Abstract
This study evaluated both the possible fungal metabolites involved in the degradation of the commercial consolidant known as Paraloid® B72 and the national artisanal consolidant named transparent dispersion of casein and the deteriorative potential of melanised fungi. Fungi were found to have the capacity to produce organic acids, proteases and esterases when they grow on consolidants, which can be used as nutrients. Mycelia produced by melanised fungi affected the appearance, as well as the integrity, of consolidants applied on painted experimental models and fragments of frescoes. In treatment trials with biocides (Biotin R®, Biotin T® and Preventol® RI 80), the morphology of the consolidants, as well as the vitality of the fungi, were assessed 30 days after the inoculation with melanic fungi. Direct observation, optical microscopy, colourimetry and microbiological analysis highlighted the degradation of the consolidants by fungi and their acquired resistance after biocidal treatments. Biotin R® applied by brushing on the surface of the consolidants proved to be the most effective treatment, followed by Biotin T®. Considering the overall results for both Paraloid® B72 and transparent dispersion of casein, use of Biotin R® applied by brushing is recommended for preventive conservation.
1. Introduction
Studying the original and restoration materials used in historical monuments, investigating their conservation and planning the appropriate methodology represent sustainable requirements in the field of restoration, and these are permanent concerns that must be taken into account in order to identify biodeterioration and the first stage of microbial colonization and ensure long-term preservation. Physical and chemical factors can act as deteriogens, but they also stimulate microbial growth [1]. Identification of microorganisms on mural paintings does not always imply a change in the composition or physical properties of the materials. Nevertheless, in special environmental conditions, bacteria and fungi act as biodeteriogens that start to grow and colonize surfaces, stimulating or accelerating the deterioration process [2]. Microorganisms are not only biodeteriogens but can also become a risk factor for the health of restorers and visitors [3,4,5].
Microbial colonization of either original or restored mural paintings takes place due to these microbes’ ability to use organic and inorganic materials as nutrients. As a result, the biodeterioration process becomes visible through aesthetic and structural changes. Microbial colonization and deterioration processes are enhanced by a wide range of abiotic factors, such as humidity, temperature, nutrients and the chemical composition of the air [6,7,8].
Execution techniques in mural painting involve a layered structure comprising the support, ground and pictorial layers. Over time, organic compounds may be added, either through the restoration process or from human activities, such as firing candles in churches [9]. Bacteria, mostly those with low nutritional needs, are the first colonizers [6], but later on, due to the application of consolidants and the accumulation of organic deposits, fungi become the main colonizers producing physical and chemical deterioration phenomena. Mural paintings stand in confined and semi-confined environments [6] with extreme humidity and abundant nutrients, which promote the germination of fungal spores and mycelia growth. Fungal colonization starts on the surface of the consolidants, in the pictorial layer or in cracks. As a consequence of in-depth development of the hyphae, degradation of consolidants, production of exfoliations and loss of the pictorial layer take place [10,11]. Dornieden et al. [12] revealed that fungal species adapt to different environmental conditions, which can be reflected both in the rate of growth and the developing life forms that contribute to changes in the substrate. Fungi produce aesthetic damage due to pigment discolorations, mycelia pigmentation and release of organic pigments of different colours [13]. Extracellular enzymes and organic acids are also released in the substrate, causing chemical alterations to the original pigments [14,15,16,17].
Black fungi produce discoloration on both the pictorial layer and the consolidants due to melanised cell walls [18,19,20].
Assessment of the biodegradation capacities of fungi, such as production of organic acids, proteseses, esterases and pigments, through rapid screening tests is important for the evaluation of their potential risk to cultural heritage [21]. To minimize and/or prevent microbial growth, contaminated areas are treated with biocides [22,23,24,25,26,27,28] and evaluated from the microbiological point of view due to the high spore resistance [29].
According to Ruggiero et al. [30], to reduce biodeterioration of outdoor surfaces, biocides could be applied directly to stone surfaces or added into coating formulations. After evaluation with FT-IR spectroscopy in ATR mode and Raman spectroscopy, promising results were obtained with two silica nanosystems loaded with 2-mercaptobenzothiazole.
Presentato et al. [31] reported a successful treatment based on a controlled release system using mesoporous silica nanoparticles as carriers loaded with Preventol RI-80.
Presently, conservation and restoration are still based on non-standardized techniques. In recent years, innovative environmentally and operator-friendly products for conservation and restoration have been developed [29,32,33].
Lo Schiavo et al. [34] investigated green conservation of cultural heritage, which refers to eco-sustainable practices used as alternatives to traditional methods. The choice of consolidants for restoration has to take into consideration their adhesive strength, the absence of significant chromatic variations and their permeability [35]. Over time, some materials used in restoration, such as consolidants, age and lose their properties. New restorations include the removal of the aged or biodeteriorated consolidants with toxic solvents alone or in combination with non-toxic or innovative green compounds [36].
The thick layers of aged acrylic coating on the surfaces of wall paintings in San Salvador church in Venice were removed with highly viscous gel-like matrices: hydrophobically modified hydroxyethyl cellulose loaded with an oil-in-water microemulsion [37].
Paraloid® B72 is a copolymer of ethyl methacrylate and methyl acrylate (ratio 70:30) and is a very stable resin, with a Tg of 40 °C and a refractive index3 of 1.49. The solvents most commonly used in combination with Paraloid® B72 are acetone, toluene, xylene, ethyl acetate and diacetone alcohol [38]. Paraloid® B72, thanks to its adhesive and consolidating properties, is one of the most frequently used acrylic polymers, and it is employed for the conservation of a wide range of materials due to its reversibility, mechanical characteristics and ease of application [39,40].
It has been suggested that fungal growth on Paraloid® B72 may interfere with the structural properties of the polymer due to modification of the water affinity [41]. In addition, it has been proved that aged synthetic acrylics can be colonized by melanised fungi, which accelerate the decay process [41,42]. Degradation of consolidants occurs at the surface and leads to the fragility and loss of the pictorial layer.
Transparent dispersion of casein (TDC) is an artisanal product obtained from lime and casein that is used as a consolidant in the restoration of mural paintings in Romanian churches [43].
The present study aimed to: (I) identify the main fungal metabolites involved in the decomposition of Paraloid® B72 and TDC; (II) evaluate in vitro the deteriorative potential of the fungi that colonize and decompose Paraloid® B72 and TDC; and (III) improve the susceptibility of consolidants to degradation through treatments with biocides.
2. Materials and Methods
2.1. Sampling
Biodeteriorated deposited (from the collection of the National University of Arts in Bucharest (NUAB)) and original (from the Nave of Humor Monastery and Tismana Monastery) frescoes were sampled for microbiological analysis.
After visual examination and recognition of the contaminated surfaces [44,45], they were sampled by swabbing 10 cm2 areas. Sampling was performed in triplicate for each area of mural painting. Then, the samples were placed in sterile tubes maintained at 4 °C and transported to the Microbiological Department of the Institute of Biology Bucharest for analysis. The series of dilutions were inoculated in Petri dishes with yeast glucose chloramphenicol agar (YGCA) nutrient growth medium [46]. All inoculated Petri dishes were incubated at 25 °C for 10 days. Based on macroscopic (shape, colour of unsporulated and sporulated aerial and submerse mycelia, odour, etc.) and microscopic (conidiophore, spores, hyphae, branches) features of the colonies, the isolates from the deposited frescoes and the frescoes from Humor Monastery were found to be closely related to Aspergillus niger (Aspergillus sp. S1) and Ulocladium chartarum (Ulocladium sp. H1), respectively. Four strains of Bipolaris sp. (P2—nave belt of the Holy Prophet; M3—nave, above the foot of the Holy Prophet; M4—nave, angelic host of Heaven; and M7—nave, Christ Pantocrator) isolated from the pictorial layer of frescoes from the Tismana Monasteries were identified using PCR amplification and sequencing of the ITS1-5.8S gene-ITS2 [16,47].
After visual inspection, pictures were acquired using a Digital Nikon D200.
2.2. Assessment of the Ability to Produce Metabolites Involved in Degradation of the Consolidants
2.2.1. Production of Organic Acids
Nutrient medium containing glucose [48], with pH readjusted to 7.0, was inoculated with 10 µL of fungal spores (from suspensions with concentrations of approximately 1.0 × 103 spores/mL). To assess the production of acids and enzymes, spores of the strains Aspergillus sp. S1, Ulocladium sp. H1, Bipolaris sp. P2, Bipolaris sp. M3, Bipolaris sp. M4 and Bipolaris sp. M7 were inoculated. They were then incubated for 7 days under stirring conditions (150 rpm). pH measurements were performed in triplicate at 3, 5 and 7 days.
2.2.2. Production of Proteases
The capability of fungi to produce hydrolytic proteases was qualitatively tested with a plate assay using the nutrient medium [49] containing 1% casein. The nutrient medium was inoculated in the centre of Petri dishes with 10 µL of fungal spores. After 10 days of incubation at 25 °C, the Petri dishes were flooded with 1 N HCl to enhance the visibility of the caseine hydrolysis zone. Experiments were conducted in duplicate, and results were qualitatively expressed as levels of enzyme activities (LEsA) using the following formula: LEsA = diameter of the hydrolysis zone divided by the diameter of the microbial colony (in millimetres) [47].
2.2.3. Production of Esterases
The qualitative assay of esterase production was performed with the same nutrient medium as for proteases, but it contained 1% Tween-80. All test conditions were similar, but esterase activity was indicated by an opaque halo around the colonies resulting from the precipitation of fatty acids due to Tween-80 hydrolysis [50]. LEsA was evaluated using the previously mentioned procedure.
2.3. Assessment of the Role of Fungi in the Deterioration of Consolidants Placed on Painted Laboratory Models
2.3.1. Experimental Design
Following the preparation of painted laboratory models, three layers of Paraloid® B72 (respectively, TDC), were applied on the painted surfaces using a brush [16]. Inocula, in the form of 50 μL of conidia suspension in distilled water, were spread onto six squares of each pigment as follows: Aspergillus sp. S1 on area 1, Bipolaris sp. P2 on area 2, Bipolaris sp. M4 on area 3, Ulocladium sp. H1 on area 4, Bipolaris sp. M3 on area 5 and Bipolaris sp. M7 on area 6. All of the inoculated painted laboratory models were incubated in special devices with relative humidity of 90% for 30 days (Figure S1). Then, the fungal cultures were removed from the surfaces using cotton swabs moistened in distilled water. The cleaned painted laboratory models were further dried and samples cut and analysed using different methods.
2.3.2. Assessment of Fungal Growth
The growth of the colonies developed on the painted laboratory models covered with Paraloid® B72 and TDC was evaluated using images taken at 10, 20 and 30 days. The area and the diameter of each colony were measured with ‘Image J’ software [46]. Measurements were converted into a mm scale using a scale bar attached to the models. Culture diameters and area measurements were acquired from cultures in each square; the standard deviation was also calculated. Division of the diameter of each colony by the number of days between the 10th, 20th and 30th days provided an estimation of the growth over time. Differences were determined by t-test, and p < 0.05 was considered significant.
2.3.3. Assessment of the Decomposition of Consolidants by Optical Microscopy
After removing the colonies and cleaning the models with a damp cloth, samples of the painted experimental models were analysed with a Nikon AZ 100 optical microscope (OM).
2.3.4. Assessment of the Decomposition of Consolidants by Colorimetry
Colorimetric assessments are used to evaluate colour changes [51] and the efficiency of the removal of consolidants, such as Paraloid B72, in various cultural heritage items, including wall paintings [52,53].
Colour changes were assessed using an X-rite portable colorimeter (Ci64UV), recording data in the 400–700 nm wavelength range with a 10 nm increment. The equipment had a variable aperture of 4 or 8 mm, and the standard illuminant D65/10° was used. All inoculated areas were measured, with a total of 48 areas being analysed. For each analysed area, three measurements were performed, and the reported data represent the means of those values. Data were represented in the CIELab colour space. The CIELab colour space, defined in 1976 by the International Commission on Illumination (CIE 1978), is one of the most widely used systems for reporting colorimetric data. It is a three-dimensional space in which colours are represented based on their lightness (L*), red-green value (a*) and yellow-blue value (b*). From the colorimetric data, the ΔE value was calculated using Equation (1) in order to describe the difference in colour between samples prior to and after the removal process [54]. ΔE can be mathematically explained as the geometric (Euclidian) distance between two points corresponding to the two samples in the colour space, where , and are the CIELab coordinates of the reference sample (the sample without surface contaminant, prior to application of the consolidant) and , and are the coordinates corresponding to the same area after the cleaning process:
Removal efficiency was assessed based on Equation (2):
where represents the difference between the samples with and without the consolidant and is the difference between the reference and treated areas.
2.4. Assessment of Resistance of Consolidants to Fungal Degradation from Treatment with Biocides
2.4.1. Experimental Design of Slide Cultures
Following the cleaning of glass microscope slides, Paraloid® B72 and TDC were applied with a brush, either alone or as mixtures with biocides (5% v/v). A detailed description is provided in Table 1. CTSR Company supplied all biocides. Inocula consisting of Aspergillus sp. S1, Bipolaris sp. M7, Bipolaris sp. P2 and Ulocladium sp. H1, deployed in the form of 50 μL of conidia suspension in distilled water, were spread onto slides covered by consolidants (Figure S2). After incubation for 30 days in special devices with a relative humidity of 90%, the fungal growth was analysed under a Nikon Z 100 optical microscope (OM).

