Corrosion Diagnostics Performed on Cores Drilled from Concrete Structures, Using the Laboratory Simulation of Temperature and Relative Humidity Impact
Abstract
:1. Introduction
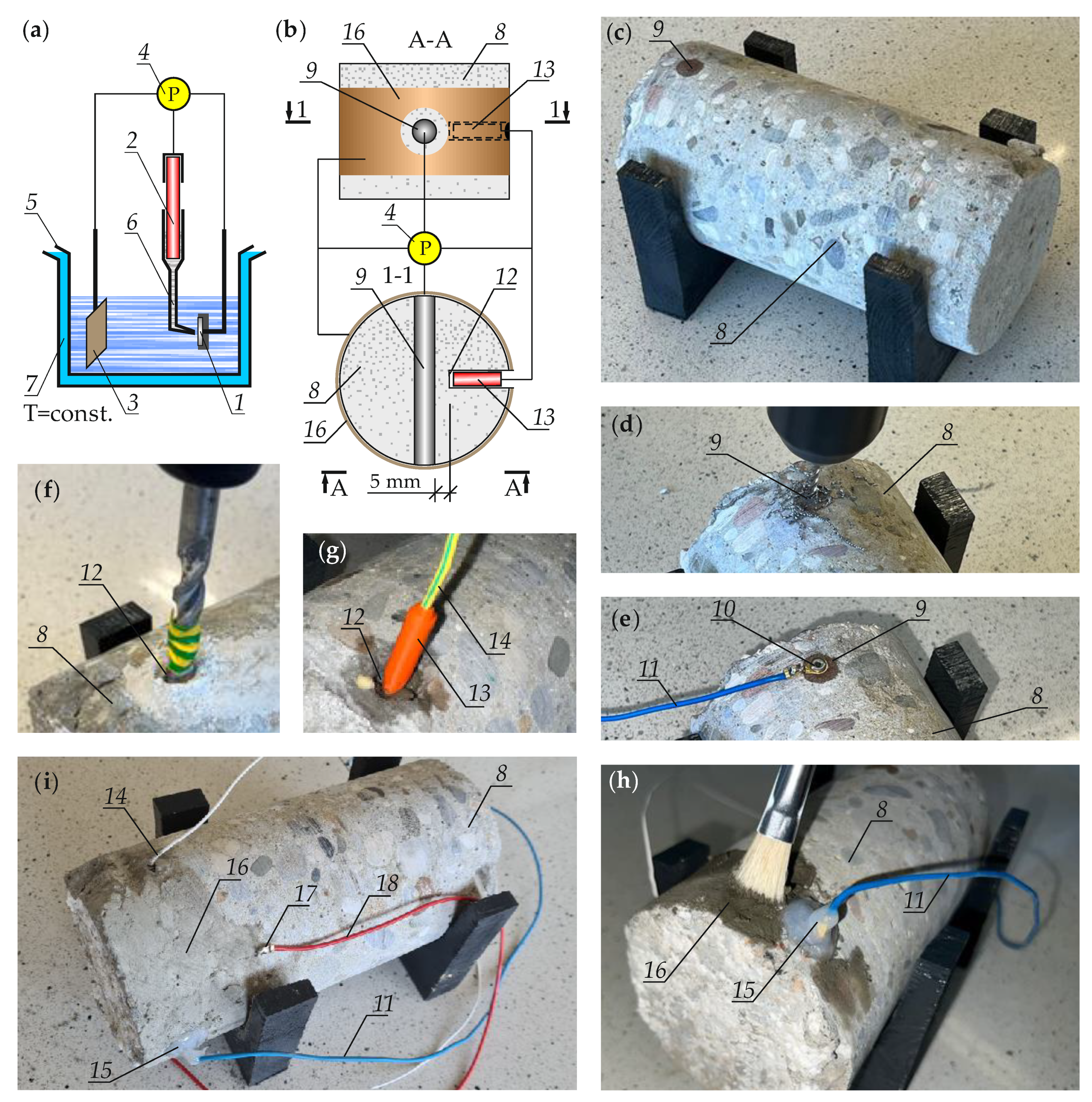
2. Methodology for Measuring the Rate of Corrosion Reinforcement in Cores Drilled from The Structure
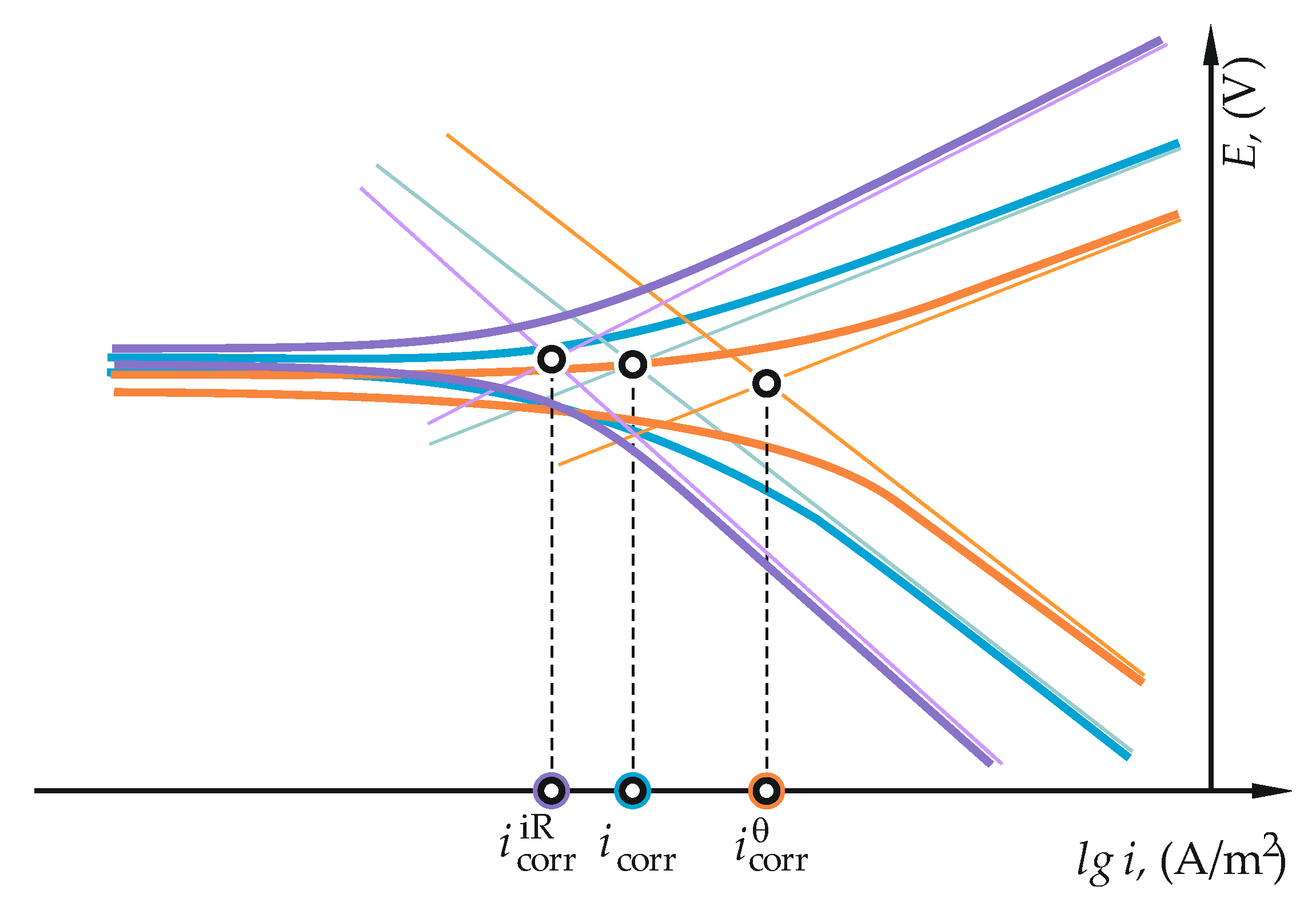
2.1. Measuring Corrosion Rate by Electrochemical Methods
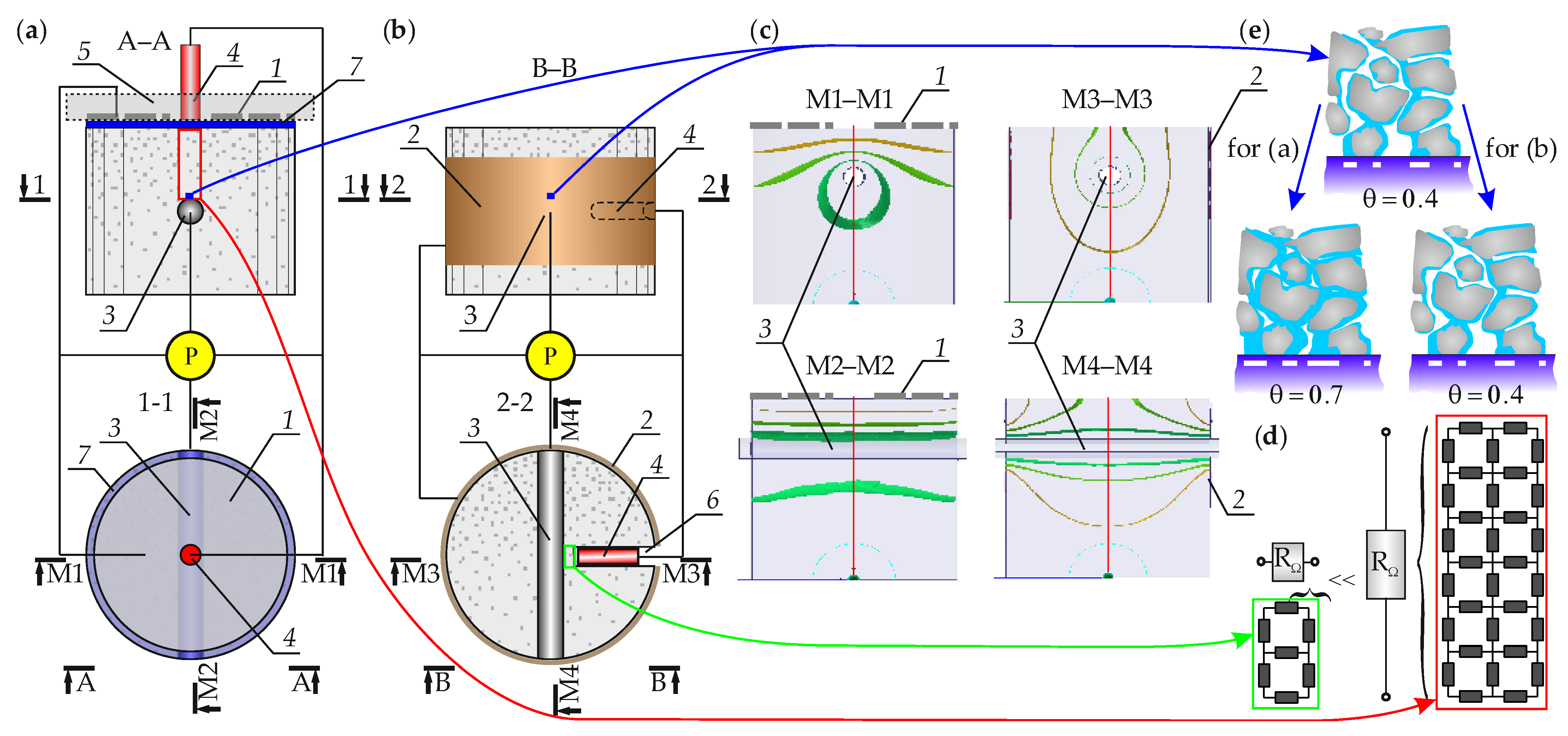
2.2. Specification for Development of the Three-Electrode System for Concrete Cores
2.3. Methodology of Measuring Corrosion Rate in the Reinforcement of Cores in the Climate Chamber
3. Validation of the Developed Methodology in Testing Reinforced Concrete Structures without Clear Signs of Corrosion Degradation
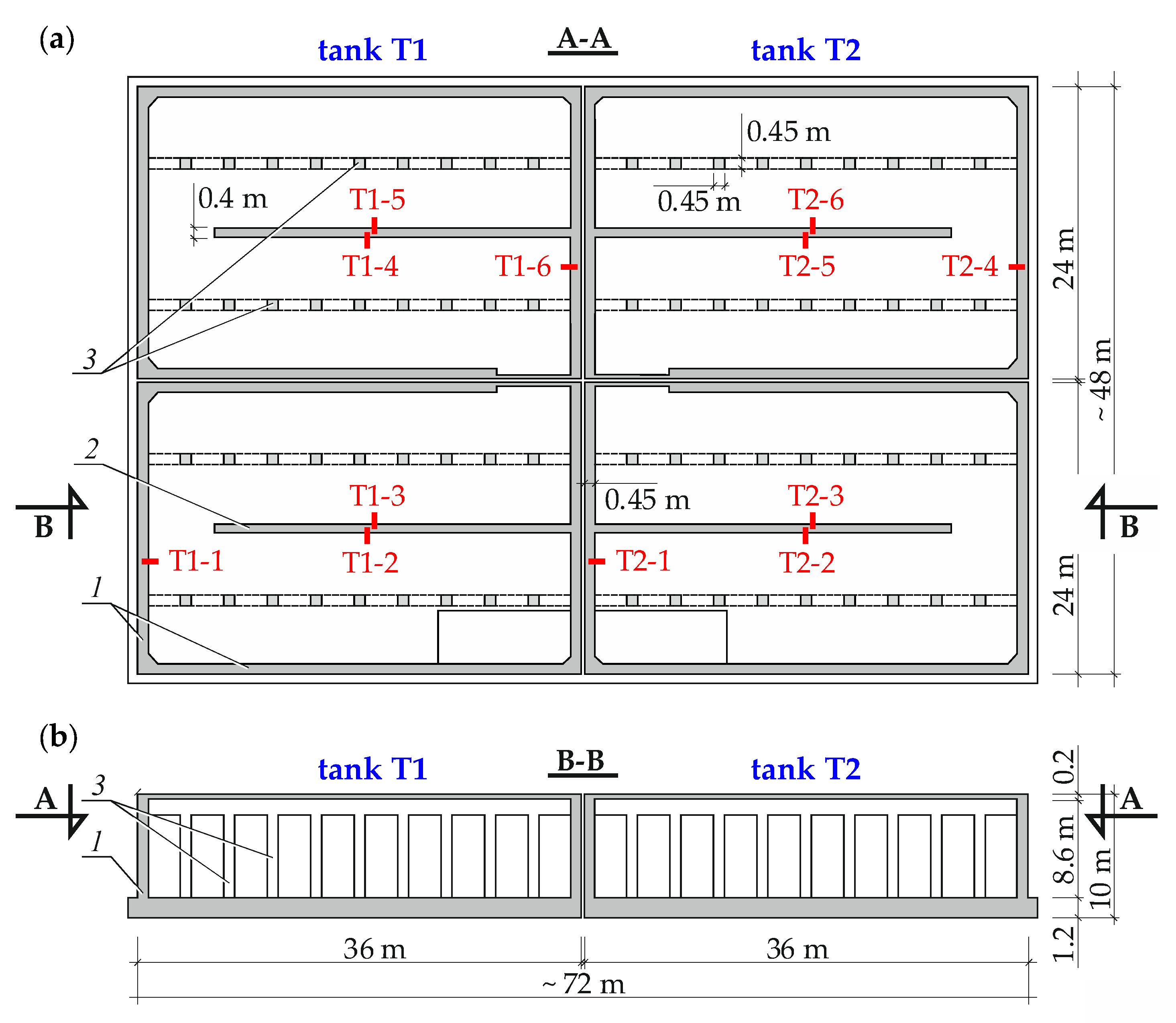
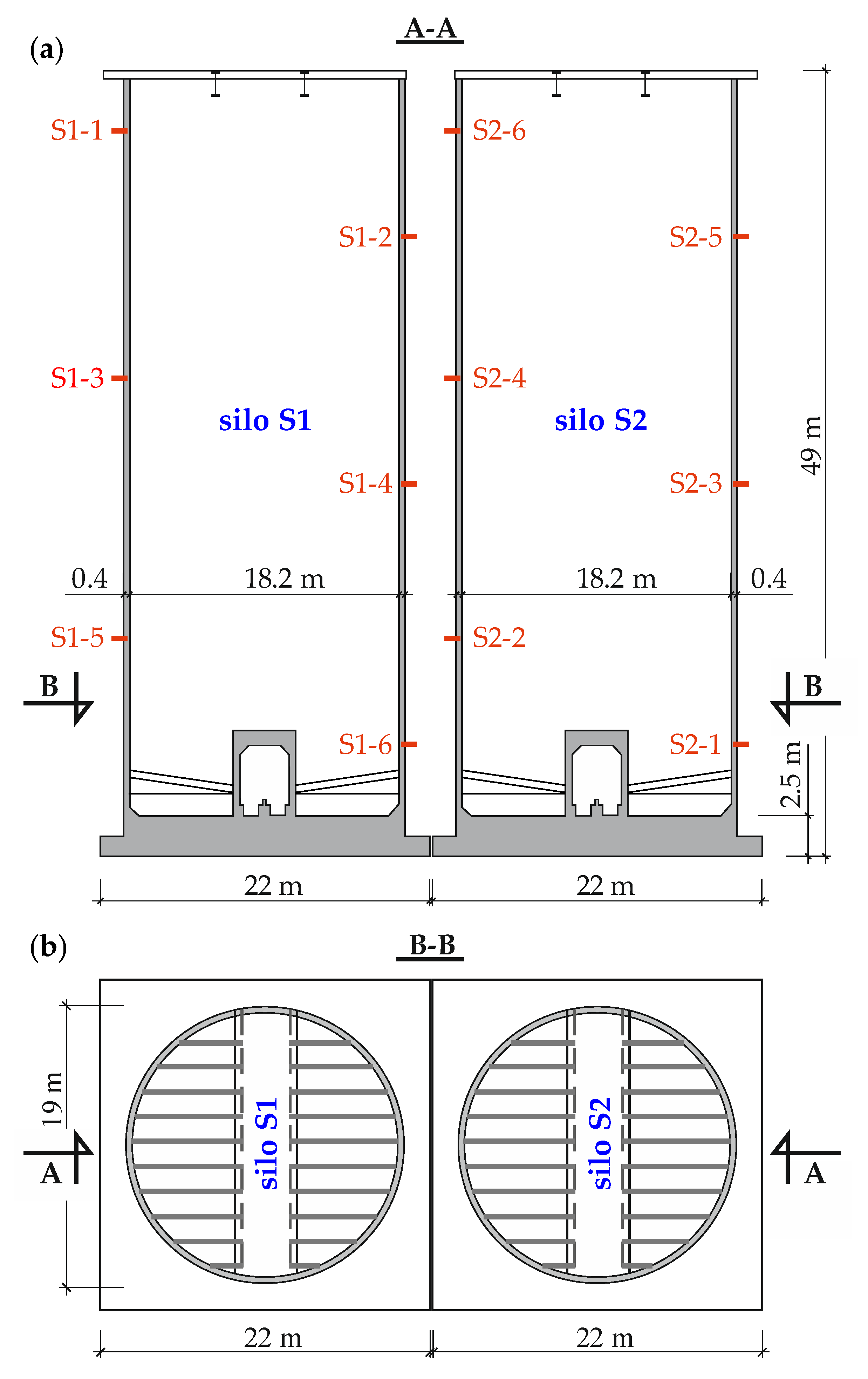
3.1. Description of the Tested Structure and Its Condition
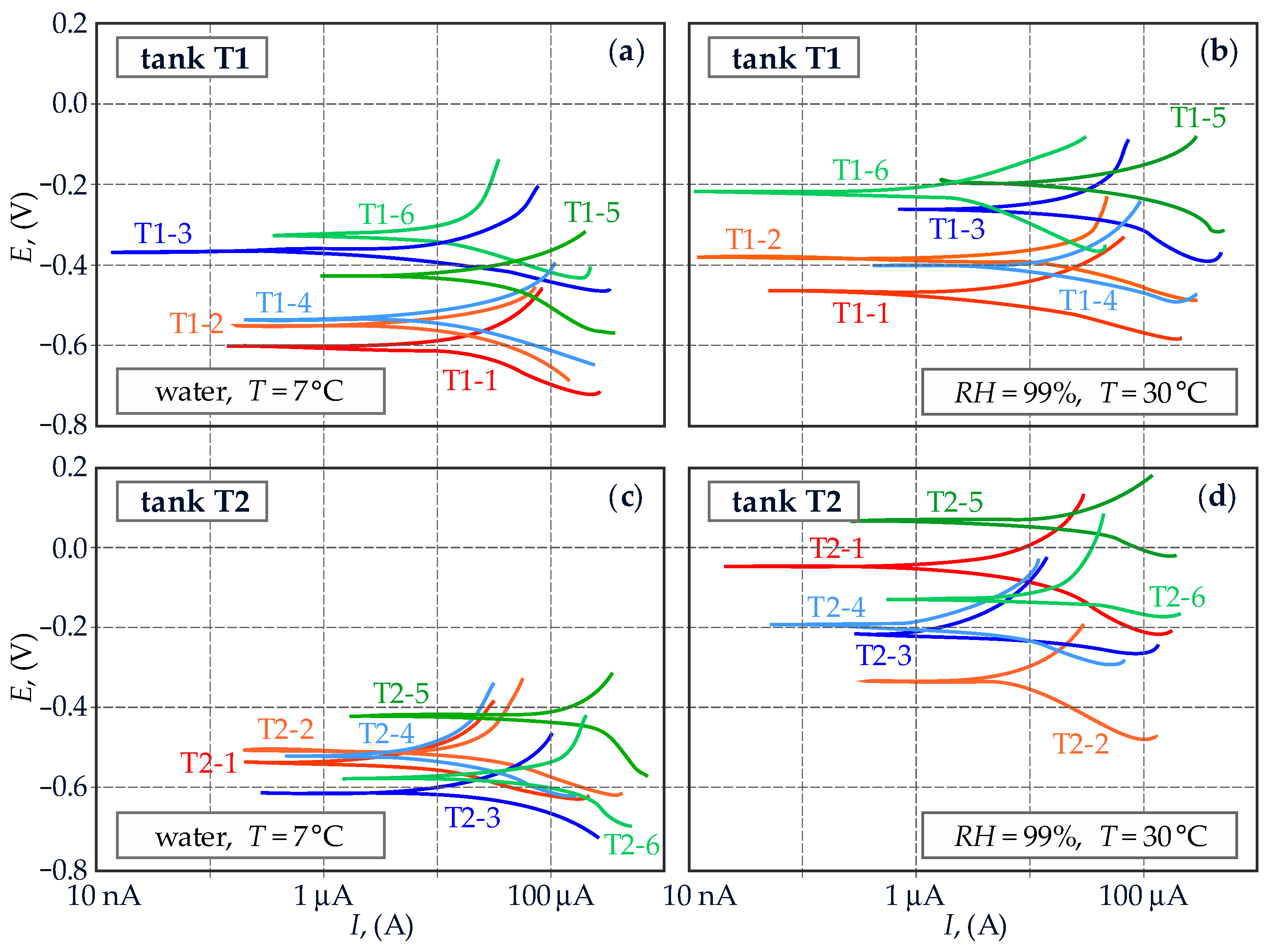
3.2. Measurements of Reinforcement Corrosion Using the Method of Linear Polarization Resistance (LPR)
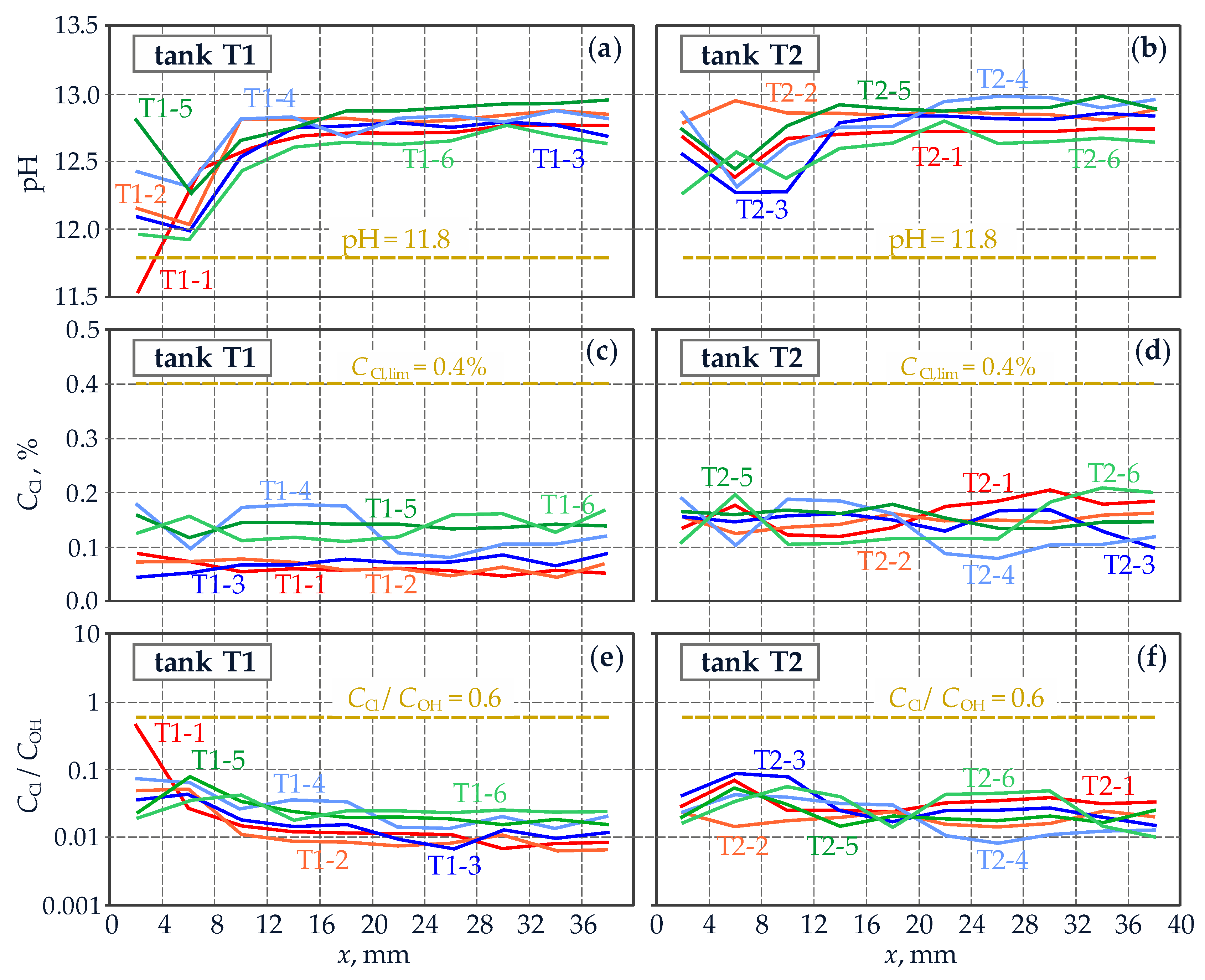
3.3. Testing Protective Properties of Concrete against Reinforcing Steel
4. Validation of the Developed Methodology in Testing Reinforced Concrete Structures with Clear Signs of Corrosion Degradation
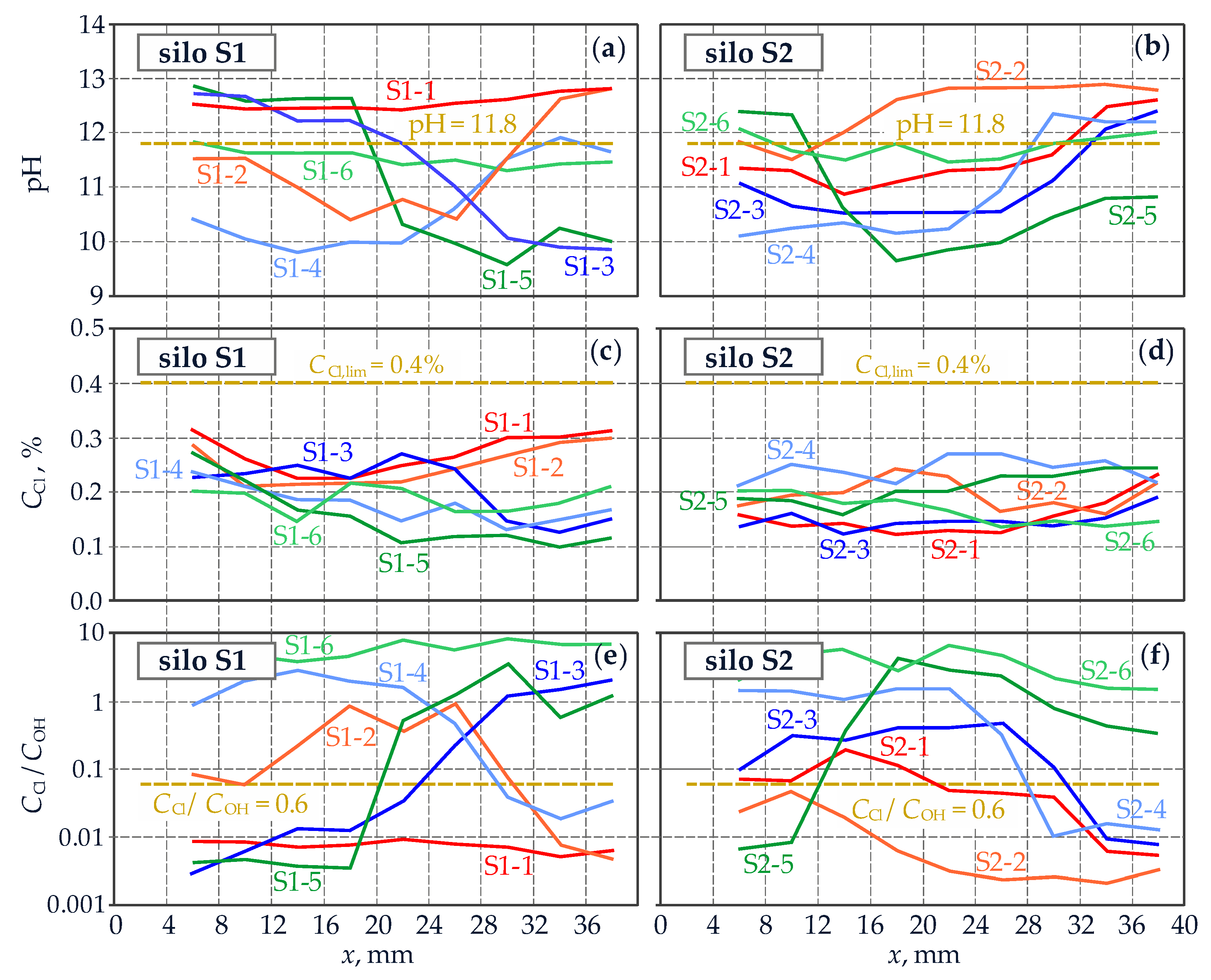
4.1. Description of the Tested Structure and Its Condition
4.2. Measurements of Reinforcement Corrosion Using the Method of Linear Polarization Resistance (LPR)
4.3. Testing Protective Properties of Concrete against Reinforcing Steel
5. Discussion
5.1. Comparative Analysis of Test Results for Two Different Structures with Reference to the Applied Diagnostics Method
5.2. Assessment of Benefits of the Modified Testing Methodology
6. Conclusions
- The traditional in-situ diagnostics testing of corrosion using the electrochemical methods (measurements of potential, resistivity, polarization) provide valuable information; however, they only indicate the temporary electrochemical state of the reinforced concrete structure, which depends on thermal and humidity conditions at the moment of measurements;
- Generally, diagnostics processes for reinforced concrete structure conducted on large areas of concrete, and/or at high altitudes, and/or in difficult-to-access areas are troublesome and dangerous. Hence, drilling cores with fragments of the secondary reinforcement (e.g., spacer bars, stirrups, binders) from the structure greatly improve safety and comfort of tests by conducting them in the laboratory conditions. There, polarization tests on corrosion rate of reinforcement, and tests on protective properties of concrete against reinforcing steel can be performed as well;
