Organ Preservation[Mesh] OR Cryopreservation[Mesh] OR glucose[Mesh] OR acids[Mesh] OR vitamins[Mesh] OR penicillins[Mesh] OR streptomycin[Mesh] OR Bicarbonates[Mesh] OR Adenosine Triphosphate[Mesh] OR Methylcellulose[Mesh] OR Culture Media[tiab] OR Cryopreservation[tiab] OR Adenosine Triphosphate[tiab] OR cornea max[tiab] OR cold storage medium[tiab] OR hypothermic storage[tiab]) AND (Chondroitin Sulfates[Mesh] OR Dextrans[Mesh] OR Gentamicins[Mesh] OR Complex Mixtures[Mesh] OR Optisol[tiab] OR Dextran[tiab] OR Gentamicin Sulfate[tiab) |
| SCOPUS | INDEXTERMS(Cornea) OR TITLE-ABS-KEY(Cornea*) OR TITLE-ABS-KEY(“cornea ex vivo”) AND INDEXTERMS(“Culture Media”) OR INDEXTERMS(HEPES) OR INDEXTERMS(“Cryoprotective Agents”) OR INDEXTERMS(“Organ Preservation solutions”) OR INDEXTERMS(“Organ Preservation”) OR INDEXTERMS(Cryopreservation) OR INDEXTERMS(glucose) OR INDEXTERMS(acids) OR INDEXTERMS(vitamins) OR INDEXTERMS(penicillins) OR INDEXTERMS(streptomycin) OR INDEXTERMS(Bicarbonates) OR INDEXTERMS(“Adenosine Triphosphate”) OR INDEXTERMS(Methylcellulose) OR TITLE-ABS-KEY(“Culture Media”) OR TITLE-ABS-KEY(Cryopreservation) OR TITLE-ABS-KEY(“Adenosine Triphosphate”) OR TITLE-ABS-KEY(“cornea max”) OR TITLE-ABS-KEY(“cold storage medium”) OR TITLE-ABS-KEY(“ hypothermic storage”) AND INDEXTERMS(“Chondroitin Sulfates”) OR INDEXTERMS(Dextrans) OR INDEXTERMS(Gentamicins) OR INDEXTERMS(“Complex Mixtures”) OR TITLE-ABS-KEY(Optisol) OR TITLE-ABS-KEY(Dextran) OR TITLE-ABS-KEY(“Gentamicin Sulfate”) |
| WOS | TS = (Cornea) OR TS = (Corneas) OR TS = (corneal) OR TS = (“cornea ex vivo”) AND TS = (“Culture Media”) OR TS = (HEPES) OR TS = (“Cryoprotective Agents”) OR TS = (“Organ Preservation solutions”) OR TS = (“Organ Preservation”) OR TS = (Cryopreservation) OR TS = (glucose) OR TS = (acids) OR TS = (vitamins) OR TS = (penicillins) OR TS = (streptomycin) OR TS = (Bicarbonates) OR TS = (“Adenosine Triphosphate”) OR TS = (Methylcellulose) OR TS = (“cornea max”) OR TS = (“cold storage medium”) OR TS = (“hypothermic storage”) AND TS = (“Chondroitin Sulfates”) OR TS = (Dextrans) OR TS = (Gentamicins) OR TS = (“Complex Mixtures”) OR TS = (Optisol) OR TS = (Dextran) OR TS = (“Gentamicin Sulfate”) |
| COCHRANE | #1 MeSH descriptor: [Cornea] explode all trees #2 (Cornea* OR cornea ex vivo):ti,ab,kw #3 = #1 OR #2 #4 MeSH descriptor: [Culture Media] explode all trees #5 MeSH descriptor: [HEPES] explode all trees #6 MeSH descriptor: [Cryoprotective Agents] explode all trees #7 MeSH descriptor: [Organ Preservation Solutions] explode all #8 MeSH descriptor: [Organ Preservation] explode all trees #9 MeSH descriptor: [Cryopreservation] explode all trees #10 MeSH descriptor: [Glucose] explode all trees #11 MeSH descriptor: [Acids] explode all trees #12 MeSH descriptor: [Vitamins] explode all trees #13 MeSH descriptor: [Penicillins] explode all trees #14 MeSH descriptor: [Streptomycin] explode all trees #15 MeSH descriptor: [Bicarbonates] explode all trees #16 MeSH descriptor: [Adenosine Triphosphate] explode all trees #17 MeSH descriptor: [Methylcellulose] explode all trees #18 (Culture Media OR Cryopreservation OR Adenosine Triphosphate OR cornea max OR cold storage medium OR hypothermic storage):ti,ab,kw #19 = #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 #16 OR #17 OR #18 #20 MeSH descriptor: [Chondroitin Sulfates] explode all trees #21 MeSH descriptor: [Dextrans] explode all trees #22 MeSH descriptor: [Gentamicins] explode all trees #23 MeSH descriptor: [Complex Mixtures] explode all trees #24 (Optisol OR Dextran OR Gentamicin Sulfate):ti,ab,kw #25 = #3 AND #19 AND #24 |
| EMBASE | ‘cornea’/exp OR cornea*:ti,ab,kw OR ‘cornea ex vivo’:ti,ab,kw AND ‘culture media’/exp OR ‘hepes’/exp OR ‘cryoprotective agents’/exp OR ‘organ preservation solutions’/exp OR ‘organ preservation’/exp OR ‘cryopreservation’/exp OR ‘glucose’/exp OR ‘acids’/exp OR ‘vitamins’/exp OR ‘penicillins’/exp OR ‘streptomycin’/exp OR ‘bicarbonates’/exp OR ‘adenosine triphosphate’/exp OR ‘methylcellulose’/exp OR ‘culture media’:ti,ab,kw OR ‘cryopreservation’:ti,ab,kw OR ‘adenosine triphosphate’:ti,ab,kw OR ‘cornea max’:ti,ab,kw OR ‘cold storage medium’:ti,ab,kw OR ‘hypothermic storage’:ti,ab,kw AND ‘chondroitin sulfates’/exp OR ‘dextrans’/exp OR ‘gentamicins’/exp OR ‘complex mixtures’/exp OR ‘optisol’:ti,ab,kw ‘dextrans’:ti,ab,kw OR ‘gentamicin sulfate’:ti,ab,kw |