Table 1.
Description of experimental variants.
2.4.2. Experimental Design for Fresco Fragments
Following the cleaning of the fresco fragments, three layers of Paraloid® B72 (respectively, TDC), as well as Biotin R®, Biotin T® and Preventol® RI 80, were applied with a brush. Experimental variants and incubation were prepared as previously described (Figure S3). Inocula were prepared using the above method for Aspergillus sp. S1 and Bipolaris sp. M7. For Bipolaris sp. P2 and Ulocladium sp. H1, squares of 2 mm2 from the margins of the colonies were transferred onto the frescoes. After 30 days of incubation, inoculated areas were analysed under a Nikon AZ 100 optical microscope (OM).
3. Results
3.1. Morphological Characterization of Contaminated Mural Paintings
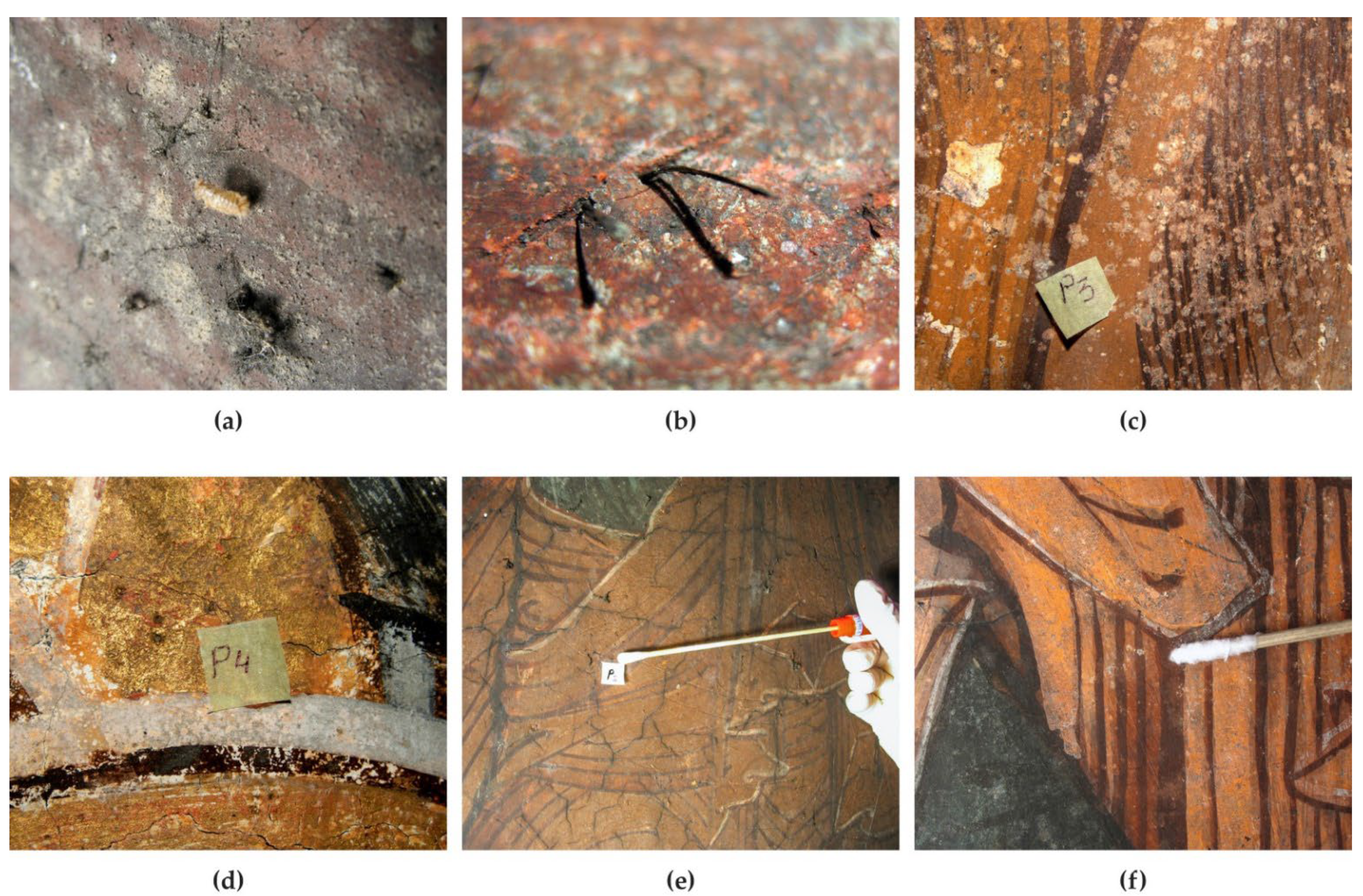
Mural paintings from different locations were analysed from the point of view of biodeterioration. The deposited frescoes were covered with spots of black mycelium (Figure 1a). The presence of a larva could be observed, which may have played an important role in the spread of microorganisms through its movements. Due to a technical error, the vegetal debris that was not covered by the pictorial layer in a fresco from the Humor Monastery became soft and black, as it was being decomposed by sporulated mycelium (Figure 1b). On the murals from the Tismana Monastery, dark grey, black and light grey spots could be observed, but the pictorial layer was either lost (Figure 1c) or firmly attached to the mortar (Figure 1d–f). The isolated species are described in Section 2.1.
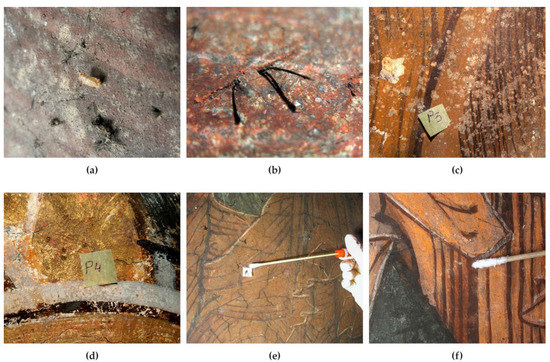
Figure 1.
The morphological aspect of the murals from which the fungi were isolated. (a) Black mycelium on the deposited frescoes (NUAB); (b) plant debris colonized by black fungi (Humor Monastery); (c–f) light grey, black and grey dark mycelia on mural paintings (Tismana Monastery).
3.2. Identification of the Main Fungal Metabolites Involved in the Decomposition of Consolidants
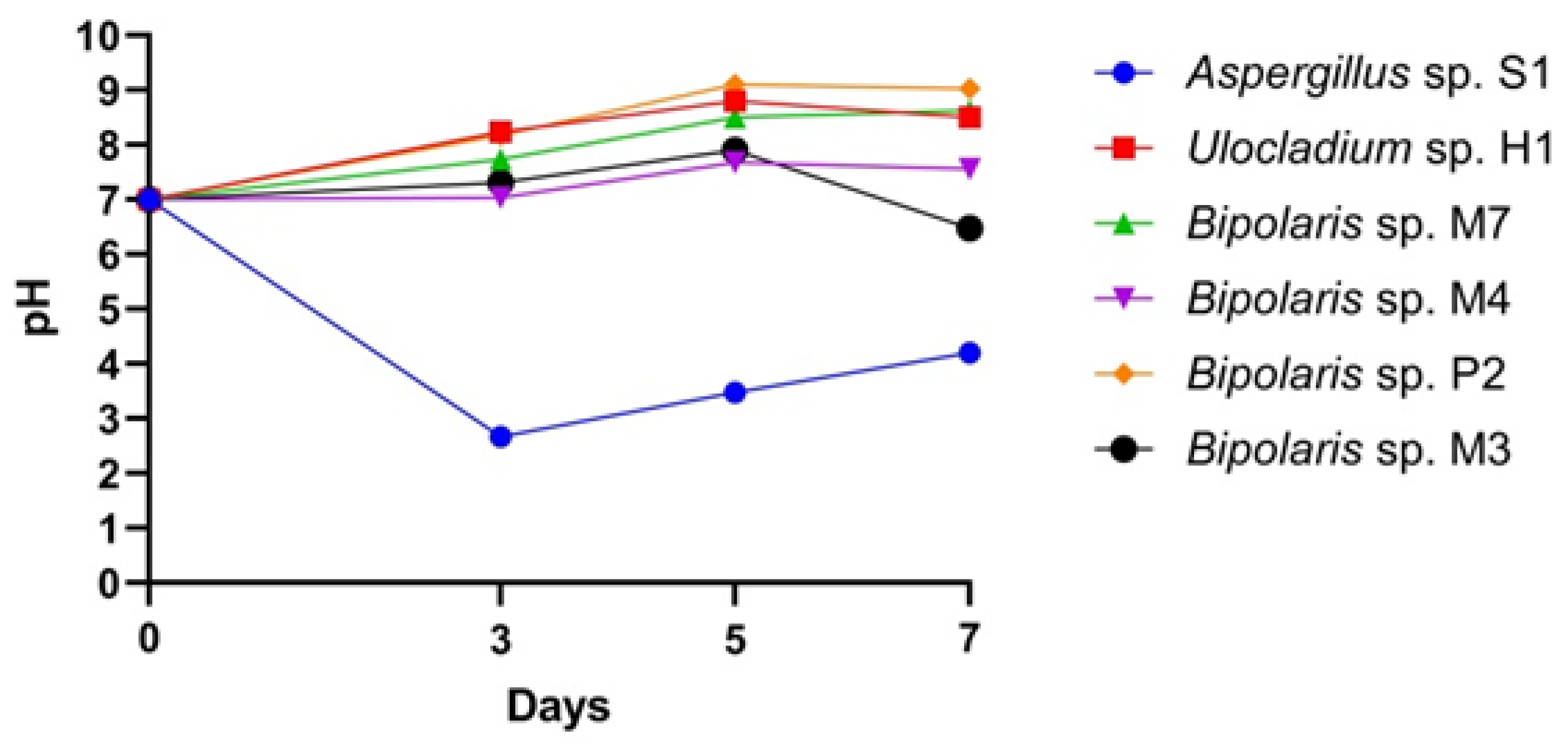
Organic Acid Production. Cultivation of fungi for 7 days in specific broth medium showed that they were able to change pH across a range from 7.00 to 2.6 or 8.24. The lowest measured pH values (2.6) were found in the case of the Aspergillus sp. S1 cultures. Ulocladium sp. H1 and all Bipolaris species increased the pH of the culture medium to between 7.1 and 8.24 (Figure 2). Aspergillus species, especially A. niger, are well-known as the best producers of citric, gluconic, malic, formic and oxalic acids [55,56]. After 5 days of Aspergillus sp. S1 incubation, the pH value of the culture medium decreased to 2.6, indicating that this strain had a higher ability to enhance acidity than the Bipolaris and Ulocladium strains.

Figure 2.
The dynamics of the production of acids by the fungal isolates.