- The extreme values of corrosion current density can be determined when extreme thermal and humidity parameters are set in the climate chamber while testing the rate of corrosion current for reinforcement in the tested cores. Selection of temperature and relative humidity in the climate chamber should be based on the analysis of historical weather and operational data from a few years, concerning the location of the tested civil structure. The set of values of corrosion current density obtained in that way, after conversion into corrosion rate values, can be included in the mechanical models representing degradation of the tested reinforced concrete structures, which provides more precise estimation of the remaining service life of this structure;
- The arrangement of the three-electrode system on the drilled concrete cores should minimize the measuring errors. Therefore, the counter electrode in the form of conductive coating applied on the side wall of the cylindrical core with the painting technique is the novelty proposed by the authors. The main advantage of this solution is the constant electric contact between the coating and the core concrete, which does not require any additional conductive medium that changes electric properties of concrete. The second improvement of the three-electrode system consists in using the solid reference electrode embedded in cement grout in the opening with bottom placed very close the working electrode. This solution minimizes the problem related to resistance compensation and increases stability of the results while polarization curves are recorded during the tests on corrosion rate. Moreover, cores with fragments of rebars which are used as working electrodes in the three-electrode system, drilled from the structure cause that the polarization surface area can be precisely determined by directly measuring side walls of rebars after crushing the cores at the end of the corrosion tests;
- The described examples of corrosion tests performed on reinforced concrete tanks for fresh water and silos for cement storage confirmed the application possibilities of the diagnostics method based on the tests on drilled cores and improved by the authors. At satisfactory protective properties of concrete against reinforcement, changes in thermal and humidity parameters in the tanks did not produce any significant differences in corrosion rate, which was generally kept at low level. The results were different for the silos, where concrete carbonation decreased protective properties of concrete. Consequently, values of corrosion current density were high under conditions favourable for corrosion. Particular attention should be paid to the results obtained in conditions unfavourable for corrosion because in that case density of corrosion current of the reinforcement indicated passive state. In-situ measurements of corrosion rate taken in such conditions could lead to incorrect conclusions about corrosion of reinforcement in the silos.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Core | T | RH | Egraphite | ba | bc | B | Rp | RpAp | icorr |
|---|---|---|---|---|---|---|---|---|---|
| No. | (°C) | (%) | (V) | (mV) | (mV) | (mV) | (kΩ) | (kΩcm2) | (µA/cm2) |