| LILACS via VHL | (mh:cornea OR cornea* OR “cornea ex vivo”) AND (mh:hepes OR mh:“culture media” OR mh:“cryoprotective agents” OR mh:“organ preservation solutions” OR mh:“organ preservation” OR mh:cryopreservation OR mh:glucose OR mh:acids OR mh:vitamins OR mh:penicillins OR mh:streptomycin OR mh:bicarbonates OR mh:“adenosine triphosphate” OR mh:methylcellulose OR “culture media” OR cryopreservation OR “adenosine triphosphate” OR “cornea max” OR “cold storage medium” OR “hypothermic storage”) AND (mh:dextrans OR mh:“chondroitin sulfates” OR mh:gentamicins OR mh:“complex mixtures” OR optisol OR dextran OR “gentamicin sulfate”) |
| OPENGREY | Cornea and Optisol |
| GOOGLE SCHOLAR | Cornea and Optisol |
| Reference | Group I: Test Substance Identification | Group II: Test System Characterization | Group III: Study Design Description | Group IV: Study Results Documentation | Group V: Plausibility of Study Design and Data | Total | Reliability Categorization |
|---|---|---|---|---|---|---|---|
| Basak and Prajna, 2016 [22] | 4 | 3 | 6 | 3 | 2 | 18 | Reliable without restrictions |
| Cid et al., 2021 [23] | 4 | 3 | 6 | 3 | 2 | 18 | Reliable without restrictions |
| Duncan et al., 2016 [24] | 4 | 3 | 6 | 3 | 2 | 18 | Reliable without restrictions |
| Greenbaum et al., 2004 [25] | 4 | 3 | 7 | 1 | 1 | 16 | Reliable without restrictions |
| Javadi et al., 2021 [26] | 4 | 4 | 6 | 3 | 2 | 19 | Reliable without restrictions |
| Kanavi et al., 2015 [27] | 4 | 3 | 6 | 3 | 2 | 18 | Reliable without restrictions |
| Layer et al., 2014 [28] | 4 | 3 | 6 | 2 | 2. | 17 | Reliable without restrictions |
| Li et al., 2012 [29] | 4 | 3 | 6 | 3 | 2 | 18 | Reliable without restrictions |
| Mistò et al., 2020 [30] | 4 | 3 | 6 | 3 | 2 | 18 | Reliable without restrictions |
| Nelson et al., 2000 [31] | 4 | 3 | 6 | 3 | 2 | 18 | Reliable without restrictions |
| Parekh et al., 2014 [13] | 4 | 3 | 6 | 3 | 2 | 18 | Reliable without restrictions |
| Perry et al., 2020 [32] | 4 | 3 | 6 | 3 | 2 | 18 | Reliable without restrictions |
| Smith et al., 1995 [8] | 4 | 3 | 6 | 3 | 2 | 18 | Reliable without restrictions |
| Tachibana and Sawa, 2001 [20] | 4 | 3 | 6 | 2 | 2 | 17 | Reliable without restrictions |
| Author Year | Country | Eye Bank/Source | Sample Type | Exposition Media | Sample Size (n) | Storage and Assessment Period (Days) | Storage Temperature | Parameters (Assessment Methods) | Study Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Basak and Prajna, 2016 [22] | India | Rotary Aravind Eye Bank, Madurai; Aravind Eye Bank, Coimbatore; and Prova Eye Bank, Barrackpore. | Human corneas | Cornisol | 64 | 3, 7, 10, 14 | 2–8 °C | Corneal structure grading (slit lamp and specular microscope/histopathology); endothelial cell vitality (alizarin red S and trypan blue); ECD and ECL (specular microscopy) | This study concludes that CS is as effective as OS for storing corneal tissues at 2–8 °C for 14 days. |
| Cid et al., 2021 [23] | Brazil | Eye Bank of the General Hospital of Fortaleza. | Human corneas | Coconut water-based solution | 28 | 0, 1, 3, 7 | 2–8 °C | Viability: osmolarity (specular microscopy and slit lamp biomicroscopy) | Coconut water-based preservative partially maintained corneal transparency and epithelial integrity, especially during the first three days of follow-up. The coconut water-based solutions used were not effective for use as preservatives in a human eye bank. |
| Duncan et al., 2016 [24] | USA | SightLife | Human Corneas | Optisol-GS with amphotericin B | 24 | 0, 7, 14 | 4 °C | Endothelial cell density—ECD (specular microscopy); percentage of intact epithelium (slit lamp); damaged or dead endothelial cells (trypan blue); cell borders and areas of denuded Descemet membrane (alizarin red) | This study confirmed the efficacy and safety of amphotericin B as an antifungal supplement in Optisol-GS. Although all concentrations of amphotericin B effectively eliminated fungal contaminants within 7 days, only the 0.255-μg/mL concentration eliminated all fungal contaminants within 2 days. |