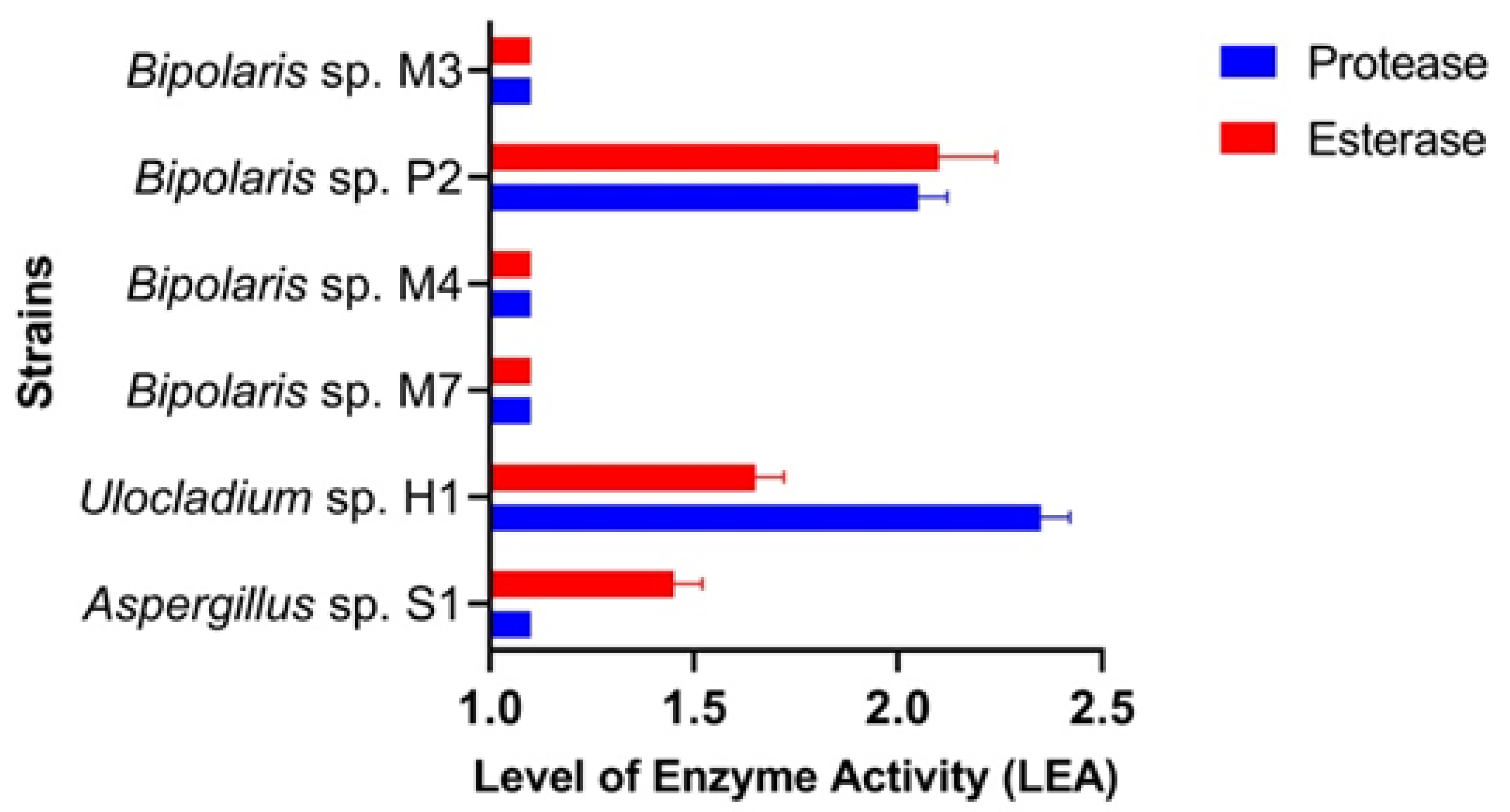
Protease Activity. Investigation using Petri dishes revealed that all isolates had LEsA values between 1.0 (Aspergillus sp. S1, Bipolaris sp. M3, Bipolaris sp. M4, Bipolaris sp. M7) and 2.3 (Ulocladium sp. H1). Remarkable protease activity (LEsA = 2.0) was also harboured by Bipolaris sp. P2 (Figure 3 and Figure S4).
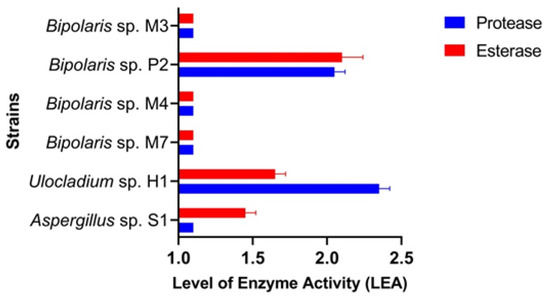
Figure 3.
The level of enzyme activity in the fungal isolates.
Esterase Activity. Investigation using Petri dishes revealed that all isolates had LEsA values between 1.0 (Bipolaris sp. M3, Bipolaris sp. M7, Bipolaris sp. M4) and 2.0 (Bipolaris sp. P2). Remarkable esterase activities were also harboured by Aspergillus sp. S1 and Ulocladium sp. H1 (Figure 3 and Figure S5).
3.3. Colonization and Degradation of Consolidants
Preliminary visual inspection showed that efflorescences had formed on the on the red and yellow pigments in the control and TDC-coated samples (Figure S1).
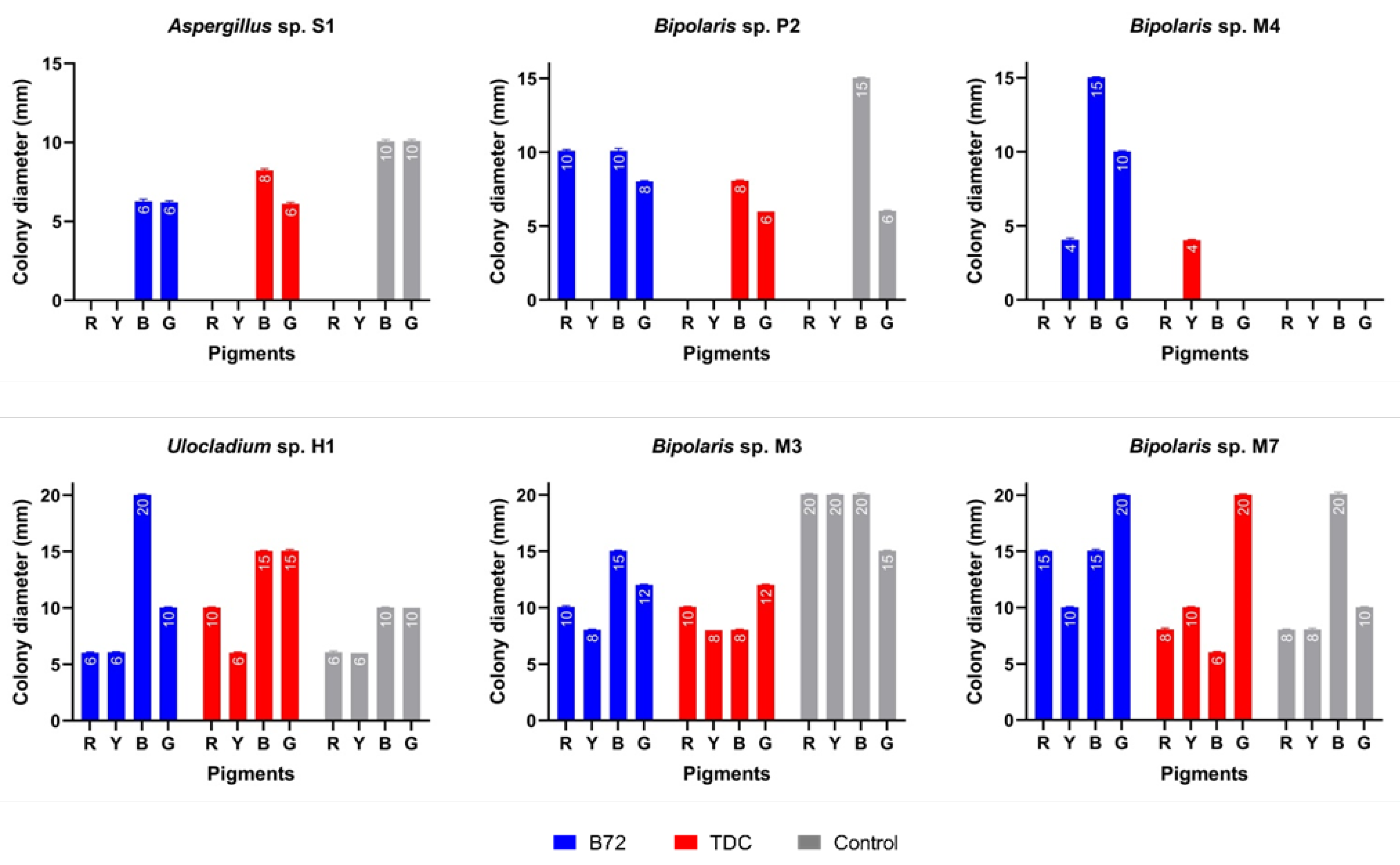
Direct examination revealed that all painted experimental models (control, covered with Paraloid® B72 and covered with TDC) were colonized by almost all of the inoculated fungal strains. Colonization of these substrates is evidence that fungi use them as a nutrient source, thus causing their degradation. The diameter of the colonies varied between 4 and 20 mm (Figure 4). Ulocladium sp. H1 produced the largest colonizations on the blue pigment covered with Paraloid® B72 and on the blue and green pigments covered with TDC (Figure 4). Bipolaris sp. M7 and Bipolaris sp. M3 showed the largest colonizations on all pigments covered with Paraloid® B72 and TDC. Bipolaris sp. M7 developed the largest colonies on the green pigment covered with both consolidants.

Figure 4.
Growth of fungal strains on painted experimental models covered with Paraloid® B72 and TDC. Abbreviations: R (red), Y (yellow), B (blue), G (green). Experimental models without consolidants were used as a control.
Bipolaris sp. M4 was the only strain that did not grow on the control. Aspergillus sp. S1 and Bipolaris sp. P2 did not grow on yellow pigment (all samples), suggesting its possible sensitivity to some toxic compound that might be present in the chemical composition of this pigment. The situation was similar for Aspergillus sp. S1 and Bipolaris sp. M4 in the case of red pigment (covered with TDC). Those fungal strains that grew on consolidants were considered to be directly involved in their decomposition because they used them as nutrient sources.
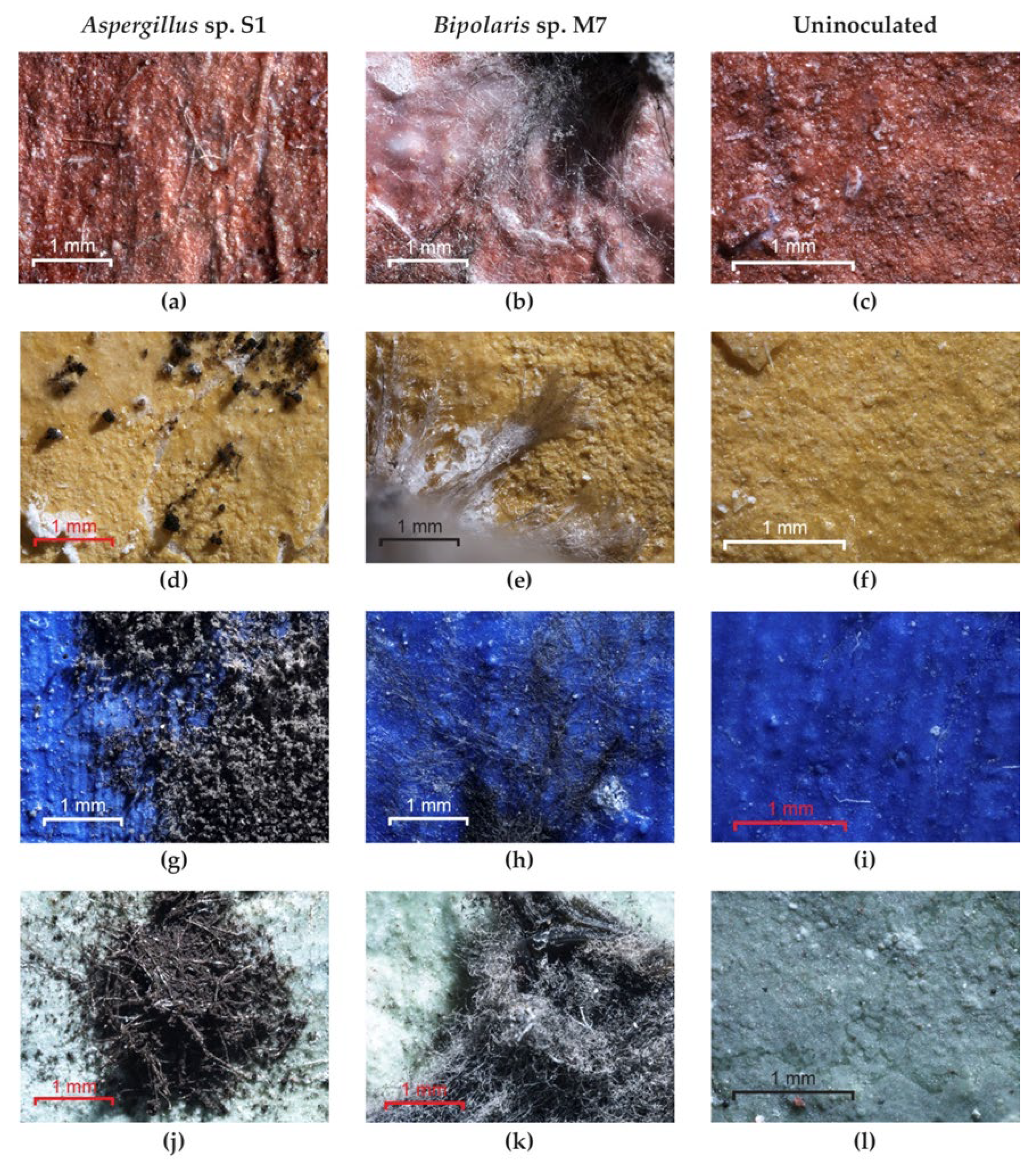
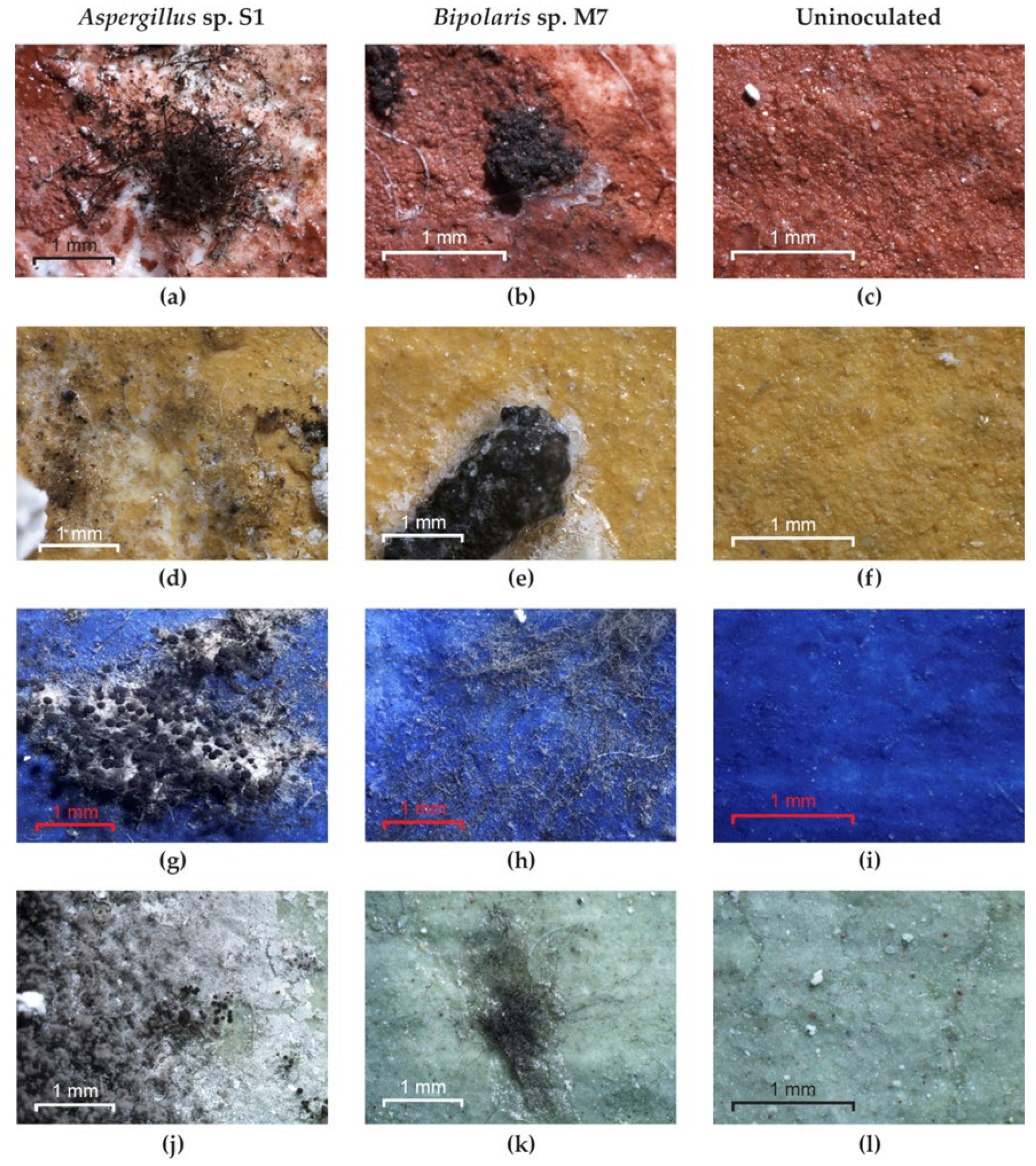
Optical microscopy examination of the painted experimental models covered with TDC showed the following: non-germinated spores and an absence of mycelium for Bipolaris sp. P2 and Aspergillus sp. S1 on red and yellow pigments (Figure 5a,d) but good growth on blue and green pigments (Figure 5g,j); non-germinated spores and an absence of mycelium for the Bipolaris sp. M4 strain on red, blue and green pigments; well-developed mycelium in the cases of the Ulocladium sp. H1, Bipolaris