| T1-1 | 30 | 100 | −0.432 | 125 | 29 | 10.2 | 2.51 | 88.4 | 0.12 |
| 7 | water | −0.546 | 153 | 43 | 14.6 | 1.10 | 38.8 | 0.38 | |
| T1-2 | 30 | 100 | −0.316 | 485 | 42 | 16.8 | 0.95 | 35.1 | 0.48 |
| 7 | water | −0.534 | 66 | 72 | 15.0 | 1.14 | 42.0 | 0.36 | |
| T1-3 | 30 | 100 | −0.182 | 337 | 52 | 19.6 | 0.85 | 42.2 | 0.46 |
| 7 | water | −0.303 | 183 | 23 | 8.9 | 1.43 | 70.6 | 0.13 | |
| T1-4 | 30 | 100 | −0.345 | 228 | 41 | 15.1 | 0.55 | 43.4 | 0.35 |
| 7 | water | −0.490 | 96 | 36 | 11.4 | 1.08 | 84.6 | 0.14 | |
| T1-5 | 30 | 100 | −0.169 | 80 | 69 | 16.1 | 0.34 | 56.5 | 0.28 |
| 7 | water | −0.414 | 71 | 66 | 14.9 | 0.40 | 67.5 | 0.22 | |
| T1-6 | 30 | 100 | −0.165 | 40 | 48 | 9.5 | 8.52 | 332 | 0.03 |
| 7 | water | −0.237 | 34 | 74 | 10.1 | 1.94 | 75.8 | 0.13 |
| Core | T | RH | Egraphite | ba | bc | B | Rp | RpAp | icorr |
|---|---|---|---|---|---|---|---|---|---|
| No. | (°C) | (%) | (V) | (mV) | (mV) | (mV) | (kΩ) | (kΩcm2) | (µA/cm2) |
| T2-1 | 30 | 100 | 0.052 | 141 | 43 | 14.3 | 3.18 | 83.7 | 0.17 |
| 7 | water | −0.437 | 170 | 30 | 11.1 | 2.31 | 61.1 | 0.18 | |
| T2-2 | 30 | 100 | −0.270 | 141 | 69 | 20.1 | 1.18 | 31.4 | 0.64 |
| 7 | water | −0.428 | 262 | 36 | 13.7 | 1.17 | 31.2 | 0.44 | |
| T2-3 | 30 | 100 | −0.080 | 142 | 11 | 4.4 | 0.55 | 14.5 | 0.31 |
| 7 | water | −0.554 | 60 | 44 | 11.0 | 0.50 | 13.2 | 0.84 | |
| T2-4 | 30 | 100 | −0.109 | 162 | 31 | 11.3 | 5.17 | 13.7 | 0.08 |
| 7 | water | −0.435 | 127 | 29 | 10.3 | 1.94 | 51.3 | 0.20 | |
| T2-5 | 30 | 100 | 0.098 | 78 | 56 | 14.2 | 0.48 | 72.5 | 0.20 |
| 7 | water | −0.398 | 140 | 140 | 30.4 | 0.30 | 45.2 | 0.67 | |
| T2-6 | 30 | 100 | 0.006 | 213 | 21 | 8.3 | 0.47 | 36.1 | 0.23 |
| 7 | water | −0.515 | 378 | 97 | 33.5 | 0.64 | 49.0 | 0.68 |
| Core | T | RH | Egraphite | ba | bc | B | Rp | RpAp | icorr |
|---|---|---|---|---|---|---|---|---|---|
| No. | (°C) | (%) | (V) | (mV) | (mV) | (mV) | (kΩ) | (kΩcm2) | (µA/cm2) |
| S1-1 | 30 | 100 | −0.283 | 192 | 43 | 15.3 | 0.37 | 20.8 | 0.73 |
| 13 | 40 | −0.169 | 185 | 31 | 11.5 | 2.88 | 160 | 0.07 | |
| S1-2 | 30 | 100 | −0.421 | 50 | 64 | 12.2 | 0.22 | 13.2 | 0.93 |
| 13 | 40 | −0.356 | 43 | 18 | 5.5 | 2.32 | 138 | 0.04 | |
| S1-3 | 30 | 100 | −0.135 | 132 | 41 | 13.6 | 0.16 | 9.57 | 1.42 |
| 13 | 40 | +0.008 | 41 | 76 | 11.6 | 2.38 | 142 | 0.08 | |
| S1-4 | 30 | 100 | −0.098 | 106 | 73 | 18.8 | 1.21 | 69.8 | 0.27 |
| 13 | 40 | +0.024 | 121 | 18 | 6.8 | 4.19 | 241 | 0.03 | |
| S1-5 | 30 | 100 | −0.467 | 56 | 56 | 12.2 | 0.99 | 58.6 | 0.21 |
| 13 | 40 | −0.386 | 70 | 48 | 12.4 | 2.60 | 154 | 0.08 | |
| S1-6 | 30 | 100 | −0.383 | 171 | 49 | 16.5 | 0.39 | 22.4 | 0.74 |
| 13 | 40 | −0.238 | 117 | 51 | 15.4 | 2.66 | 152 | 0.10 |
| Core | T | RH | Egraphite | ba | bc | B | Rp | RpAp | icorr |
|---|---|---|---|---|---|---|---|---|---|
| No. | (°C) | (%) | (V) | (mV) | (mV) | (mV) | (kΩ) | (kΩcm2) | (µA/cm2) |
| S2-1 | 30 | 100 | +0.001 | 36.7 | 31.1 | 7.3 | 1.41 | 65.4 | 0.11 |
| 13 | 40 | +0.080 | 67 | 39 | 10.7 | 4.00 | 185 | 0.06 | |