| Greenbaum et al., 2004 [25] | Canada | Eye Bank of Canada (Ontario Division) Toronto, Canada | Human corneas | Dexsol | 24 | 1, 2, 4 | 4 °C | Loss of donor epithelium (light microscopy); epithelium damage: basement membrane, cellular integrity, intercellular junctions, and intracellular organelles (transmission electron microscopy) | Loss of donor epithelium is related mainly to the length of storage and is similar in both Optisol GS and Dexsol. The storage time should be less than 4 days. |
| Javadi et al., 2021 [26] | Iran | Central Eye Bank of Iran | Human corneas | Sinasol | 128 corneas (7 d); 59 corneas (14 d) | 7, 14 | 4 °C | ECD (specular microscopic) viability of the ECs (trypan blue staining) | The overall results indicate that Sinasol is a safe, effective, and affordable intermediate cold storage medium for preservation of corneas. |
| Kanavi et al., 2015 [27] | Iran | Central Eye Bank of Iran (CEBI) | Human corneas | Eusol | 180 | 1, 7 | 4 °C | Epithelial defects, stromal edema, Descemet’s folding and an endothelial rating (slit lamp biomicroscopy) ECD (specular microscopic) | There was no significant change between Optisol-GS and Eusol-C and none of them seem to be superior to another (day 1 to day 7). |
| Layer et al., 2014 [28] | USA | SightLife | Human corneas | OGS with amphotericin B or voriconazole | 30 | 0, 7, 14 | 2–8 °C | Change in epithelium (slit lamp); endothelial cell count ECC (specular microscopy); vital dye staining (trypan blue) | A low concentration of amphotericin B might be a safe and efficacious addition to storage media, and a larger study is warranted to confirm these findings. No difference in the percentage of nonviable endothelial cells between paired controls and antifungal-supplemented Optisol-GS. |
| Li et al., 2012 [29] | USA | Eye Bank Association of America | Human Corneas | OGS with linezolid; daptomycin; calcium | 10 | 0, 7 ECD 0, 10 DCT | 4 °C | ECD (specular microscopy); DCT (ultrasound pachymetry) | The addition of daptomycin to Optisol-GS significantly increases the anti-MRSA activity of the medium without any apparent negative effects on donor corneal tissue. |
| Mistò et al., 2020 [30] | Italy | Eye Bank of Monza | Human corneas | Kerasave with amphotericin B and Optisol. | 32 | 1, 7, 14 | 2–8 °C | CCT, ECD (Azul trypan, specular microscopy), corneal transparency, EC morphology (slit lamp biomicroscopy) and inverted-phase microscopy. | No differences were found in the qualitative (corneal transparency, EC morphology), and quantitative metrics. |
| Nelson et al., 2000 [31] | USA | Eye Bank Specular Microscope | Human corneas | Chen Medium | 18 | 7, 10, 14, 21 | 4 °C | CT, ECD (specular microscopy), scanning electron microscopy (SEM), | Corneas stored in CM were thicker during storage than those stored in OM. |
| Parekh et al., 2014 [13] | Italy | The Veneto Eye Bank Foundation | Human corneas | Cornea Cold | 60 | 7, 14, 21, 28 | 2–6 °C | CT, ECD, and morphology (optical microscopic evaluation); transparency (specific device) | Cornea Cold is a promising hypothermic corneal storage medium with preservation time until 21 days. |
| Perry et al., 2020 [32] | USA | Sight Eye Bank | Human corneas | Kerasave | 88 | 12 | 4 °C | ECD, CCT (slit lamp; specular microscope) | Kerasave should notice little difference when compared with Optisol-GS. This was not statistically significant. |
| Smith et al., 1995 [8] | USA | Los Angeles Doheny Eye Bank | Human corneas | OGS with gentamicin and streptomycin | 4 | 5 | 4 °C | Cytotoxicity; morphological or cell changes (SEM) | The shape and boundaries of the EC of each donor pair appeared to be similar. The quality of EC was poor, many cells were shrunken, and cytoplasm was separated from the plasma membranes, which were thick and irregular. |
| Tachibana and Sawa, 2002 [20] | Japan | - | Rabbit corneas | Medium with 2.5% chondroitin sulfate in different molecular weights: Medium I and II | 12 | 7, 14 | 4 °C | Cell morphology (SEM and TEM) | No significant difference in histological findings between Optisol-GS and test medium at days 5 and 10. On day 14, corneal endothelial cells with marked degeneration of intracellular organelles in both media at day 14. |
| Components | Optisol-GS | Kerasave [24,33] | Cornisol [21] | Cornea Cold Medium [11] | Eusol-C [36] | Chen Medium [20] | Dexsol [22] | Sinasol [26] |
|---|---|---|---|---|---|---|---|---|
