Examination of Postmortem β-Hydroxybutyrate Increase in Forensic Autopsy Cases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Characteristics of the Patients
2.3. Statistical Analysis
3. Results
3.1. Results of Statistical Intergroup Analysis
3.2. Result of Wilcoxon’s Rank Sum Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UpToDate. Approach to the Adult with Metabolic Acidosis. Available online: https://www.uptodate.com/contents/approach-to-the-adult-with-metabolic-acidosis?search=metabolic%20acidosis&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 (accessed on 4 June 2022).
- Jeffrey, A.K.; Nicolaos, E.M. Treatment of Acute Metabolic Acidosis: A Pathophysiologic Approach. Nat. Rev. Nephrol. 2012, 8, 589–601. [Google Scholar] [CrossRef]
- UpToDate. Acid-Base and Electrolyte Abnormalities with Diarrhea. Available online: https://www.uptodate.com/contents/acid-base-and-electrolyte-abnormalities-with-diarrhea?search=metabolic%20acidosis&topicRef=2291&source=see_link#H626692994 (accessed on 5 June 2022).
- UpToDate. Overview and Pathophysiology of Renal Tubular Acidosis and the Effect on Potassium Balance. Available online: https://www.uptodate.com/contents/overview-and-pathophysiology-of-renal-tubular-acidosis-and-the-effect-on-potassium-balance?sectionName=PROXIMAL%20(TYPE%202)%20RTA&search=metabolic%20acidosis&topicRef=2291&anchor=H42786907&source=see_link#H42786907 (accessed on 5 June 2022).
- UpToDate. Causes of Lactic Acidosis. Available online: https://www.uptodate.com/contents/causes-of-lactic-acidosis?search=metabolic%20acidosis&topicRef=2291&source=see_link#H7 (accessed on 5 June 2022).
- Benoit, S.R.; Zhang, Y.; Geiss, L.S.; Gregg, E.W.; Albright, A. Trends in Diabetic Ketoacidosis Hospitalizations and in-Hospital Mortality—United States, 2000–2014. MMWR Morb. Mortal. Wkly Rep. 2018, 67, 362–365. [Google Scholar] [CrossRef] [PubMed]
- McGuire, L.C.; Cruickshank, A.M.; Munro, P.T. Alcoholic Ketoacidosis. Emerg. Med. J. 2006, 23, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, M.M.; Hajja, W.; Al-Khatib, S.; Hazeghazam, M.; Sreedhar, D.; Li, R.N.; Wong-McKinstry, E.; Carlson, R.W. Toxigenic and Metabolic Causes of Ketosis and Ketoacidotic Syndromes. Crit. Care Clin. 2012, 28, 601–631. [Google Scholar] [CrossRef]
- Luna, J.; Gilliland, M.G.; Hewan-Lowe, K.O.; Tanenberg, R.J. Postmortem Diagnosis of Diabetic Ketoacidosis Presenting as the “Dead-in-Bed Syndrome”. Endocr. Pract. 2014, 20, e123–e125. [Google Scholar] [CrossRef]
- Irwin, J.; Cohle, S.D. Sudden Death Due to Diabetic Ketoacidosis. Am. J. Forensic Med. Pathol. 1988, 9, 119–121. [Google Scholar] [CrossRef]
- Kjærulff, M.L.B.G.; Astrup, B.S. Sudden Death Due to Diabetic Ketoacidosis Following Power Failure of an Insulin Pump: Au topsy and Pump Data. J. Forensic Leg. Med. 2019, 63, 34–39. [Google Scholar] [CrossRef]
- UpToDate. Diabetic Ketoacidosis and Hyperosmolar Hyperglycemic State in Adults: Epidemiology and Pathogenesis. Available online: https://www.uptodate.com/contents/diabetic-ketoacidosis-and-hyperosmolar-hyperglycemic-state-in-adults-epidemiology-and-pathogenesis?search=diabetic%20ketoacidosis&source=search_result&selectedTitle=5~150&usage_type=default&display_rank=5#H2 (accessed on 4 June 2022).
- Barnes, A.J.; Bloom, S.R.; Goerge, K.; Alberti, G.M.; Smythe, P.; Alford, F.P.; Chisholm, D.J. Ketoacidosis in Pancreatectomized Man. N. Engl. J. Med. 1977, 296, 1250–1253. [Google Scholar] [CrossRef]
- Miles, J.M.; Haymond, M.W.; Nissen, S.L.; Gerich, J.E. Effects of Free Fatty Acid Availability, Glucagon Excess, and Insulin Defi ciency on Ketone Body Production in Postabsorptive Man. J. Clin. Investig. 1983, 71, 1554–1561. [Google Scholar] [CrossRef]
- UpToDate. Fasting Ketosis and Alcoholic Ketoacidosis. Available online: https://www.uptodate.com/contents/fasting-ketosis-and-alcoholic-ketoacidosis?search=alcoholic%20ketoacidosis&source=search_result&selectedTitle=1~17&usage_type=default&display_rank=1 (accessed on 5 June 2022).