| S2-2 | 30 | 100 | −0.267 | 409 | 88 | 31.4 | 0.95 | 55.2 | 0.57 |
| 13 | 40 | −0.206 | 57 | 128 | 17.1 | 20.99 | 1222 | 0.01 | |
| S2-3 | 30 | 100 | −0.284 | 15.6 | 43.9 | 5.0 | 1.43 | 72.3 | 0.07 |
| 13 | 40 | −0.113 | 56 | 64 | 13.0 | 12.44 | 629 | 0.02 | |
| S2-4 | 30 | 100 | −0.466 | 90 | 89 | 19.4 | 0.27 | 16.2 | 1.20 |
| 13 | 40 | −0.330 | 25 | 236 | 9.8 | 32.63 | 1931 | 0.01 | |
| S2-5 | 30 | 100 | −0.333 | 178 | 44 | 15.3 | 0.26 | 15.3 | 1.00 |
| 13 | 40 | −0.381 | 236 | 34 | 12.9 | 0.56 | 33.7 | 0.38 | |
| S2-6 | 30 | 100 | −0.396 | 142 | 46 | 15.1 | 0.24 | 11.9 | 1.27 |
| 13 | 40 | −0.006 | 64 | 60 | 13.4 | 8.04 | 404 | 0.04 |
References
- Stefanoni, M.; Angst, U.; Elsener, B. Corrosion rate of carbon steel in carbonated concrete—A critical review. Cem. Concr. Res. 2018, 103, 35–48. [Google Scholar] [CrossRef]
- Marciniak, A.; Grymin, W.; Margiewicz, T.; Koniorczyk, M. Influence of freezing-induced damage on the carbonation rate of concrete. Eur. J. Environ. Civ. Eng. 2016, 21, 1–16. [Google Scholar] [CrossRef]
- Liu, X.; Niu, D.; Li, X.; Lv, Y.; Fu, Q. Pore solution pH for the corrosion initiation of rebars embedded in concrete under a long-term natural carbonation reaction. Appl. Sci. 2018, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Cheng, X.; Li, X.; Zhou, C.; Tan, H. Effect of carbonation on the electrochemical behavior of corrosion resistance low alloy steel rebars in cement extract solution. Constr. Build. Mater. 2017, 130, 193–201. [Google Scholar] [CrossRef]
- Al-Saleh, S.A. Analysis of total chloride content in concrete. Case Stud. Constr. Mater. 2015, 3, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Ann, K.Y.; Song, H.W. Chloride threshold level for corrosion of steel in concrete. Corros. Sci. 2007, 49, 4113–4133. [Google Scholar] [CrossRef]
- Figueira, R.B. Electrochemical sensors for monitoring the corrosion conditions of reinforced concrete structures: A review. Appl. Sci. 2017, 7, 1157. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S. Reinforcement corrosion in concrete structures, its monitoring and service life prediction—A review. Cem. Concr. Compos. 2003, 25, 459–471. [Google Scholar] [CrossRef]
- Montemor, M.F.; Alves, J.H.; Simões, A.M.; Fernandes, J.C.S.; Lourenço, Z.; Costa, A.J.S.; Appleton, A.J.; Ferreira, M.G.S. Multiprobe chloride sensor for in situ monitoring of reinforced concrete structures. Cem. Concr. Compos. 2006, 28, 233–236. [Google Scholar] [CrossRef]
- Dong, S.G.; Lin, C.J.; Hu, R.G.; Li, L.Q.; Du, R.G. Effective monitoring of corrosion in reinforcing steel in concrete constructions by a multifunctional sensor. Electrochim. Acta 2011, 56, 1881–1888. [Google Scholar] [CrossRef]
- Yu, H.; Caseres, L. An embedded multi-parameter corrosion sensor for reinforced concrete structures. Mater. Corros. 2012, 63, 1011–1016. [Google Scholar] [CrossRef]