| Base medium | Tissue culture medium 199, Eagle’s balance salt solution and MEM | MEM | MEM | MEM | MEM-Earle | Modified medium 199 | MEM | MEM |
| Buffer | HEPES | Sodium bicarbonate | HEPES | HEPES | HEPES | HEPES | HEPES | HEPES |
| Antibiotics | Gentamicin, streptomicin | gentamicin, streptomicin | Gentamicin, streptomicin | Yes | Gentamicin | Gentamicin, streptomicin | Gentamicin | Gentamicin, penicillin, streptomicin |
| Chondroitin sulfate | 2.5% | No | Yes | No | No | No | 1.35% | Yes |
| Dextran | T-40 | Yes | T-40 | T-500 | T-500 | Yes | T-40 | T-70 |
| ATP precursors | Yes | No | Yes | Yes | Yes | No | Yes | No |
| Sodium bicarbonate | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Ascorbic acid | Yes | No | No | No | No | No | No | No |
| Sodium pyruvate | Yes | No | No | Yes | Yes | No | Yes | No |
| Additional supplements | Yes | No | No | Yes | Yes | Yes | Yes | No |
| Constituent | Optisol with Supplements [5,23,29,35] | MEM with Supplements [18] | Coconut Water Solution [27] |
|---|---|---|---|
| Base medium | Tissue culture medium 199 Eagle’s balance salt solution and MEM | MEM | ACP-412 powdered coconut water |
| Buffer | HEPES | HEPES | Yes |
| Antibiotics | Gentamicin, streptomicin, linezolid and daptomycin | No | Gentamicin 200 μg/mL |
| Chondroitin sulfate | 2.5% | 2.5% | 2.5% |
| Dextran | T-40 | No | 1% |
| ATP precursors | Yes | No | Not reported |
| Sodium bicarbonate | Yes | Yes | Not reported |
| Ascorbic acid | Yes | No | Not reported |
| Sodium pyruvate | Yes | No | Not reported |
| Antifungal additives | Voriconazole or amphotericin B or vancomycin | No | Not reported |
| Additional supplements | α-tocopherol | α-tocopherol | Not reported |
| Author Year | Viable Endothelial Cell Density (ECD) (cells/mm2) * Optisol-GS | Viable Endothelial Cell Density (ECD) (cells/mm2) * Distinct Media |
|---|---|---|
| Basak and Prajna, 2016 [22] | Optisol-GS Day 1: 2868 ± 318 Day 3: 2664 ± 352 Day 7: 2599 ± 334 Day 10: 2482 ± 296 Day 14: 2417 ± 384 | Cornisol Day 1: 2731 ± 394; p = 0.065 Day 3: 2623 ± 356; p = 0.655 Day 7: 2475 ± 377; p = 0.177 Day 10: 2349 ± 398; p = 0.049 Day 14: 2256 ± 336; p = 0.110 |
| Duncan et al., 2016 [24] | Optisol-GS mean change in ECD 0–14 days, control cornea: 0.06 μg/mL: −191.5; p = 0.73 0.12 μg/mL: −206.84; p = 0.01 0.255: −46.75; p = 0.45 | Optisol-GS with amphotericin B 0.06 μg/mL: 29.92; p = 0.73 0.12 μg/mL: 114.25; p = 0.01 0.255: −288.42; p = 0.45 |
| Javadi et al., 2021 [26] | Optisol-GS comparison of 7 days: day 1: 2946 ± 457 day 7: 2835 ± 493; comparison of 14 days: day 1: 2282 ± 699 day 14: 2880 ± 383 | Sinasol comparison of 7 days: day 1: 2829 ± 423; p = 0.135 day 7: 2723 ± 419; p = 0.18; comparison of 14 days: day 1: 2804 ± 423; p = 0.782 day 14: 2245 ± 589; p = 0.851 |
| Kanavi et al., 2015 [27] | Optisol-GS baseline (3151 ± 612); p = 0.319 1 w (3058 ± 481); p = 0.319 | Eusol-C baseline (2925 ± 431); p = 0.319 1 w (2909 ± 474); p = 0.319 |
| Layer et al., 2014 * [28] | Optisol-GS Day 0 to Day 7 Voriconazole 50 × MIC: −72.8 (233.7); p = 0.6 Amphotericin B 0.25 × MIC: −509.9 (497.5); p = 0.74 Amphotericin B 0.5 × MIC: −107.6 (94.4); p = 0.27 Amphotericin B 1 × MIC: 84.3 (59.8); p = 0.07 Amphotericin B 10 × MIC: −99.9 (141.8); p = 0.04 Day 0 to Day 14 Voriconazole 50 × MIC: −182.3 (115.0); p = 0.41 Amphotericin B 0.25 × MIC: −1026.3 (670.3); p = 0.38 Amphotericin B 0.5 × MIC: −567.9 (296.3); p = 0.50 Amphotericin B 1 × MIC: −363.6 (770.8); p = 0.45 Amphotericin B 10 × MIC: −206.3 (233.9); p = 0.16 | Optisol-GS with amphotericin B or voriconazole Day 0 to Day 7 Voriconazole 50 × MIC: −4.3 (60.3); p = 0.6 Amphotericin B 0.25 × MIC: −567.3 (705.4), p = 0.74 Amphotericin B 0.5 × MIC: 180.9 (243.6); p = 0.27 Amphotericin B 1 × MIC: −138.4 (140.2); p = 0.07 Amphotericin B 10 × MIC: indeterminate Day 0 to Day 14 Voriconazole 50 × MIC: −259.2 (10.1); p = 0.41 Amphotericin B 0.25 × MIC: −291.8 (204.4); p = 0.38 Amphotericin B 0.5 × MIC: −362.7 (102.8); p = 0.50 Amphotericin B 1 × MIC: −473.1 (288.4); p = 0.45 Amphotericin B 10 × MIC: indeterminate |