sp. M3 and Bipolaris sp. M7 strains on all pigments (Figure 5b,e,h,k); and no growth on the uninoculated control (Figure 5c,f,i,l). After cleaning the experimental models, discoloured areas corresponding to the removal of spores or mycelium were found for all strains and pigments due to the fact that these fungi firmly adhere to the TDC layer through the ornamentation (spores) and the exopolysaccharide layer (hyphae). In addition, in the cases of the yellow and green pigments, the diffusion of melanin pigments in the substrate was observed (Figure S6).

Figure 5.
The growth of fungi on the painted experimental models covered with TDC. (a,d,g,j) Aspergillus sp. S1; (b,e,h,k) Bipolaris sp. M7; (c,f,i,l) control.
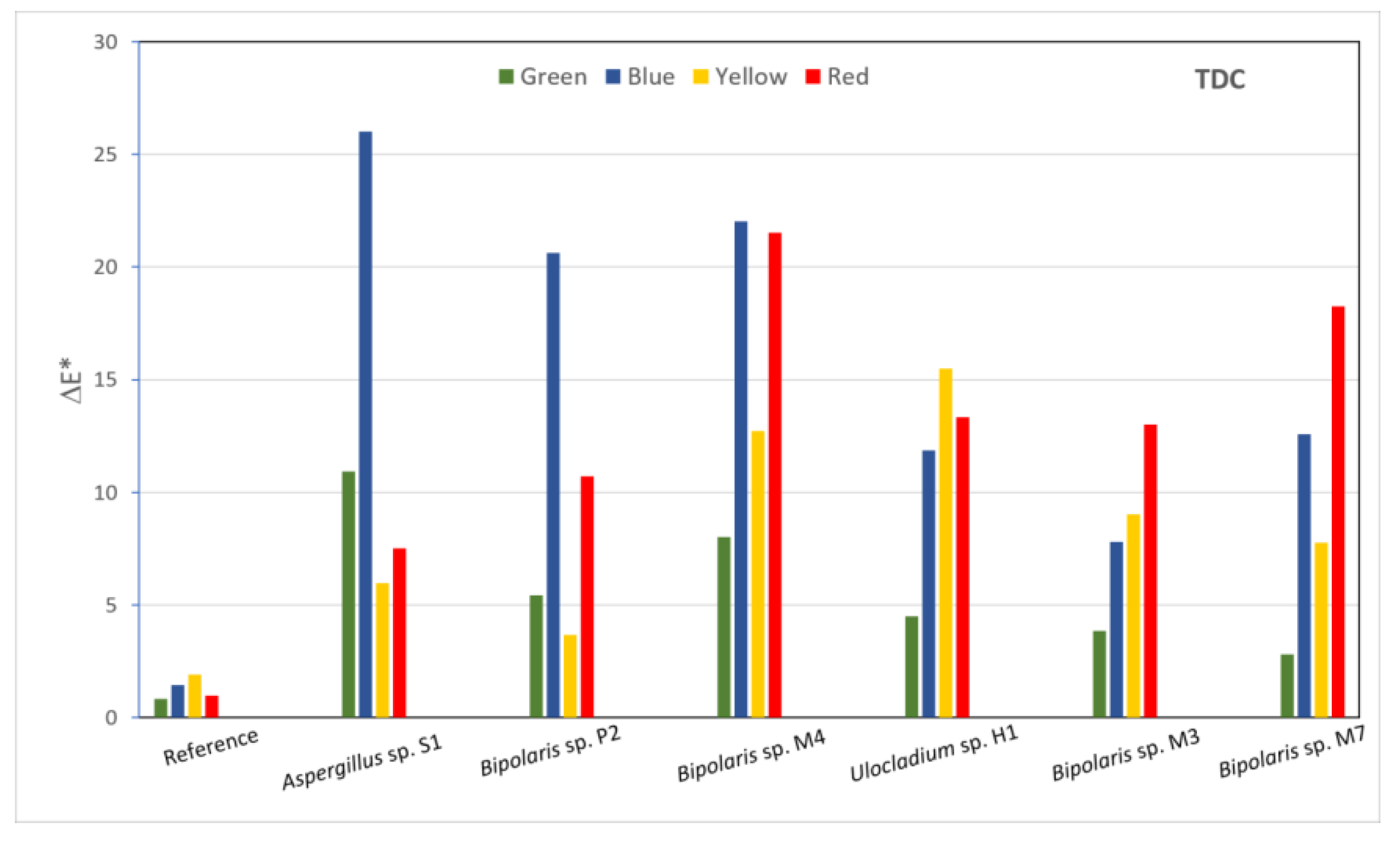
The effects of the fungi on the consolidants were also evaluated on the basis of the colour changes induced by inoculated fungi on the surfaces of the painted laboratory models covered with consolidants. The luminosity, red-green and yellow-blue coordinates of each inoculated area were recorded and, based on them, the ΔE value were calculated (Figure 6) in order to highlight the differences between areas prior to the application of consolidants and after various treatments had been applied. The calculated ΔE values are shown in (Table S1), where represents the difference between the samples with and without consolidants and (1–6) represents the difference between the reference and inoculated areas. In the case of the sample covered with TDC, all values were greater than the values, which means that, from the colourimetric point of view, there were greater changes induced between the inoculated areas and the reference areas than between the reference areas with and without consolidants. All areas showed colour changes that were perceptible with close observations, with values between 1 and 2 (Table S1). The lowest a* values were measured for the area inoculated with Bipolaris sp. M3 on red pigment and for the areas inoculated with Bipolaris sp. M4 and M7 on green pigment. The removal efficiency calculated from the colourimetry data (Table S2) showed very high negative values for all inoculated areas.
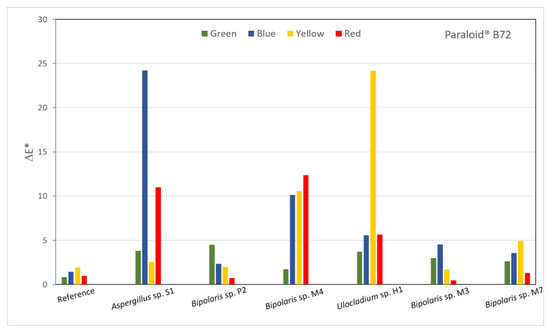
Figure 6.
Calculated ΔE* values for all inoculated areas of the sample covered with TDC.
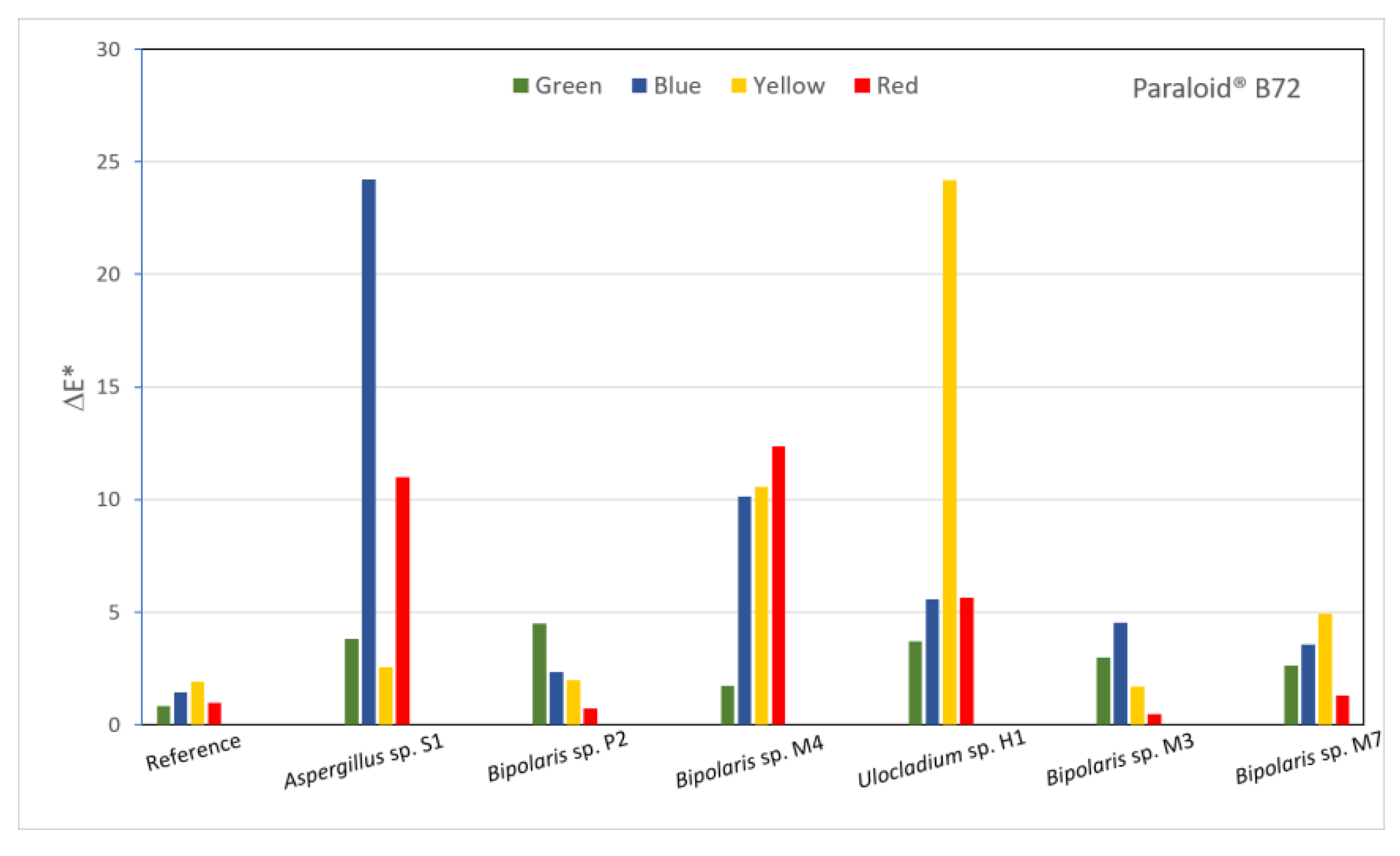
Most of the values calculated for the Paraloid® B72-covered sample (Figure 7) were between 1 and 2, which indicated colour changes perceptible through close observation. The lowest values were calculated for the Aspergillus sp. S1 and Bipolaris sp. M4 strains applied on yellow pigment, for the Bipolaris sp. P2, M4 and M7 strains applied on red pigment and for the Bipolaris sp. M3 strain applied on green pigment. These values were the closest to the reference, which may be an indication of the role of fungi in the decomposition of Paraloid® B72. At the opposite end, the highest differences were calculated for Aspergillus sp. S1 and Bipolaris sp. M3 applied on the blue and red pigments and for Bipolaris sp. M3 and Ulocladium sp. H1 applied on the yellow pigment.