- Felby, S.; Nielsen, E.; Thomsen, J.L. The Postmortem Distribution of Ketone Bodies Between Blood, Vitreous Humor, Spinal Fluid, and Urine. Forensic Sci. Med. Pathol. 2008, 4, 100–107. [Google Scholar] [CrossRef]
- Thomsen, J.L.; Felby, S.; Theilade, P.; Nielsen, E. Alcoholic Ketoacidosis as a Cause of Death in Forensic Cases. Forensic Sci. Int. 1995, 75, 163–171. [Google Scholar] [CrossRef]
- Kanetake, J.; Kanawaku, Y.; Mimasaka, S.; Sakai, J.; Hashiyada, M.; Nata, M.; Funayama, M. The Relationship of a High Level of Serum Beta-Hydroxybutyrate to Cause of Death. Leg. Med. Tokyo 2005, 7, 169–174. [Google Scholar] [CrossRef]
- Osuna, E.; Vivero, G.; Conejero, J.; Abenza, J.M.; Martínez, P.; Luna, A.; Pérez-Cárceles, M.D. Postmortem Vitreous Humor β-Hydroxybutyrate: Its Utility for the Postmortem Interpretation of Diabetes Mellitus. Forens. Sci. Int. 2005, 153, 189–195. [Google Scholar] [CrossRef]
- Heninger, M. Postmortem Vitreous Beta-Hydroxybutyrate: Interpretation in a Forensic Setting. J. Forens. Sci. 2012, 57, 1234–1240. [Google Scholar] [CrossRef]
- Palmiere, C.; Bardy, D.; Letovanec, I.; Mangin, P.; Augsburger, M.; Ventura, F.; Iglesias, K.; Werner, D. Biochemical Markers of Fatal Hypothermia. Forens. Sci. Int. 2013, 226, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Eriksson Hydara, Y.; Zilg, B. Postmortem Diagnosis of Ketoacidosis: Levels of Beta-Hydroxybutyrate, Acetone and Isopropanol in Different Causes of Death. Forens. Sci. Int. 2020, 314, 110418. [Google Scholar] [CrossRef]
- Ahlström, S.; Ahlner, J.; Jönsson, A.K.; Green, H. The Importance of BHB Testing on the Post-Mortem Diagnosis of Ketoacidosis. Biomolecules 2021, 12, 9. [Google Scholar] [CrossRef]
- Iliescu, D.B.; Furnica, C.; Girlescu, N.; Chistol, R.O.; Perianu, L.; Diac, M.; Timofte, A.D.; Knieling, A.; Ciureanu, I.-A. Postmortem Diagnosis of Ketoacidosis by Determining Beta-Hydroxybutyrate Levels in Three Types of Body Fluids by Two Different Methods. Appl. Sci. 2022, 12, 5541. [Google Scholar] [CrossRef]
- Rousseau, G.; Reynier, P.; Jousset, N.; Rougé-Maillart, C.; Palmiere, C. Updated Review of Postmortem Biochemical Exploration of Hypothermia with a Presentation of Standard Strategy of Sampling and Analyses. Clin. Chem. Lab. Med. 2018, 56, 1819–1827. [Google Scholar] [CrossRef] [Green Version]
- Woydt, L.; Bernhard, M.; Kirsten, H.; Burkhardt, R.; Hammer, N.; Gries, A.; Dreßler, J.; Ondruschka, B. Intra-individual Alterations of Serum Markers Routinely Used in Forensic Pathology Depending on Increasing Post-Mortem Interval. Sci. Rep. 2018, 8, 12811. [Google Scholar] [CrossRef]
- Pomeranz, Y.; Meloan, C.E. Enzymatic Methods. In Food Analysis; Springer: Boston, MA, USA, 1994. [Google Scholar] [CrossRef]
- Ren, F.Q.; Yan, S.K.; Mao, D.Y.; Li, Y.X.; Xiao, X.H.; Xu, E.M. Evaluation of Enzymatic Method for Determination of Serum Beta-Hydroxybutyrate and Its Clinical Application. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2003, 25, 702–705. (In Chinese) [Google Scholar]
- Palmiere, C.; Mangin, P. Postmortem Chemistry Update Part I. Int. J. Legal Med. 2012, 126, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Iten, P.X.; Meier, M. Beta-Hydroxybutyric Acid—An Indicator for an Alcoholic Ketoacidosis as Cause of Death in Deceased Alcohol Abusers. J. Forens. Sci. 2000, 45, 624–632. [Google Scholar] [CrossRef]
- Jungermann, K.; Katz, N. Functional Specialization of Different Hepatocyte Populations. Physiol. Rev. 1989, 69, 708–764. [Google Scholar] [CrossRef]
- Watanabe, S.; Hirakawa, A.; Aoe, S.; Fukuda, K.; Muneta, T. Basic Ketone Engine and Booster Glucose Engine for Energy Production. Open J. 2016, 2, 14–23. [Google Scholar] [CrossRef]
- Nakamura, M.T.; Yudell, B.E.; Loor, J.J. Regulation of Energy Metabolism by Long-Chain Fatty Acids. Prog. Lipid Res. 2014, 53, 124–144. [Google Scholar] [CrossRef]
- Kiranjit, K.D.; Sonu, G. Biochemistry, Ketogenesis. StatPearls. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493179/ (accessed on 5 June 2022).