| Li et al., 2012 [29] | Optisol-GS Pair 1: without daptomycin: 580 (day 0) without daptomycin: 783 (day 10) Pair 2: without daptomycin: 573 (day 0) without daptomycin: 635 (day 10) Pair 3: without daptomycin: 668 (day 0) without daptomycin: 682 (day 10) | Optisol-GS with linezolid, daptomycin, calcium Pair 1: with daptomycin: 592 (day 0); p > 0.05 with daptomycin: 715 (day 10); p > 0.05 Pair 2: with daptomycin: 552 (day 0); p > 0.05 with daptomycin: 610 (day 10); p > 0.05 Pair 3: with daptomycin: 649 (day 0); p > 0.05 with daptomycin: 642 (day 10); p > 0.05 |
| Mistò et al., 2020 [30] | Optisol-GS Keratoanalyzer baseline: ECD ranged from 2000 cells/mm2 to 3448 cells/mm2 Day 1: (2578 ± 96) Day 7: 2321 ± 145) Day 14: (2335 ± 128) Stocker method Day 1: (2481 ± 71); p = 0.8974 Day 14: (2050 ± 122); p = 0.5096 | Kerasave with amphotericin B and Optisol Keratoanalyzer baseline: ECD ranged from 2000 cells/mm2 to 3448 cells/mm2 Day 1: (2521 ± 82); p = 0.6567 Day 7: (2437 ± 58); p = 0.4767 Day 14: (2312 ± 98); p = 0.8863 Stocker method Day 1: (2469 ± 64); p = 0.8974 Day 14: (2150 ± 87); p = 0.5096 |
| Nelson et al., 2000 [31] | Optisol-GS baseline: 2603 ± 356 (n = 9); p = 0.44 Day 21: 2464 ± 243; p = 0.02 * (n = 9) | Chen medium baseline: 2544 ± 312 (n = 9); p = 0.44 Day 21: 2264 ± 217 (n = 7); p = 0.02 |
| Parekh et al., 2014 [13] | Optisol-GS baseline av 1900 (1500—2150) 1 w loss of 8.03% (± 6.6); 2 w loss increased 8.01% (± 6.5); 3 w loss 10.99 (± 10.03). 4 w loss 16.48 (± 13.5) | Cornea Cold baseline average of 1900 (1500–2150) p = 0.60 1 w loss of 2.94% (± 3.72) in Cornea Cold; p < 0.05 2 w loss increased 4.83% (±5.03); p < 0.05 3 w loss of 2.66% (±4.44) p < 0.05 4 w loss of 5.75% (±6.51) p < 0.05 |
| Perry et al., 2020 [32] | Optisol-GS baseline: 2918 ± 55 PK group: Day 1: 3052 ± 66 Day 6: 2976 ± 94 Day 12: 3060 ± 116 DSAEK group: Day 1: 2846 ± 91 Day 4: 2847 ± 115 Day 6: 2847 ± 71 DMEK group: Day 1: 2750 ± 121 Day 4: 2779 ± 145 Day 6: 2777 ± 140 | * Kerasave baseline: 2887 ± 69; p = 0.734 (n = 22) PK group (n = 10): Day 1: 3096 ± 77; p = 0.669 Day 6: 3025 ± 118 (n = 5); p = 0.756 Day 12: 2999 ± 132 (n = 5); p = 0.735 DSAEK group (n = 7): Day 1: 2740 ± 134; p = 0.527 Day 4: 2824 ± 174; (n = 6) p = 0.916 Day 6: 2810 ± 173; (n = 6)p = 0.844 DMEK group: (n = 5) Day 1: 2677 ± 82; p = 0.632 Day 4: 2814 ± 94 (n = 5); p = 0.843 Day 6: 2682 ± 74 (n = 5); p = 0.563 |
| Author Year | Central Corneal Thickness (CCT) (μm) (Mean ± sd) Optisol-GS | Central Corneal Thickness (CCT) (μm) (Mean ± sd) Distinct Media | ||||||
|---|---|---|---|---|---|---|---|---|
| Li et al., 2012 [29] | Days | (n) | CCT | p value | Days | (n) | CCT | p value |
| Optisol-GS Day 0: Optisol-GS without daptomycin Pair 1 | 2 | 580 (media of pairs) | Optisol-GS with linezolid, daptomycin, calcium Day 0: Optisol-GS with daptomycin Pair 1 | 2 | 592 | p > 0.05 | ||
| Day 10: Pair 1 | 2 | 783 (media of pairs | Day 10: Pair 1 | 2 | 715 | p > 0.05 | ||
| Day 0: Optisol without daptomycin Pair 2 | 2 | 573 | Day 0: Optisol with daptomycin Pair 2 | 2 | 552 | p > 0.05 | ||
| Day 10: Optisol-GS without daptomycin Pair 2 | 2 | 635 | Day 10: Optisol-GS with daptomycin Pair 2 | 2 | 610 | p > 0.05 | ||
| Day 0: Optisol without daptomycin Pair 3 | 2 | 668 | Day 0: Optisol with daptomycin Pair 3 | 2 | 649 | p > 0.05 | ||
| Day 10: Pair 3 | 2 | 682 | Day 10: Pair 3 | 2 | 642 | p > 0.05 | ||
| Day 0: Optisol-GS without daptomycin Pair 1 | 2 | 580 (media of pairs) | Day 0: Optisol-GS with daptomycin Pair 1 | 2 | 592 | p > 0.05 | ||
| Mistò et al., 2020 [30] | Optisol-GS baseline: 551 μm to 740 μm | Kerasave with amphotericin B and Optisol. baseline: 551 μm to 740 μm | (p = 0.026) | |||||
| 1 | 16 | (637 ± 10) | 1 | 16 | (629 ± 13) | 0.6281 | ||
| 7 | 16 | (640 ± 19) | 7 | 16 | (672 ± 15) | 0.1939 | ||
| 14 | 16 | (697 ± 19) | 14 | 16 | (717 ± 17) | 0.4543 | ||
| Nelson et al., 2000 [31] | Optisol-GS Day 0: | 9 | 0.65 ± 0.06 | Chen medium Day 0: | 9 | 0.69 ± 0.05 | 0.001 | |
| 7 | 9 | 0.59 ± 0.07 | 7 | 9 | 0.69 ± 0.06 | 0.0001 | ||
| 10 | 6 | 0.63 ± 0.03 | 10 | 6 | 0.73 ± 0.08 | 0.01 | ||
| 14 | 4 | 0.60 ± 0.02 | 14 | 4 | 0.87 ± 0.04 | 0.0001 | ||
| 21 | 2 | 0.69 ± 0.02 | 21 | 2 | 0.87 ± 0.03 | 0.02 | ||
| Day 0: | 9 | 0.65 ± 0.06 | Day 0: | 9 | 0.69 ± 0.05 | 0.001 | ||
| Parekh et al., 2014 [13] | Optisol-GS baseline: | 550 ± 50 | Cornea Cold baseline: | 550 ± 50 | ||||
| 7 | 12 | 8.5% (±8.0) increase | 7 | 12 | 6.3% (±8.2) | (p < 0.05) | ||
| 14 | 12 | 7.8% (±7.5 | 14 | 12 | 5.3% ± 7.33 | (p < 0.05) | ||