Figure 7.
Calculated ΔE* values for all inoculated areas of the sample covered with Paraloid® B72.
Although all treated areas had higher luminosity values compared to the reference for the samples covered with Paraloid® B72, much higher L* values for the red and blue pigments could be seen in areas where Aspergillus sp. S1 and Bipolaris sp. M3 were inoculated. This correlated with the lowest a* values for the red pigment and higher a* values for the green pigment, which may have indicated colour changes in comparison to the other areas. Similarly, the lowest b* value was recorded for the area inoculated with Ulocladium sp. H1 on the yellow pigment, while the highest b* value was seen for the area inoculated with Bipolaris sp. P2 on the blue pigment. For both TDC and Paraloid® B72, a* values were lower than the reference for the red pigment, while b* values were mostly all higher than the reference for the green pigment, with the exception of the area treated with Bipolaris sp. P2. Similarly, for the blue pigment, b* values were all higher compared to the reference. Likewise, for the TDC-covered sample, the calculated removal efficiency for the treatments applied to the Paraloid® B72-covered sample (Table S2) indicated very high negative values. However, some exceptions were seen in three areas that were treated with Bipolaris sp. M3 (on yellow and red pigment) and Bipolaris sp. P2 (on red pigment), for which the calculated percentage values varied between 10 and 50%.
Optical microscopy examination of the experimental models covered with Paraloid® B72 showed the following: germinated spores and an absence of mycelium for Aspergillus sp. S1 (Figure 8a) and Bipolaris sp. M4 strains on red pigment; germinated spores and an absence of mycelium for Aspergillus sp. S1 (Figure 8d) and Bipolaris sp. P2 strains on yellow pigment; poorly developed mycelium for Aspergillus sp. S1 on blue and green pigments (Figure 8g,j); well-developed mycelium in the case of Bipolaris sp. P2 and Bipolaris sp. M4 on blue and green pigments; well-developed mycelium in the case of the strains Ulocladium sp. H1, Bipolaris sp. M3 and Bipolaris sp. M7 (Figure 8b,e,h,k) on all pigments; and no growth on the uninoculated control (Figure 8c,f,i,l). After cleaning the experimental models, discoloured areas corresponding to the removal of spores or mycelium could be observed due to the fact that these fungi adhere firmly to the Paraloid® B72 layer through the ornamentation and the exopolysaccharide layer. In addition, in the cases of the yellow and green pigments, diffusion of melanin pigments in the substrate was observed (Figure S6).

Figure 8.
The growth of fungi on the painted experimental models covered with Paraloid® B72. (a,d,g,j) Aspergillus sp. S1; (b,e,h,k) Bipolaris sp. M7; (c,f,i,l) control.
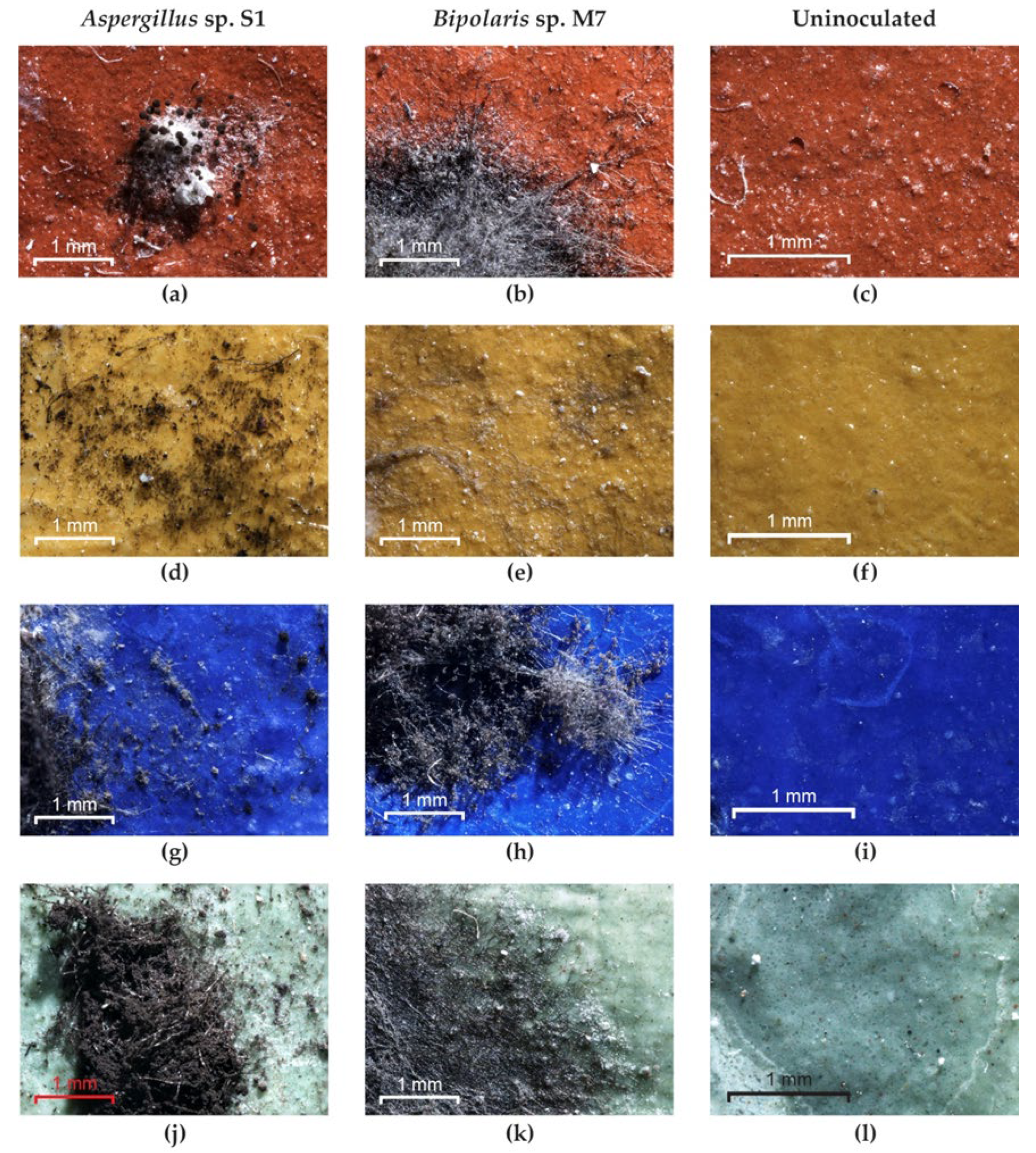
Optical microscopy examination of the experimental models used as inoculated controls revealed the following: the strain Bipolaris sp. M4 did not grow on any pigment; the Aspergillus sp. S1 (Figure 9a,d) and Bipolaris sp. P2 strains did not grow on red and yellow pigments but grew on green and blue pigments (Figure 9g,j); the strains Ulocladium sp. H1, Bipolaris sp. M3 and Bipolaris sp. M7 (Figure 9b,e,h,k) showed well-developed mycelium on all pigments; and no growth was noticed in the uninoculated control (Figure 9c,f,i,l).
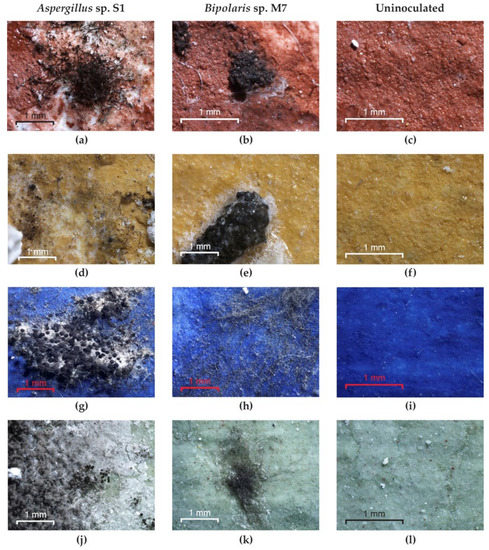
Figure 9.
The growth of fungi on the experimental models without consolidants. (a,d,g,j) Aspergillus sp. S1; (b,e,h,k) Bipolaris sp. M7; (c,f,i,l) control.
3.4. Improving Susceptibility of Consolidants to Degradation through Treatments with Biocides
The efficacy of applying commercial products based on quaternary ammonium salts with biocidal properties (Biotin R®, Biotin T® and Preventol® RI 80) to prevent fungal growth was evaluated.
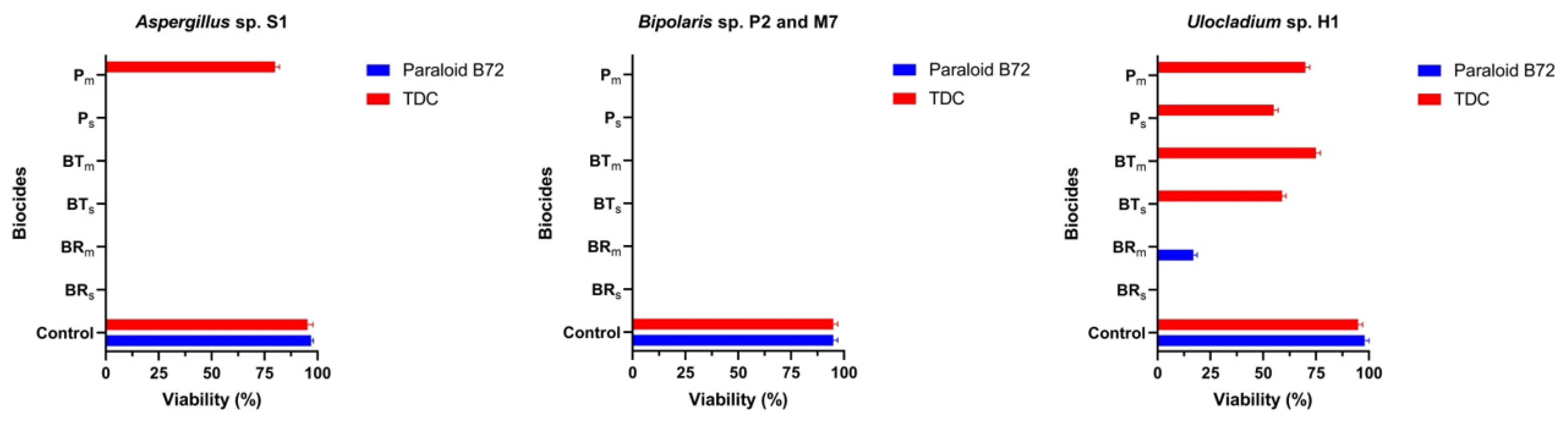
On slide cultures. Almost all tested biocides had toxic effects on the following strains: Aspergillus sp. S1, Bipolaris sp. P2 and Bipolaris sp. M7 (Figure 10). Although the germination of Aspergillus sp. S1 spores on consolidants was microscopically observed, the viability test was negative in all cases (Figure 10 and Figure S7). In the case of Bipolaris sp. P2 and Bipolaris sp. M7, for both consolidants, although some spores germinated or poor mycelium developed, the viability test was negative. Ulocladium sp. H1 did not germinate on either of the consolidants treated with Biotin R® but developed mycelium on TDC treated with Biotin T® and Preventol® RI 80.

Figure 10.
Viability of fungal strains on Paraloid® B72 and TDC coupled with biocides.
On the frescoes. Treatments of Paraloid® B72 and TDC consolidants with biocides applied to the surface or to the mixture prepared before application to the frescoes resulted in the prevention of colonization by the fungal strains used in experiments.