- Andrade, C.; Martinez, I. Corrosion Rate Monitoring of Deteriorated and Repaired Structures through On-Site Linear Polarization Measurements Using Surface or Embedded Sensors. In 2nd International RILEM Symposium on Advances in Concrete through Science and Engineering; RILEM Publications SARL: Quebec City, QC, Canada, 2006. [Google Scholar]
- Barroca, N.; Borges, L.M.; Velez, F.J.; Monteiro, F.; Górski, M.; Castro-Gomes, J. Wireless sensor networks for temperature and humidity monitoring within concrete structures. Constr. Build. Mater. 2013, 40, 1156–1166. [Google Scholar] [CrossRef] [Green Version]
- Taheri, S. A review on five key sensors for monitoring of concrete structures. Constr. Build. Mater. 2019, 204, 492–509. [Google Scholar] [CrossRef]
- Abbas, Y.; Have, B.T.; Hoekstra, G.I.; Douma, A.; De Bruijn, D.; Olthuis, W.; Van Den Berg, A. Connecting to concrete: Wireless monitoring of chloride ions in concrete structures. Procedia Eng. 2015, 120, 965–968. [Google Scholar] [CrossRef] [Green Version]
- Duffó, G.S.; Farina, S.B. Electrochemical behaviour of steel in mortar and in simulated pore solutions: Analogies and differences. Cem. Concr. Res. 2016, 88, 211–216. [Google Scholar] [CrossRef]
- Jiang, J.Y.; Wang, D.; Chu, H.Y.; Ma, H.; Liu, Y.; Gao, Y.; Shi, J.; Sun, W. The passive film growth mechanism of new corrosion-resistant steel rebar in simulated concrete pore solution: Nanometer structure and electrochemical study. Materials 2017, 10, 412. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.; Liu, J.; Chen, Z. Probabilistic evaluation method for corrosion risk of steel reinforcement based on concrete resistivity. Constr. Build. Mater. 2017, 138, 101–113. [Google Scholar] [CrossRef]
- ASTM C876; 2015 Standard Test Method for Corrosion Potentials of Uncoated Reinforcing Steel in Concrete. ASTM International: West Conshohocken, PA, USA, 2015.
- Millard, S.G.; Law, D.; Bungey, J.H.; Cairns, J. Environmental influences on linear polarisation corrosion rate measurement in reinforced concrete. NDT E Int. 2001, 34, 409–417. [Google Scholar] [CrossRef]
- Sadowski, Ł. New non-destructive method for linear polarisation resistance corrosion rate measurement. Arch. Civ. Mech. Eng. 2010, 10, 109–116. [Google Scholar] [CrossRef]
- Ribeiro, D.V.; Souza, C.A.C.; Abrantes, J.C.C. Use of Electrochemical Impedance Spectroscopy (EIS) to monitoring the corrosion of reinforced concrete. Rev. IBRACON Estruturas Mater. 2015, 8, 529–546. [Google Scholar] [CrossRef]
- Ribeiro, D.V.; Abrantes, J.C.C. Application of electrochemical impedance spectroscopy (EIS) to monitor the corrosion of reinforced concrete: A new approach. Constr. Build. Mater. 2016, 111, 98–104. [Google Scholar] [CrossRef]
- Raczkiewicz, W.; Wójcicki, A. Temperature impact on the assessment of reinforcement corrosion risk in concrete by galvanostatic pulse method. Appl. Sci. 2020, 10, 1089. [Google Scholar] [CrossRef] [Green Version]
- Sathiyanarayanan, S.; Natarajan, P.; Saravanan, K.; Srinivasan, S.; Venkatachari, G. Corrosion monitoring of steel in concrete by galvanostatic pulse technique. Cem. Concr. Compos. 2006, 28, 630–637. [Google Scholar] [CrossRef]