- Akram, M. Citric Acid Cycle and Role of Its Intermediates in Metabolism. Cell Biochem. Biophys. 2014, 68, 475–478. [Google Scholar] [CrossRef]
- Kelly, D.J.; Hughes, N.J. Chapter 12. The Citric Acid Cycle and Fatty Acid Biosynthesis. In Helicobacter pylori: Physiology and Genetics; Mobley, H.L.T., Mendz, G.L., Hazell, S.L., Eds.; ASM Press: Washington, DC, USA, 2001. [Google Scholar]
- World Health Organization. Clinical Criteria for the Determination of Death—WHO Technical Expert Consultation; WHO Headquarters, Geneva, Switzerland, 22–23 September 2014; World Health Organization: Geneva, Switzerland, 2017; Available online: https://apps.who.int/iris/handle/10665/254737 (accessed on 30 May 2022).
- Latil, M.; Rocheteau, P.; Châtre, L.; Sanulli, S.; Mémet, S.; Ricchetti, M.; Tajbakhsh, S.; Chrétien, F. Skeletal Muscle Stem Cells Adopt a Dormant Cell State Postmortem and Retain Regenerative Capacity. Nat. Commun. 2012, 3, 903. [Google Scholar] [CrossRef] [Green Version]
- Verwer, R.W.; Hermens, W.T.; Dijkhuizen, P.; ter Brake, O.; Baker, R.E.; Salehi, A.; Sluiter, A.A.; Kok, M.J.; Muller, L.J.; Verhaagen, J.; et al. Cells in Human Postmortem Brain Tissue Slices Remain Alive for Several Weeks in Culture. FASEB J. 2002, 16, 54–60. [Google Scholar] [CrossRef]
- Pozhitkov, A.E.; Neme, R.; Domazet-Lošo, T.; Leroux, B.G.; Soni, S.; Tautz, D.; Noble, P.A. Tracing the Dynamics of Gene Transcripts After Organismal Death. Open Biol. 2017, 7, 160267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peter, K.; Jože, B.; Vera, F.M. Alcoholic ketoacidosis: A Cause of Sudden Death of Chronic Alcoholics. Forens. Sci. Int. 1999, 103, S53–S59. [Google Scholar]