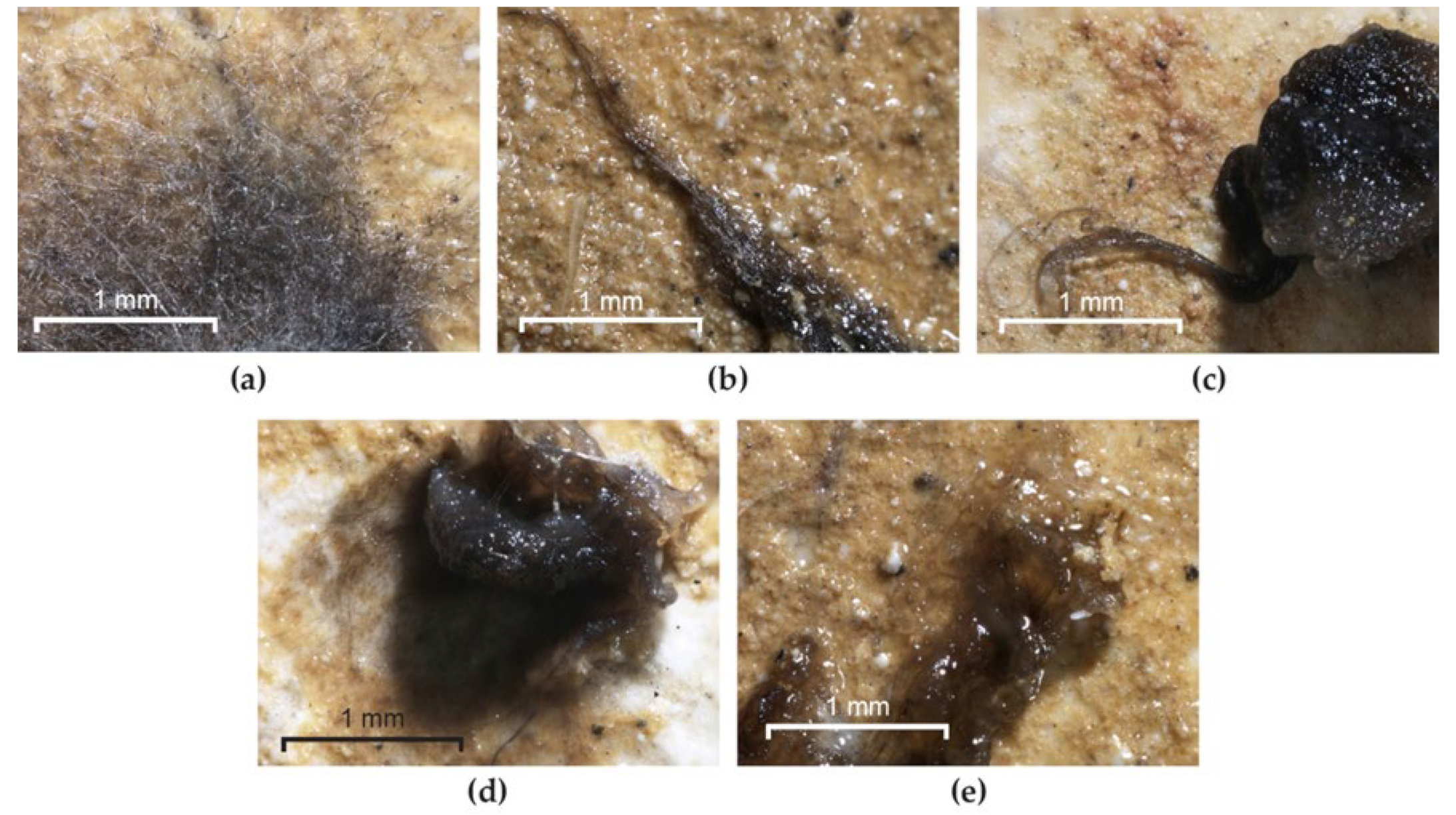
All the fungal species inoculated developed colonies on the frescoes used as controls. Colonial growth was not noticed in any of the experimental variants treated with Biotin R® (Figure 11b,c) or Biotin T® (Figure 11d). In the case of Preventol® RI 80, the treatment applied to the surface of the Paraloid® B72 only allowed partial germination of the spores and no formation of mycelium (Figure 11e). In none of these experiments were the spores or hyphae found to be viable, which demonstrates the toxic effect of biocide on fungal spores and, in consequence, the prevention of consolidant degradation.

Figure 11.
Resistance of Paraloid® B72 to Aspergillus sp. S1 colonization after biocide treatment. (a) Culture developed on frescoes without biocide; (b) masses of spores on BRs; (c) masses of spores on BRm; (d) masses of spores on BTs; (e) masses of spores on Ps.
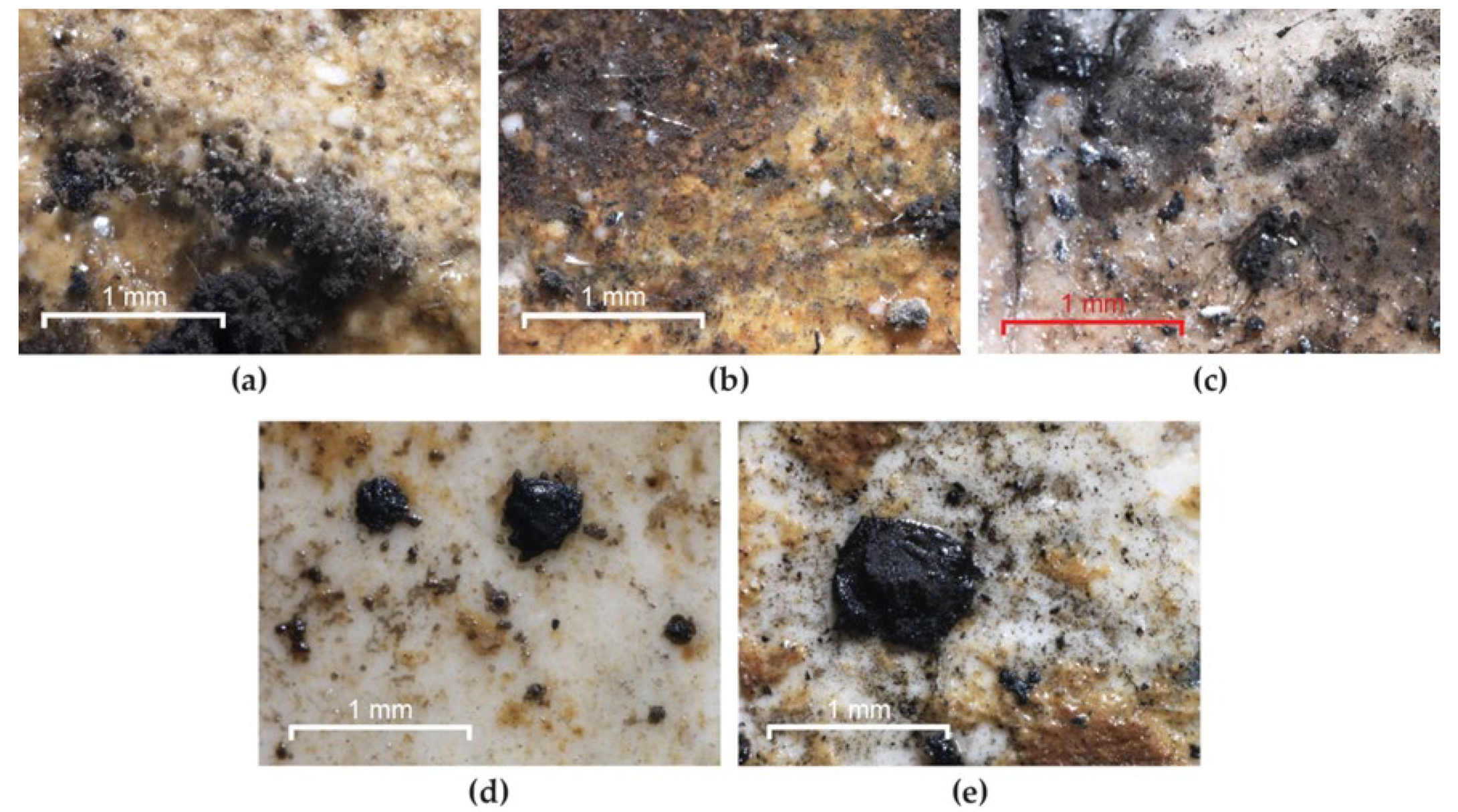
Frescoes consolidated with TDC and treated with biocides on the surface and in the form of a mixture were judged to be protected from fungal decomposition because they were not colonized (Figure 12). However, three exceptions were identified: the strain Bipolaris sp. M7 developed a brown-black mycelium on the frescoes treated with Biotin R® (Figure 12b), Biotin T® (Figure 12c) and Preventol® RI 80 on the surface (Figure 12d,e). In all cases, the mycelium ceased its vital activity until the end of the experiment. Combining these results with those obtained from the experimental painted models, we do not recommend the use of Preventol® RI 80 to prevent colonization because the hyphae firmly adhered to the TDC surface and cleaning attempts would cause tearing.

Figure 12.
Resistance of TDC to Bipolaris sp. M7 colonization after biocide treatment. (a) Culture developed on frescoes without biocide; (b) masses of spores on BRs; (c) masses of spores on BTs; (d) masses of spores on Ps; (e) masses of spores on Pm.
4. Discussion
According to Zucconi’s classification, the environment in the churches where the fungi were isolated for the present research was a confined non-hypogean environment (C-NHE). Monasteries are partially isolated from the external environment and have relatively more stable humidity and temperature than outdoor conditions, but they are influenced by external day–night cycles, seasonal variations in temperature and relative humidity values. They are more prone to biodeterioration since they include sites that are often visited.
The main genera found on the mural paintings as biodeteriogens were: Aspergillus, Penicillium, Chaetomium, Cladosporium, Acremonium and Fusarium [57,58,59,60,61]. Our research into their role as biodeteriogens of consolidants is in progress.
Aspergillus niger and Ulocladium chartarum are common contaminants of mural paintings [15,62]. A space experiment performed with U. chartarum isolated from Humor Monastery proved its adaptability to growth in microgravity conditions [46]. The present results revealed the resistance of Ulocladium sp. H1 to biocides.
The fungal strains isolated from the murals could have possibly originated from contaminated plant debris used in the production of the murals, outdoor airspora, percolation of water from the upper soil or outside soil brought in by visitors and small animals. Similar results were obtained in the case of Fusarium solani found in the Lascaux caves, France [63]. Jurado et al. [64] isolated entomogenous fungi from caves in northern Spain and showed that species of fungi can grow both on arthropod bodies and on wet mural paintings. In Romania, Acremonium roseum, Trichothecium roseum and Tritirachium album were found in 1973 in the parochial church of Sucevita that had been spread by an arthropod [65]. Later, the same species were also found on mural paintings and historical buildings [60,66,67], most probably spread by spiders and mites. Our results highlight the role of the larva identified among the deposited frescoes in the spreading of microorganisms.
Dornieden et al. [12] demonstrated that microcolonial black fungi are among the most dangerous fungi for cultural heritage and can influence the resistance of mortar and marble to shear and torsion stress, contributing to the separation of different layers of material in mural paintings. Our results revealed that, except for the species A. niger, all the other fungi produced melanin pigments that were fixed in the cell wall or diffused over the surface of the consolidants.
Consolidants used in restoration, such as Paraloid® B72 and TDC, can be used as nutrients by microorganisms. Other nutrients may also arrive from the external environment as airborne particles [4,68,69,70].
The polymer B72 was found to protect and fix the surface of the pictorial layer, but it reduced the permeability to vapours and thus altered the physicochemical activity in the interface between the mural and the environment. Humidity and microbial activity accelerate oxidation and cause blackening of damaged areas fixed with Paraloid® B72 [71]. Our results also revealed fungal growth after 48 of incubation and morphological damage after 30 days of incubation in the painted laboratory models.
The secretion of organic acids plays a significant role in the chemical degradation of consolidants by causing acidification of the substrate, which then becomes favourable for acidophilic microorganisms [14,17,72]. These microorganisms can cause the dissolution of cations and chelation of metal ions from mortar and mineral pigments, leading to the formation of stable metal complexes whose crystallization results in an increase in internal pressure, resulting in cracking, peeling and the eventual loss of mural fragments [49]. Aspergillus sp. S1 is a suitable example.
The finding that some strains increased the pH of the medium could have been a result of the production of alkaline metabolites, such as NH3 and polypeptides. According to Unković et al. [49], acids are dangerous metabolites, but deterioration can also occur via alkaline reactions through degradation of different nitrogen components or Na salts in organic acids.
Our results showed that the tested fungi had both the ability to produce organic acids and significant hydrolytic capacities, indicating their capacity to decompose the tested consolidants and other deposits of organic material on the pictorial layer. All of these activities were expressed in structural and aesthetic alterations in the mural paintings [21]. These broad enzymatic activities allow fungi to grow on every type of material or anywhere they find organic matter [68,73,74]. Proteolytic activity suggests the potential of proteases to degrade casein in amino acids, which is needed for colony growth and production of metabolites [69,75,76]. Bipolaris sp. M4 and Ulocadium sp. H1 are good producers of proteases and, therefore, considered as two of the primary degraders of TDC. Lipases and esterases are involved in the removal of layers of aged Paraloid® B72 [77]. Since the optimum pH for their activity is 8.0 [16], we deduced that, as a first step, the isolates change the substrate pH and then produce hydrolytic enzymes, which show high activity at this pH value.