- Jaśniok, M.; Jaśniok, T. Measurements on Corrosion Rate of Reinforcing Steel under various Environmental Conditions, Using an Insulator to Delimit the Polarized Area. Procedia Eng. 2017, 193, 431–438. [Google Scholar] [CrossRef]
- Jaśniok, T.; Jaśniok, M. Range of polarization limited by a dielectric during electrochemical measurements of corrosion rate of steel reinforcement in concrete. Ochr. Przed Korozją 2016, 1, 29–35. [Google Scholar] [CrossRef]
- Law, D.W.; Millard, S.G.; Bungey, J.H. Linear polarization resistance measurements using a potentiostatically controlled guard ring. NDT E Int. 2000, 33, 15–21. [Google Scholar] [CrossRef]
- Poursaee, A.; Hansson, C.M. Galvanostatic pulse technique with the current confinement guard ring: The laboratory and finite element analysis. Corros. Sci. 2008, 50, 2739–2746. [Google Scholar] [CrossRef]
- Wojtas, H. Determination of corrosion rate of reinforcement with a modulated guard ring electrode; analysis of errors due to lateral current distribution. Corros. Sci. 2004, 46, 1621–1632. [Google Scholar] [CrossRef]
- Andrade, C.; Alonso, C.; Sarŕa, J. Corrosion rate evolution in concrete structures exposed to the atmosphere. Cem. Concr. Compos. 2002, 24, 55–64. [Google Scholar] [CrossRef]
- Jaśniok, T.; Jaśniok, M. Effects of electrodes location in a three-electrode system on polarization measurements of reinforcing steel in concrete cores drilled from a structure. Ochr. Przed Korozją 2019, 62, 252–258. [Google Scholar] [CrossRef]
- Jaśniok, M.; Jaśniok, T. Evaluation of Maximum and Minimum Corrosion Rate of Steel Rebars in Concrete Structures, Based on Laboratory Measurements on Drilled Cores. Procedia Eng. 2017, 193, 486–493. [Google Scholar] [CrossRef]
- Kisza, A. Elektrochemia II. Elektrodyka; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2001; ISBN 83-204-2564-6. [Google Scholar]
- Correia, M.J.; Pereira, E.V.; Salta, M.M.; Fonseca, I.T.E. Sensor for oxygen evaluation in concrete. Cem. Concr. Compos. 2006, 28, 226–232. [Google Scholar] [CrossRef]
- Brewer, P.J.; Leese, R.J.; Brown, R.J.C. An improved approach for fabricating Ag/AgCl reference electrodes. Electrochim. Acta 2012, 71, 252–257. [Google Scholar] [CrossRef]
- Jin, M.; Jiang, L.; Xu, J.; Chu, H.; Tao, D.; Bai, S.; Jia, Y. Electrochemical characterization of Solid Ag/AgCl reference electrode with different electrolytes for corrosion monitoring of steel in concrete. Electrochemistry 2016, 84, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Rogulski, Z.; Siwek, H.; Paleska, I.; Czerwiński, A. Electrochemical behavior of manganese dioxide on a gold electrode. J. Electroanal. Chem. 2003, 543, 175–185. [Google Scholar] [CrossRef]
- Muralidharan, S.; Ha, T.H.; Bae, J.H.; Ha, Y.C.; Lee, H.G.; Park, K.W.; Kim, D.K. Electrochemical studies on the solid embeddable reference sensors for corrosion monitoring in concrete structure. Mater. Lett. 2006, 60, 651–655. [Google Scholar] [CrossRef]