- Palmiere, C.; Mangin, P.; Werner, D. Postmortem Distribution of 3-Beta-Hydroxybutyrate. J. Forens. Sci. 2014, 59, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Stokol, T.; Nydam, D.V. Effect of Hemolysis on Nonesterified Fatty Acid and Beta-Hydroxybutyrate Concentrations in Bovine Blood. J. Vet. Diagn. Investig. 2006, 18, 466–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
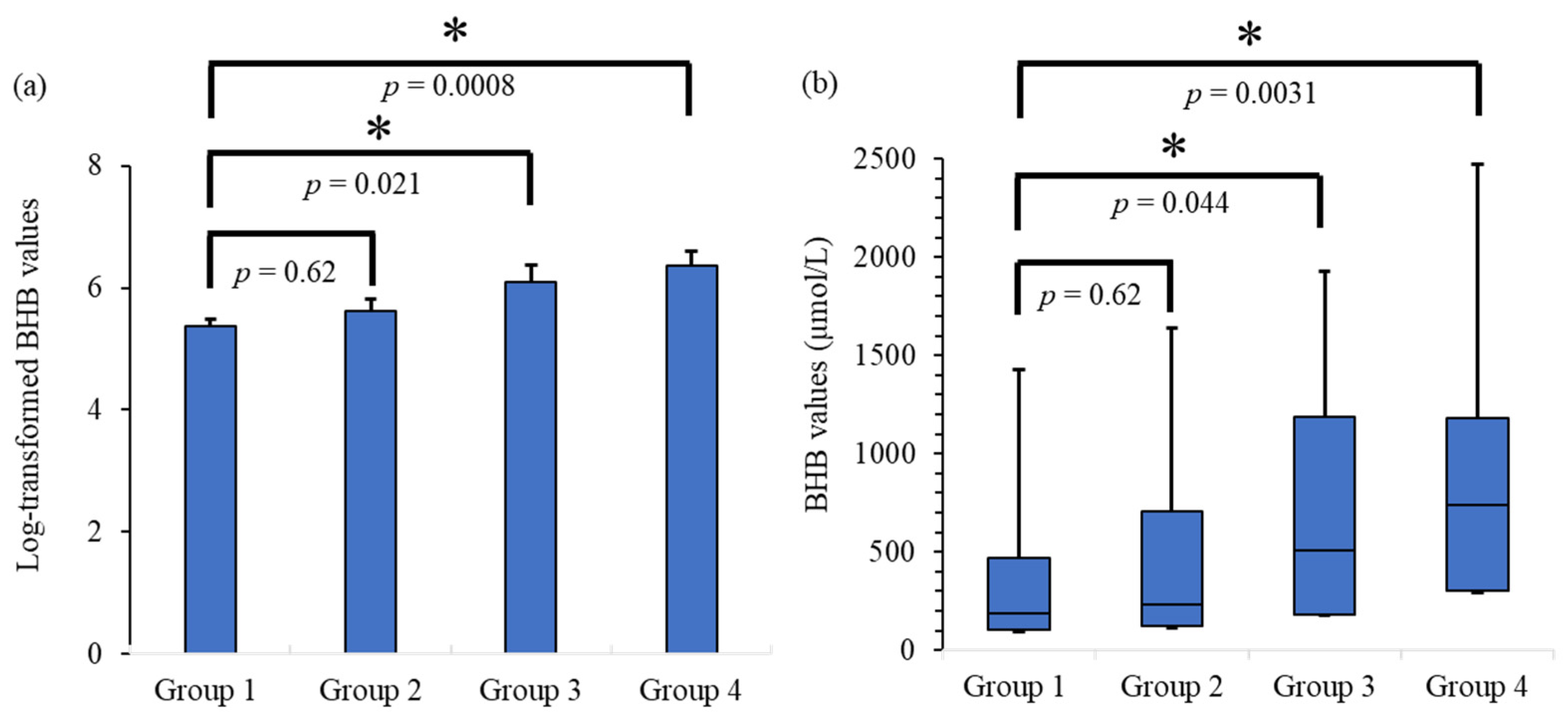

| Number of Patients | Median BHB (IQR) µmol/L | Average BHB (SE) (Log-Transformed) | |
|---|---|---|---|
| Group 1 | Males: 56 | 190 | 5.38 |
| (PMI ≤ 24 h) | Females: 18 | (104–472.5) | (0.11) |
| Group 2 | Males: 15 | 232 | 5.62 |
| (PMI ≤ 48 h) | Females: 10 | (122–705.5) | (0.19) |
| Group 3 | Males: 9 | 505.5 | 6.10 |
| (PMI ≤ 72 h) | Females: 7 | (183.25–1185) | (0.28) |
| Group 4 | Males: 14 | 741 | 6.37 |
| (PMI > 72 h) | Females: 2 | (302–1182.5) | (0.24) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondou, H.; Bandou, R.; Ichioka, H.; Matsunari, R.; Kawamoto, M.; Idota, N.; Ting, D.; Kimura, S.; Ikegaya, H. Examination of Postmortem β-Hydroxybutyrate Increase in Forensic Autopsy Cases. Appl. Sci. 2022, 12, 7181. https://doi.org/10.3390/app12147181
Kondou H, Bandou R, Ichioka H, Matsunari R, Kawamoto M, Idota N, Ting D, Kimura S, Ikegaya H. Examination of Postmortem β-Hydroxybutyrate Increase in Forensic Autopsy Cases. Applied Sciences. 2022; 12(14):7181. https://doi.org/10.3390/app12147181
Chicago/Turabian StyleKondou, Hiroki, Risa Bandou, Hiroaki Ichioka, Ryota Matsunari, Masataka Kawamoto, Nozomi Idota, Deng Ting, Satoko Kimura, and Hiroshi Ikegaya. 2022. "Examination of Postmortem β-Hydroxybutyrate Increase in Forensic Autopsy Cases" Applied Sciences 12, no. 14: 7181. https://doi.org/10.3390/app12147181