| 21 | 12 | 6.2% (±7.17); | 21 | 12 | 3.9% (±5.5) | (p < 0.05) | ||
| 28 | 12 | 3.84% (±6.15). | 28 | 12 | 2.86% (±6.28) | (p < 0.05) | ||
| Perry et al., 2020 [32] | Optisol-GS baseline: | 22 | 526 ± 10 | 0.006 | Kerasave baseline: | 22 | 571 ± 12 | 0.006 |
| Day 1:PK group | 522 ± 17 | 0.132 | Day 1:PK group | 563 ± 19 | 0.132 | |||
| Day 6: | 5 | 556 ± 30 | 0.311 | Day 6: | 5 | 596 ± 23 | 0.311 | |
| Day 12 | 5 | 594 ± 30 | 0.756 | Day 12 | 5 | 608 ± 32 | 0.756 | |
| DSAEK group: | 521 ± 18 | 0.161 | DSAEK group: | 561 ± 20 | 0.161 | |||
| Day 4: | 6 | 547 ± 19 | 0.078 | Day 4: | 6 | 600 ± 20 | 0.078 | |
| Day 6: | 6 | 115 ± 9 | 0.784 | Day 6: | 6 | 112 ± 10 | 0.784 | |
| DMEK group: | DMEK group: | |||||||
| Day 4: | 5 | 540 ± 18 | 0.072 | Day 4: | 5 | 602 ± 24 | 0.072 | |
| Day 6: | 5 | not reported | Day 6: | 5 | not reported | |||
| Author Year | Endothelial Cell (EC) Mortality (%)<2% Optisol-GS | Endothelial Cell (EC) Mortality (%) <2% Distinct Media | Study Conclusion |
|---|---|---|---|
| Basak and Prajna, 2016 [22] | Optisol-GS Endothelial cell loss (%) Day 3: 7.1 (n = 31) Day 7: 9.4 (n = 30) Day 10: 13.5 (n = 27) Day 14: 15.7 (n = 19) | Cornisol Endothelial cell loss (%) p = 0.1563 Day 3: 3.9; p = 0.759 Day 7: 9.4; p = 0.392 Day 10: 14.0; p = 0.637 Day 14: 17.4; p = 0.824 | This study concludes that CS is as effective as OS for storing corneal tissues at 2–8 °C. |
| Mistò et al., 2020 [30] | Optisol-GS %EC mortality (n = 16) Central cornea: Day 14: 0.14; p = 0.0029 Peripheral cornea: Day 1: 0.38 ± 0.20 Day 14: 3.38% ± 0.78%; p = 0.002 | Kerasave with amphotericin B and Optisol. %EC mortality (n = 16) Central cornea: Day 14: 0.54; p = 0.021 Peripheral cornea: Day 1: 0.56 ± 0.34; p = 0,97 Day 14: 3.07% ± 0.93%; p = 0.033 | Day 14, EC mortality (3.07% ± 0.93% in Kerasave and 3.38% ± 0.78% in Optisol-GS) was higher in both groups as compared to the initial values (p = 0.033 for corneas in Kerasave and p = 0.002 in Optisol-GS); it was comparable between groups (p = 0.62). Day 14, the extent of mortality was higher in the peripheral area of corneas than in the central area for both storage groups (p = 0.021 for Kerasave and p = 0.0029 for Optisol-GS), whereas it was comparable at the initial evaluation (p = 0.17 for Kerasave and p = 0.15 for Optisol-GS). |
| Nelson et al., 2000 [31] | Optisol-GS Cell loss (%) (n = 9) After 21 days: 5 ± 5 | Chen medium Cell loss (%) (n = 9) After 21 days: 11 ± 10; p = 0.18 | The two storage media did not differ with respect to endothelial cell loss during storage or to the percentage of TUNEL-positive cells or keratocyte density at the end of the storage period. |
| Author Year | Endothelial Cell (EC) morphology Optisol-GS | Endothelial Cell (EC) Morphology Distinct Media | Study Conclusion |
|---|---|---|---|
| Cid et al., 2021 [23] | Optisol-GS accentuated corneal edema in all groups after 7 days. | Coconut water-based solution accentuated corneal edema in all groups after 7 days. | The preservative solution with coconut water was not effective for human eye bank use. |
| Greenbaum et al., 2004 [25] | Optisol-GS Day 1: All corneas showed progressive epithelial damage. Cells exhibited mild separation and some began to fall off. Some epithelial cells were flattened and all were tightly adherent with a normal appearance of the cytoplasm and nucleus. Day 2: Cells exhibited less defined cell shapes and borders. Sloughing of the external medium and epithelial cell layers. The epithelium was reduced in thickness and was composed generally of two to three cell layers. The basal cell layer appeared normal with intact cell junctions and preserved intracelular organelles; this layer remained attached to the basement membrane. Day 4: All superficial epithelial cell layers were lost and were left with only a basal cell layer, which in some sections showed a mild separation from the basement membrane. Some deep squamous cells sent projections between two adjacent basal cells, reaching the basal membrane, and even separating the basal surface of the cell from the basement membrane. The basal cells did, however, contain normal cellular organelles. | Dexsol Medium Day 1: All corneas showed progressive epithelial damage. Cells exhibited mild separation and some began to fall off. Some epithelial cells were