Synthetic resins applied to the monuments result in advanced chemical and physical degradation, such as yellowing and cracking, after about 30 years, facilitating biological degradation [42,78]. Our results confirmed this hypothesis.
All the fungal strains except Bipolaris sp. M4 could colonize both the control and painted experimental models covered with Paraloid® B72 and TDC because of their versatile metabolism and their ability to grow on substrates with low nutrient content. The pigments from the painted experimental models most likely contained growth inhibitors, but the consolidants removed this effect because they blocked the direct contact with the spores and mycelium.
The investigated fungal species were demonstrated to have the potential to decompose the consolidants used during previous restorations, resulted in the loss of their role fixing the pictorial layer and implicitly rendering them powdery. The use of rapid biodegradation screening tests is mandatory for the assessment of the physiological capacity of autochthonous microbiota (contaminated areas and air spores), which are causative agents of structural and aesthetic weathering.
Our results showed that black fungi could colonize not only aged synthetic acrylics but also those that had been recently applied.
In situ, the decomposition of consolidants and the biodeterioration of the pictorial layer can be avoided through strict control of the temperature and RH of the microclimate of the indoor environment, thus enabling long-term conservation of wall paintings [79].
Removal of surface adherences from mural paintings is expected to yield colour changes, which is usually an indicator of the efficiency of the removal process [51]. From the colorimetric point of view, there were indeed changes observed for all treated areas, for both the TDC- and Paraloid® B72-covered samples. The fact that several fungi yielded the greatest and smallest differences for different pigments might suggest that fungi efficiency in the decomposition of aged consolidants also depends on the interaction of the consolidant with the painted layer. The fact that the removal efficiency values were mostly highly negative can be correlated with the input from the black melanic pigment. The appearance of black fungal pigments created more variability on the surface and did not allow the final stage of the decomposition of the consolidants to be highlighted by colourimetry.
Several biocides were tested with laboratory cultures with the aim of identifying which was the most effective for the prevention of consolidants’ degradation from fungal growth. Three biocides were tested in vitro on slide cultures and fresco fragments. On the basis of the obtained results, Biotin R® was judged to be significantly more effective than Biotin T® and Preventol RI80® for both consolidants and for all the black fungal strains tested. The initiation of germination or the formation of a few mm of mycelium on a surface do not indicate the inefficiency of a biocide, but we considered such cases as weak biological activities that eventually ceased and did not involve the formation of colonies. All biocides had as their main target the cell membrane, thus affecting the permeability, the denaturation of proteins and the inhibition of cytochrome C, producing hypoxia [25].
Based on the results obtained in this work, we recommend that Paraloid® B72 and TDC be treated with Biotin R® by brushing in buildings with high RH or with Biotin T® in buildings with low RH.
Future research should aim to identify the duration of the resistance of biocide-treated consolidants in order to ensure long-time conservation and determine their effectiveness in the restoration of mural paintings.
5. Conclusions
The present research identified new species of fungi involved in the deterioration of mural paintings, and two consolidants used in restoration were assessed using microbiological and biochemical methods and colourimetry. They were isolated from deposited frescoes or from mural paintings from two Romanian monasteries. The fungal isolates contributed to deterioration through both biochemical ways—producing organic acids, hydrolytic enzymes and black pigments—and a mechanical way—stripping the pictorial layer or consolidant when the mycelium was removed. Experiments were performed with consolidants applied to an inert support and to fragments of frescoes treated with three different types of biocides, either applied to the surface or mixed with the consolidants. Finding that Paraloid® B72 and TDC treated with biocides were resistant to colonization by fungi encourages us to recommend them for use in consolidation because they can be expected to ensure the long-term conservation of restored mural paintings.
The obtained results can be considered as a first attempt in an innovative research field that has two main goals: improving the resistance of consolidants to fungal colonization and long-term conservation of restored mural paintings. Experimental studies will be carried out to evaluate the properties of the consolidants after treatment with biocides and their compatibility with the pictorial layer.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app12147229/s1, Figure S1: Painted laboratory models before and after inoculation with Aspergillus sp. S1, Bipolaris sp. P2, Bipolaris sp. M3, Ulocladium sp. H1, Bipolaris sp. M4, Bipolaris sp. M7. Figure S2: Experimental design to evaluate the resistance of consolidants with biocides integrated to fungal colonization. Figure S3: Experimental design to evaluate the resistance of consolidants with biocides integrated to fungal colonization. Figure S4: Identification of protease activity by plate assay method. Figure S5: Identification of esterase activity by plate assay method. Figure S6: Changes produced by fungi on the surface of consolidants. Figure S7: The effect of biocides applied on consolidants on the growth of Aspergillus sp. S1. Table S1: Calculated ΔE for all treated areas. Table S2: Cleaning efficient percentage as calculated from the colorimetry data.
Author Contributions
Conceptualization, I.G., R.C., R.R. (Roxana Rădvan) and L.G.; methodology I.G., R.C., R.R. (Robert Ruginescu), S.N., M.D. and L.G.; formal analysis, M.E.; investigation, I.G., R.C., R.R. (Robert Ruginescu), S.N., M.D., R.R. (Roxana Rădvan) and L.G.; resources, I.G.; writing—original draft preparation, I.G., L.G., M.D., R.R. (Robert Ruginescu), I.O. and R.C.; writing—review and editing, I.G., R.R. (Robert Ruginescu), I.O. and R.C.; project administration, I.G.; funding acquisition, I.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Executive Agency for Higher Education, Research, Development and Innovation Funding (UEFISCDI), grant number 570PED/2020, and by the Romanian Academy, grant number RO1567-IBB05/2021.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pinna, D. Microbial Growth and Its Effects on Inorganic Heritage Materials. In Microorganisms in the Deterioration and Preservation of Cultural Heritage; Joseph, E., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 3–35. ISBN 978-3-030-69410-4. [Google Scholar]
- Isola, D.; Zucconi, L.; Cecchini, A.; Caneva, G. Dark-Pigmented Biodeteriogenic Fungi in Etruscan Hypogeal Tombs: New Data on Their Culture-Dependent Diversity, Favouring Conditions, and Resistance to Biocidal Treatments. Fungal Biol. 2021, 125, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Terada, M.; Ohki, E.; Yamagishi, Y.; Nishiyama, Y.; Satoh, K.; Uchida, K.; Yamaguchi, H.; Mikamo, H. Fungal Peritonitis Associated with Curvularia Geniculata and Pithomyces Species in a Patient with Vulvar Cancer Who Was Successfully Treated with Oral Voriconazole. J. Antibiot. 2014, 67, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ma, X.; Ma, Y.; Mao, L.; Wu, F.; Ma, X.; An, L.; Feng, H. Molecular Characterization of Airborne Fungi in Caves of the Mogao Grottoes, Dunhuang, China. Int. Biodeterior. Biodegrad. 2011, 65, 726–731. [Google Scholar] [CrossRef]
- Wang, W.; Ma, Y.; Ma, X.; Wu, F.; Ma, X.; An, L.; Feng, H. Diversity and Seasonal Dynamics of Airborne Bacteria in the Mogao Grottoes, Dunhuang, China. Aerobiologia 2012, 28, 27–38. [Google Scholar] [CrossRef]
- Zucconi, L.; Canini, F.; Isola, D.; Caneva, G. Fungi Affecting Wall Paintings of Historical Value: A Worldwide Meta-Analysis of Their Detected Diversity. Appl. Sci. 2022, 12, 2988. [Google Scholar] [CrossRef]
- Dyda, M.; Laudy, A.; Decewicz, P.; Romaniuk, K.; Ciezkowska, M.; Szajewska, A.; Solecka, D.; Dziewit, L.; Drewniak, L.; Skłodowska, A. Diversity of Biodeteriorative Bacterial and Fungal Consortia in Winter and Summer on Historical Sandstone of the Northern Pergola, Museum of King John III’s Palace at Wilanow, Poland. Appl. Sci. 2021, 11, 620. [Google Scholar] [CrossRef]
- Pinna, D. Coping with Biological Grown on Stone Heritage Objects: Methods, Products, Applications, and Perspectives; Apple Academic Press: Palm Bay, FL, USA, 2021; ISBN 978-1-77463-672-5. [Google Scholar]
- Giannini, C.; Tapete, D. Materiali e Procedimenti Esecutivi Della Pittura Murale; Il Prato: Saonara, Italy, 2009; ISBN 978-88-6336-052-3. [Google Scholar]
- Unković, N.; Grbić, M.L.; Stupar, M.; Savković, Ž.; Jelikić, A.; Stanojević, D.; Vukojević, J. Fungal-Induced Deterioration of Mural Paintings: In Situ and Mock-Model Microscopy Analyses. Microsc. Microanal. 2016, 22, 410–421. [Google Scholar] [CrossRef] [Green Version]
- Ciferri, O. Microbial Degradation of Paintings. Appl. Environ. Microbiol. 1999, 65, 879–885. [Google Scholar] [CrossRef] [Green Version]
- Dornieden, T.; Gorbushina, A.A.; Krumbein, W.E. Biodecay of Cultural Heritage as a Space/Time-Related Ecological Situation—An Evaluation of a Series of Studies. Int. Biodeterior. Biodegrad. 2000, 46, 261–270. [Google Scholar] [CrossRef]
- Sáiz-Jiménez, C.; Samson, R.A. Biodegradacion de Obras de Arte. Hongos Implicados En La Degradacion de Los Frescos Del Monasterio de La Rabida (Huelva). Bot. Macaronesica 1981, 8–9, 255–264. [Google Scholar]
- Ma, W.; Wu, F.; Tian, T.; He, D.; Zhang, Q.; Gu, J.-D.; Duan, Y.; Ma, D.; Wang, W.; Feng, H. Fungal Diversity and Its Contribution to the Biodeterioration of Mural Paintings in Two 1700-Year-Old Tombs of China. Int. Biodeterior. Biodegrad. 2020, 152, 104972. [Google Scholar] [CrossRef]
- Gomoiu, I.; Cojoc, R.L.; Enache, M.I.; Neagu, S.E.; Mohanu, D.; Mohanu, I. Microbial Ability to Colonize Mural Painting and Its Substrate. Acta Phys. Pol. A 2018, 134, 383–386. [Google Scholar] [CrossRef]
- Ruginescu, R.; Enache, M.; Popescu, O.; Gomoiu, I.; Cojoc, R.; Batrinescu-Moteau, C.; Maria, G.; Dumbravician, M.; Neagu, S. Characterization of Some Salt-Tolerant Bacterial Hydrolases with Potential Utility in Cultural Heritage Bio-Cleaning. Microorganisms 2022, 10, 644. [Google Scholar] [CrossRef]
- Rosado, T.; Gil, M.; Caldeira, A.T.; Martins, M.d.R.; Dias, C.B.; Carvalho, L.; Mirão, J.; Candeias, A.E. Material Characterization and Biodegradation Assessment of Mural Paintings: Renaissance Frescoes from Santo Aleixo Church, Southern Portugal. Int. J. Archit. Herit. 2014, 8, 835–852. [Google Scholar] [CrossRef]
- De Leo, F.; Antonelli, F.; Pietrini, A.M.; Ricci, S.; Urzì, C. Study of the Euendolithic Activity of Blackmeristematic Fungi Isolated from a Marble Statue in the Quirinale Palace’s Gardens InRome, Italy. Facies 2019, 65, 18. [Google Scholar] [CrossRef]
- Toreno, G.; Isola, D.; Meloni, P.; Carcangiu, G.; Selbmann, L.; Onofri, S.; Caneva, G.; Zucconi, L. Biological Colonization on Stone Monuments: A New Low Impact Cleaning Method. J. Cult. Herit. 2018, 30, 100–109. [Google Scholar] [CrossRef]