- Muralidharan, S.; Saraswathy, V.; Madhavamayandi, A.; Thangavel, K.; Palaniswamy, N. Evaluation of embeddable potential sensor for corrosion monitoring in concrete structures. Electrochim. Acta 2008, 53, 7248–7254. [Google Scholar] [CrossRef]
- Maruthapandian, V.; Saraswathy, V. Solid nano ferrite embeddable reference electrode for corrosion monitoring in reinforced concrete structures. Procedia Eng. 2014, 86, 623–630. [Google Scholar] [CrossRef] [Green Version]
- Qiao, G.; Hong, Y.; Song, G.; Li, H.; Ou, J. Electrochemical characterization of the solid-state reference electrode based on NiFe2O4 film for the corrosion monitoring of RC structures. Sensors Actuators B Chem. 2012, 168, 172–177. [Google Scholar] [CrossRef]
- Hong, Y.; Qiao, G.; Song, G. Preparation of the NiFe 2O 4 electrochemical film for the solid-state reference electrode based on EB-PVD. Appl. Surf. Sci. 2012, 258, 8934–8939. [Google Scholar] [CrossRef]
- Andrade, C.; Alonso, C. Corrosion rate monitoring in the laboratory and on-site. Constr. Build. Mater. 1996, 10, 315–328. [Google Scholar] [CrossRef]
- Liu, Y.; Weyers, R.E. Modeling the time-to-corrosion cracking in chloride contaminated reinforced concrete structures. ACI Mater. J. 1998, 95, 675–681. [Google Scholar] [CrossRef]
- Gulikers, J. Theoretical considerations on the supposed linear relationship between concrete resistivity and corrosion rate of steel reinforcement. Mater. Corros. 2005, 56, 393–403. [Google Scholar] [CrossRef]
- Nygaard, P.V.; Geiker, M.R.; Elsener, B. Corrosion rate of steel in concrete: Evaluation of confinement techniques for on-site corrosion rate measurements. Mater. Struct. Constr. 2009, 42, 1059–1076. [Google Scholar] [CrossRef]
- Morris, W.; Vico, A.; Vázquez, M. Chloride induced corrosion of reinforcing steel evaluated by concrete resistivity measurements. Electrochim. Acta 2004, 49, 4447–4453. [Google Scholar] [CrossRef]
- Morris, W.; Vico, A.; Vazquez, M.; De Sanchez, S.R. Corrosion of reinforcing steel evaluated by means of concrete resistivity measurements. Corros. Sci. 2002, 44, 81–99. [Google Scholar] [CrossRef]
- Hope, B.B.; Ip, A.K.; Manning, D.G. Corrosion and electrical impedance in concrete. Cem. Concr. Res. 1985, 15, 525–534. [Google Scholar] [CrossRef]
- Feliu, S.; González, J.A.; Feliu, S.; Andrade, C. Relationship between conductivity of concrete and corrosion of reinforcing bars. Br. Corros. J. 1989, 24, 195–198. [Google Scholar] [CrossRef]
- Hornbostel, K.; Larsen, C.K.; Geiker, M.R. Relationship between concrete resistivity and corrosion rate—A literature review. Cem. Concr. Compos. 2013, 39, 60–72. [Google Scholar] [CrossRef]
- Sang, Y.; Yang, Y.; Zhao, Q. Electrical resistivity of plain cement-based materials based on ionic conductivity: A review of applications and conductive models. J. Build. Eng. 2022, 46, 103642. [Google Scholar] [CrossRef]
- Sengul, O. Use of electrical resistivity as an indicator for durability. Constr. Build. Mater. 2014, 73, 434–441. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A.L. Electrochemical Polarization. J. Electrochem. Soc. 1957, 104, 56. [Google Scholar] [CrossRef]