flattened and all were tightly adherent with a normal appearance of the cytoplasm and nucleus. Day 2: Cells exhibited less defined cell shapes and borders. Sloughing of the external medium and epithelial cell layers. The epithelium was reduced in thickness and was composed generally of two to three cell layers. The basal cell layer appeared to be normal with intact cell junctions and preserved intracelular organelles; this layer remained attached to the basement membrane. Day 4: All superficial epithelial cell layers were lost and were left with only a basal cell layer, which in some sections showed a mild separation from the basement membrane. Some deep squamous cells sent projections between two adjacent basal cells, reaching the basal membrane and even separating the basal surface of the cell from the basement membrane. The basal cells did, however, contain normal cellular organelles. | According to this study, Optisol appears to be no more effective than Dexsol in preserving the integrity of human corneal epithelium. |
| Javadi et al., 2021 [26] | Optisol-GS: Day 1: stromal edema not mild: 28 (100%); p > 0.999 moderately severe: 0 (0.0%); p > 0.999 Descemet’s folding: not mild: 27 (96.4%); p = 0.038 moderately severe: 1 (3.6%); p = 0.038 Day 14: stromal edemanot mild: 12 (42.9%); p > 0.999 moderately severe: 16 (57.1%); p > 0.999 Descemet’s folding:not mild: 0 (0.0%); p > 0.999 moderately severe: 28 (100.0%); p > 0.999 | Sinasol: Day 1: stromal edema not mild: 30 (96.8%); p > 0.999 moderately severe: 1 (3.2%); p > 0.999 Descemet’s folding: not mild: 23 (74.2%); p = 0.038 moderately severe: 8 (25.8%); p = 0.038 Day 14: stromal edema not mild: 10 (32.3%); p = 0.488 moderately severe: 21 (67.7%); p = 0.488 Descemet’s folding: not mild: 0 (0.0%); p > 0.999 moderately severe: 31 (100.0%); p > 0.999 | Our study demonstrated no significant difference in the mean area of dead ECs and denuded Descemet’s membrane between the two storage media after 7- and 14-day periods. |
| Kanavi et al., 2015 [27] | Optisol-GS stromal edema: Day 1: no edema: 85 (100.0%); p = 0.554 mild edema: 0 (0.0%); p = 0.554 mod. edema: 0 (0.0%); p = 0.554 severe edema: 0 (0.0%); p = 0.554 Day 7: no edema: 83 (97.6%); p = 0.554 mild edema: 2 (2.4%); p = 0.554 mod. edema: 0 (0.0%); p = 0.554 severe edema: 0 (0.0%); p = 0.554 Descemet’s folding: Day 1: significant DF: 0 (0.0%); p = 0.325 non-significant DF: 85 (100.0%); p = 0.325 significant vac: 10 (11.8%); p = 0.687 non-significant vac: 75 (88.2%); p = 0.687 Day 7: significant DF: 4 (4.7%); p = 0.325 non-significant DF: 81 (95.3%); p = 0.325 significant vac: 30 (35.3%); p = 0.687 non-significant vac: 55 (64.7%); p = 0.687 | Eusol-C stromal edema: Day 1: no edema: 86 (100.0%); p = 0.554 mild edema: 0 (0.0%); p = 0.554 mod. edema: 0 (0.0%); p = 0.554 severe edema: 0 (0.0%); p = 0.554 Day 7: no edema: 85 (98.8%); p = 0.554 mild edema: 1 (1.2%); p = 0.554 mod. edema: 0 (0.0%); p = 0.554 severe edema: 0 (0.0%); p = 0.554 Descemet’s folding: Day 1: significant DF: 0 (0.0%); p = 0.325 non-significant DF: 86 (100.0%); p = 0.325 significant vac: 16 (18.6%); p = 0.687 non-significant vac: 70 (81.4%); p = 0.687 Day 7: significant DF: 19 (22.1%); p = 0.325 non-significant DF: 67 (77.9%); p = 0.325 significant vac: 31 (36.9%); p = 0.687 non-significant vac: 53 (63.1%); p = 0.687 | In conclusion, the changes of overall cornea rating, endothelial cell indices, stromal edema, and Descemet’s folding from Day 1 to Day 7 were not significantly different between Optisol-GS and Eusol-C and none of them seem to be superior to another. |
| Mistò et al., 2020 [30] | Optisol-GS: polymorphism: mild/medium and altered, n Day 1: 10/6 (16)); p < 0.05 Day 14: 5/10 (15); p < 0.05 endothelial cell borders homogeneous and partly homogeneous/irregular and altered, n Day 1: 5/11 (16); p < 0.05 Day 14: 1/12 (13); p < 0.05 | Kerasave: polymorphism: mild/medium and altered, n Day 1: 11/5 (16); p < 0.05 Day 14: 5/10 (15); p < 0.05 endothelial cell borders homogeneous and partly homogeneous/irregular and altered, n Day 1: 5/11 (16); p < 0.05 Day 14: 0/14 (14); p < 0.05 | No significant differences were found in all corneal gradings between Day 1 and Day 14 (all p values > 0.05). Ten corneas stored in Optisol-GS (out of 16 = 62.5%) and five in Kerasave (out of 16 = 33.3%) changed transparency grade over time without significant difference. Both groups showed increased polymorphism on Day 14, yet without statistically significant difference between groups. In both groups, EC borders showed an increase in irregularities after 14-day storage, without significant difference between groups. |