- PN-EN 206+A1:2016-12; Concrete Specification, Performance, Production and Conformity. PKN/KT 274: Warszawa, Poland, 2016.
- Thomas, M. Chloride thresholds in marine concrete. Cem. Concr. Res. 1996, 26, 513–519. [Google Scholar] [CrossRef]
- Femenias, Y.S.; Angst, U.; Moro, F.; Elsener, B. Development of a novel methodology to assess the corrosion threshold in concrete based on simultaneous monitoring of pH and free chloride concentration. Sensors 2018, 18, 3101. [Google Scholar] [CrossRef] [Green Version]
- Hausmann, D.A. Steel Corrosion in Concrete—How Does It Occur? Mater. Prot. 1967, 6, 19–23. [Google Scholar]
- Karthick, S.P.; Muralidharan, S.; Saraswathy, V.; Thangavel, K. Long-term relative performance of embedded sensor and surface mounted electrode for corrosion monitoring of steel in concrete structures. Sens. Actuators B Chem. 2014, 192, 303–309. [Google Scholar] [CrossRef]
- Clément, A.; Laurens, S.; Arliguie, G.; Deby, F. Numerical study of the linear polarisation resistance technique applied to reinforced concrete for corrosion assessment. Eur. J. Environ. Civ. Eng. 2012, 16, 491–504. [Google Scholar] [CrossRef]
- Flis, J.; Pickering, H.W.; Osseo-Asare, K. Assessment of data from three electrochemical instruments for evaluation of reinforcement corrosion rates in concrete bridge components. Corrosion 1995, 51, 602–609. [Google Scholar] [CrossRef]
- Feliu, S.; González, J.A.; Miranda, J.M.; Feliu, V. Possibilities and problems of in situ techniques for measuring steel corrosion rates in large reinforced concrete structures. Corros. Sci. 2005, 47, 217–238. [Google Scholar] [CrossRef]
- Ohno, M.; Limtong, P.; Ishida, T. Multiscale modeling of steel corrosion in concrete based on micropore connectivity. J. Build. Eng. 2022, 47, 103855. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaśniok, M.; Jaśniok, T. Corrosion Diagnostics Performed on Cores Drilled from Concrete Structures, Using the Laboratory Simulation of Temperature and Relative Humidity Impact. Appl. Sci. 2022, 12, 7134. https://doi.org/10.3390/app12147134
Jaśniok M, Jaśniok T. Corrosion Diagnostics Performed on Cores Drilled from Concrete Structures, Using the Laboratory Simulation of Temperature and Relative Humidity Impact. Applied Sciences. 2022; 12(14):7134. https://doi.org/10.3390/app12147134
Chicago/Turabian StyleJaśniok, Mariusz, and Tomasz Jaśniok. 2022. "Corrosion Diagnostics Performed on Cores Drilled from Concrete Structures, Using the Laboratory Simulation of Temperature and Relative Humidity Impact" Applied Sciences 12, no. 14: 7134. https://doi.org/10.3390/app12147134
APA StyleJaśniok, M., & Jaśniok, T. (2022). Corrosion Diagnostics Performed on Cores Drilled from Concrete Structures, Using the Laboratory Simulation of Temperature and Relative Humidity Impact. Applied Sciences, 12(14), 7134. https://doi.org/10.3390/app12147134