| Nelson et al., 2000 [31] | Optisol-GS baseline: coefficient of variation of cell size: 0,27 ± 0.04; p = 0.02 hexagonal cell (%): 64 ± 9; p = 0.02 Day 14: coefficient of variation of cell size: 0.31 ± 0.05; p = 0.65 hexagonal cell (%): 63 ± 9; p = 0.48 | Chen medium baseline: coefficient of variation of cell size: 0.28 ± 0.05; p = 0.65 hexagonal cell (%): 63 ± 8; p = 0.48 Day 14: coefficient of variation of cell size: 0.32 ± 0.05; p = 0.65 hexagonal cell (%): 62 ± 4; p = 0.48 | Corneas stored in CM were thicker during storage than those stored in OGS. SEM of the endothelium of four paired corneas after storage in CM and OGS. The endothelium appears intact in all corneas. The abnormal appearance of the endothelium after storage in CM for 14 and 21 days may be related to the dramatic increase in stromal swelling. |
| Parekh et al., 2014 [13] | Optisol-GS low level of cells: 1 w: 1.6% (±10.4) with statistical significance (p < 0.05) 2 w: 4.5% (±10.7) (p < 0.05) 3 w: 5.4% (±8.3) (p < 0.05) 4 w: 4.38% (±9.8) (p < 0.05) | Cornea Cold medium low level of cells: 1 w: 14.5% (±14.86) with statistical significance (p < 0.05) 2 w: 8.9% (±11.2) (p < 0.05) 3 w: 12.9% (±15.6) (p < 0.05) 4 w: 14.75 (±15.2) (p < 0.05) | Cornea Cold is a promising hypothermic corneal storage medium with preservation time until 21 days. |
| Tachibana and Sawa, 2002 [20] | Optisol-GS Day 7: in cornea stored in Optisol-GS, SEM revealed distinctive corneal endothelial cell boundaries and TEM showed almost normal findings in intracellular organelles. Day 14: In cornea stored in Optisol-GS or Medium l, cell boundaries became blurred in several places and vacuoles in the cytoplasm were observed. These findings indicated that corneal endothelial cells were well preserved in their morphological aspects. | MEM with 2.5% chondroitin sulfate with different molecular weights in Medium I and Medium II Day 7: in cornea stored in Medium I, SEM revealed distinctive corneal endothelial cell boundaries and TEM showed almost normal findings in intracellular organelles. Day 14: Medium I could be similarly potent and maintain morphological characteristics as well as Optisol-GS in a 14-day preservation period. Medium II Day 7: cornea in Medium II showed a paving-stone-like bulging of endothelial cell surface but had almost normal intracellular organelles.Day 14: Cornea in Medium II demonstrated irregularly shrunken cell surfaces by SEM examination, and the degenerated cells showed less staining pattern, destroyed cristae in mitochondria, and aggregation of intranuclear chromatin by TEM examination. Cornea stored in Medium II showed greater deterioration than the corneas stored in the other two media. | In the present morphological study, a novel formulated solution with simple ingredients showed a capability for corneal preservation similar to Optisol-GS. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gimenes, I.; Pintor, A.V.B.; da Silva Sardinha, M.; Marañón-Vásquez, G.A.; Gonzalez, M.S.; Presgrave, O.A.F.; Maia, L.C.; Alves, G.G. Cold Storage Media versus Optisol-GS in the Preservation of Corneal Quality for Keratoplasty: A Systematic Review. Appl. Sci. 2022, 12, 7079. https://doi.org/10.3390/app12147079
Gimenes I, Pintor AVB, da Silva Sardinha M, Marañón-Vásquez GA, Gonzalez MS, Presgrave OAF, Maia LC, Alves GG. Cold Storage Media versus Optisol-GS in the Preservation of Corneal Quality for Keratoplasty: A Systematic Review. Applied Sciences. 2022; 12(14):7079. https://doi.org/10.3390/app12147079
Chicago/Turabian StyleGimenes, Izabela, Andréa V. Braga Pintor, Mariana da Silva Sardinha, Guido A. Marañón-Vásquez, Marcelo Salabert Gonzalez, Octavio Augusto França Presgrave, Lucianne Cople Maia, and Gutemberg Gomes Alves. 2022. "Cold Storage Media versus Optisol-GS in the Preservation of Corneal Quality for Keratoplasty: A Systematic Review" Applied Sciences 12, no. 14: 7079. https://doi.org/10.3390/app12147079