- Trovão, J.; Tiago, I.; Soares, F.; Paiva, D.S.; Mesquita, N.; Coelho, C.; Catarino, L.; Gil, F.; Portugal, A. Description of Aeminiaceae Fam. Nov., Aeminium Gen. Nov. and Aeminium ludgeri sp. Nov. (Capnodiales), Isolated from a Biodeteriorated Art-Piece in the Old Cathedral of Coimbra, Portugal. MycoKeys 2019, 45, 57–73. [Google Scholar] [CrossRef]
- Savković, Ž.; Stupar, M.; Unković, N.; Ivanović, Ž.; Blagojević, J.; Vukojević, J.; Ljaljević Grbić, M. In Vitro Biodegradation Potential of Airborne Aspergilli and Penicillia. Sci. Nat. 2019, 106, 8. [Google Scholar] [CrossRef]
- Sanmartín, P.; Carballeira, R. Changes in Heterotrophic Microbial Communities Induced by Biocidal Treatments in the Monastery of San Martiño Pinario (Santiago de Compostela, NW Spain). Int. Biodeterior. Biodegrad. 2021, 156, 105130. [Google Scholar] [CrossRef]
- Sanmartín, P.; DeAraujo, A.; Vasanthakumar, A. Melding the Old with the New: Trends in Methods Used to Identify, Monitor, and Control Microorganisms on Cultural Heritage Materials. Microb. Ecol. 2018, 76, 64–80. [Google Scholar] [CrossRef]
- Sanmartín, P.; Fuentes, E.; Montojo, C.; Barreiro, P.; Paz-Bermúdez, G.; Prieto, B. Tertiary Bioreceptivity of Schists from Prehistoric Rock Art Sites in the Côa Valley (Portugal) and Siega Verde (Spain) Archaeological Parks: Effects of Cleaning Treatments. Int. Biodeterior. Biodegrad. 2019, 142, 151–159. [Google Scholar] [CrossRef]
- Sanmartín, P.; Rodríguez, A.; Aguiar, U. Medium-Term Field Evaluation of Several Widely Used Cleaning-Restoration Techniques Applied to Algal Biofilm Formed on a Granite-Built Historical Monument. Int. Biodeterior. Biodegrad. 2020, 147, 104870. [Google Scholar] [CrossRef]
- Fiorillo, F.; Fiorentino, S.; Montanari, M.; Roversi Monaco, C.; Del Bianco, A.; Vandini, M. Learning from the Past, Intervening in the Present: The Role of Conservation Science in the Challenging Restoration of the Wall Painting Marriage at Cana by Luca Longhi (Ravenna, Italy). Herit. Sci. 2020, 8, 10. [Google Scholar] [CrossRef]
- Kakakhel, M.A.; Wu, F.; Gu, J.-D.; Feng, H.; Shah, K.; Wang, W. Controlling Biodeterioration of Cultural Heritage Objects with Biocides: A Review. Int. Biodeterior. Biodegrad. 2019, 143, 104721. [Google Scholar] [CrossRef]
- Leplat, J.; Francois, A.; Bousta, F. White Fungal Covering on the Wall Paintings of the Saint-Savin-Sur-Gartempe Abbey Church Crypt: A Case Study. Int. Biodeterior. Biodegrad. 2017, 122, 29–37. [Google Scholar] [CrossRef]
- Di Turo, F.; Medeghini, L. How Green Possibilities Can Help in a Future Sustainable Conservation of Cultural Heritage in Europe. Sustainability 2021, 13, 3609. [Google Scholar] [CrossRef]
- Ruggiero, L.; Sodo, A.; Cestelli-Guidi, M.; Romani, M.; Sarra, A.; Postorino, P.; Ricci, M.A. Raman and ATR FT-IR Investigations of Innovative Silica Nanocontainers Loaded with a Biocide for Stone Conservation Treatments. Microchem. J. 2020, 155, 104766. [Google Scholar] [CrossRef]
- Presentato, A.; Armetta, F.; Spinella, A.; Chillura Martino, D.F.; Alduina, R.; Saladino, M.L. Formulation of Mesoporous Silica Nanoparticles for Controlled Release of Antimicrobials for Stone Preventive Conservation. Front. Chem. 2020, 8, 699. [Google Scholar] [CrossRef]
- Arreche, R.; Vázquez, P. Green Biocides to Control Biodeterioration in Materials Science and the Example of Preserving World Heritage Monuments. Curr. Opin. Green Sustain. Chem. 2020, 25, 100359. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green Extraction of Natural Products. Origins, Current Status, and Future Challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Lo Schiavo, S.; De Leo, F.; Urzì, C. Present and Future Perspectives for Biocides and Antifouling Products for Stone-Built Cultural Heritage: Ionic Liquids as a Challenging Alternative. Appl. Sci. 2020, 10, 6568. [Google Scholar] [CrossRef]
- Negri, A.; Nervo, M.; Di Marcello, S.; Castelli, D. Consolidation and Adhesion of Pictorial Layers on a Stone Substrate. The Study Case of the Virgin with the Child from Palazzo Madama, in Turin. Coatings 2021, 11, 624. [Google Scholar] [CrossRef]
- Artesani, A.; Di Turo, F.; Zucchelli, M.; Traviglia, A. Recent Advances in Protective Coatings for Cultural Heritage–An Overview. Coatings 2020, 10, 217. [Google Scholar] [CrossRef] [Green Version]
- Baglioni, P.; Carretti, E.; Chelazzi, D. Nanomaterials in Art Conservation. Nat. Nanotech 2015, 10, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Chapman, S.; Mason, D. Literature Review: The Use of Paraloid B-72 as a Surface Consolidant for Stained Glass. J. Am. Inst. Conserv. 2003, 42, 381–392. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Mohamed, W.S.; Mohamed, H.M. Comparative and Experimental Studies for Evaluation of Paraloid B-72 in Traditional and Nano Forms for Joining of Pottery Samples. JNanoR 2020, 61, 61–71. [Google Scholar] [CrossRef]
- Vinçotte, A.; Beauvoit, E.; Boyard, N.; Guilminot, E. Effect of Solvent on PARALOID® B72 and B44 Acrylic Resins Used as Adhesives in Conservation. Herit. Sci. 2019, 7, 42. [Google Scholar] [CrossRef]
- Elhagrassy, A.F. Modification of EAPC-XYL by Pseudomonas Lipases Bacteria to Remove Acrylic from the Mural Oil Paintings. Shedet 2019, 6, 189–202. [Google Scholar] [CrossRef]
- Cappitelli, F.; Nosanchuk, J.D.; Casadevall, A.; Toniolo, L.; Brusetti, L.; Florio, S.; Principi, P.; Borin, S.; Sorlini, C. Synthetic Consolidants Attacked by Melanin-Producing Fungi: Case Study of the Biodeterioration of Milan (Italy) Cathedral Marble Treated with Acrylics. Appl. Environ. Microbiol. 2007, 73, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Istudor, I.; Ciobanu, G. Dispersii de Cazeinat de Calciu Folosite În Conservarea Picturilor Murale Iîn Frescă Și Tempera.; Muzeul Naţional de Istorie: Bucharest, Romania, 1982; pp. 126–130. [Google Scholar]
- Okpalanozie, O.E.; Adebusoye, S.A.; Troiano, F.; Cattò, C.; Ilori, M.O.; Cappitelli, F. Assessment of Indoor Air Environment of a Nigerian Museum Library and Its Biodeteriorated Books Using Culture-Dependent and –Independent Techniques. Int. Biodeterior. Biodegrad. 2018, 132, 139–149. [Google Scholar] [CrossRef]
- Isola, D.; Zucconi, L.; Onofri, S.; Caneva, G.; de Hoog, G.S.; Selbmann, L. Extremotolerant Rock Inhabiting Black Fungi from Italian Monumental Sites. Fungal Divers. 2016, 76, 75–96. [Google Scholar] [CrossRef]
- Gomoiu, I.; Chatzitheodoridis, E.; Vadrucci, S.; Walther, I. The Effect of Spaceflight on Growth of Ulocladium chartarum Colonies on the International Space Station. PLoS ONE 2013, 8, e62130. [Google Scholar] [CrossRef]
- Ruginescu, R.; Gomoiu, I.; Popescu, O.; Cojoc, R.; Neagu, S.; Lucaci, I.; Batrinescu-Moteau, C.; Enache, M. Bioprospecting for Novel Halophilic and Halotolerant Sources of Hydrolytic Enzymes in Brackish, Saline and Hypersaline Lakes of Romania. Microorganisms 2020, 8, 1903. [Google Scholar] [CrossRef]
- Borrego, S.; Guiamet, P.; Gómez de Saravia, S.; Batistini, P.; Garcia, M.; Lavin, P.; Perdomo, I. The Quality of Air at Archives and the Biodeterioration of Photographs. Int. Biodeterior. Biodegrad. 2010, 64, 139–145. [Google Scholar] [CrossRef]
- Unković, N.; Dimkić, I.; Stupar, M.; Stanković, S.; Vukojević, J.; Ljaljević Grbić, M. Biodegradative Potential of Fungal Isolates from Sacral Ambient: In Vitro Study as Risk Assessment Implication for the Conservation of Wall Paintings. PLoS ONE 2018, 13, e0190922. [Google Scholar] [CrossRef]
- Menasria, T.; Aguilera, M.; Hocine, H.; Benammar, L.; Ayachi, A.; Si Bachir, A.; Dekak, A.; Monteoliva-Sánchez, M. Diversity and Bioprospecting of Extremely Halophilic Archaea Isolated from Algerian Arid and Semi-Arid Wetland Ecosystems for Halophilic-Active Hydrolytic Enzymes. Microbiol. Res. 2018, 207, 289–298. [Google Scholar] [CrossRef]
- Gioventù, E.; Lorenzi, P. Bio-Removal of Black Crust from Marble Surface: Comparison with Traditional Methodologies and Application on a Sculpture from the Florence’s English Cemetery. Procedia Chem. 2013, 8, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Baglioni, M.; Rengstl, D.; Berti, D.; Bonini, M.; Giorgi, R.; Baglioni, P. Removal of Acrylic Coatings from Works of Art by Means of Nanofluids: Understanding the Mechanism at the Nanoscale. Nanoscale 2010, 2, 1723. [Google Scholar] [CrossRef]
- Al-Emam, E.; Motawea, A.G.; Janssens, K.; Caen, J. Evaluation of Polyvinyl Alcohol–Borax/Agarose (PVA–B/AG) Blend Hydrogels for Removal of Deteriorated Consolidants from Ancient Egyptian Wall Paintings. Herit. Sci. 2019, 7, 22. [Google Scholar] [CrossRef]
- CIE Recommendations on Uniform Color Spaces, Color-Difference Equations, and Metric Color Terms. Color Res. Appl. 1977, 2, 5–6. [CrossRef]
- Znad, H.; Markoš, J.; Baleš, V. Production of Gluconic Acid from Glucose by Aspergillus niger: Growth and Non-Growth Conditions. Process Biochem. 2004, 39, 1341–1345. [Google Scholar] [CrossRef]
- Li, Z.; Bai, T.; Dai, L.; Wang, F.; Tao, J.; Meng, S.; Hu, Y.; Wang, S.; Hu, S. A Study of Organic Acid Production in Contrasts between Two Phosphate Solubilizing Fungi: Penicillium oxalicum and Aspergillus niger. Sci. Rep. 2016, 6, 25313. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Z.; Petropoulos, E.; Ma, Y.; Shen, Y. Humidity Governs the Wall-Inhabiting Fungal Community Composition in a 1600-Year Tomb of Emperor Yang. Sci. Rep. 2020, 10, 8421. [Google Scholar] [CrossRef]
- Unković, N.; Ljaljević Grbić, M.; Subakov-Simić, G.; Stupar, M.; Vukojević, J.; Jelikić, A.; Stanojević, D. Biodeteriogenic and Toxigenic Agents on 17th Century Mural Paintings and Façade of the Old Church of the Holy Ascension (Veliki Krčimir, Serbia). Indoor Built Environ. 2016, 25, 826–837. [Google Scholar] [CrossRef]
- Guglielminetti, M.; De Giuli Morghen, C.; Radaelli, A.; Bistoni, F.; Carruba, G.; Spera, G.; Caretta, G. Mycological and Ultrastructural Studies to Evaluate Biodeterioration of Mural Paintings. Detection of Fungi and Mites in Frescos of the Monastery of St Damian in Assisi. Int. Biodeterior. Biodegrad. 1994, 33, 269–283. [Google Scholar] [CrossRef]
- Berner, M.; Wanner, G.; Lubitz, W. A Comparative Study of the Fungal Flora Present in Medieval Wall Paintings in the Chapel of the Castle Herberstein and in the Parish Church of St Georgen in Styria, Austria. Int. Biodeterior. Biodegrad. 1997, 40, 53–61. [Google Scholar] [CrossRef]
- Vasanthakumar, A.; DeAraujo, A.; Mazurek, J.; Schilling, M.; Mitchell, R. Microbiological Survey for Analysis of the Brown Spots on the Walls of the Tomb of King Tutankhamun. Int. Biodeterior. Biodegrad. 2013, 79, 56–63. [Google Scholar] [CrossRef]
- Caneva, G.; Isola, D.; Lee, H.J.; Chung, Y.J. Biological Risk for Hypogea: Shared Data from Etruscan Tombs in Italy and Ancient Tombs of the Baekje Dynasty in Republic of Korea. Appl. Sci. 2020, 10, 6104. [Google Scholar] [CrossRef]
- Dupont, J.; Jacquet, C.; Dennetière, B.; Lacoste, S.; Bousta, F.; Orial, G.; Cruaud, C.; Couloux, A.; Roquebert, M.-F. Invasion of the French Paleolithic Painted Cave of Lascaux by Members of the Fusarium solani Species Complex. Mycologia 2007, 99, 526–533. [Google Scholar] [CrossRef]
- Jurado, V.; Sanchez-Moral, S.; Saiz-Jimenez, C. Entomogenous Fungi and the Conservation of the Cultural Heritage: A Review. Int. Biodeterior. Biodegrad. 2008, 62, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Ionita, I. Contributions to the Study of the Biodeterioration of the Work of Art and of Historic Monuments. II. Species of Fungi Involved in the Deterioration of Mural Paintings from the Monasteries of Moldavia. Rev. Roum. Biol. Série Bot. 1973, 18, 179–189. [Google Scholar]
- Rebrikova, N.L. Micromycetes Taking Part in Deterioration of Old Russian Wall Paintings. In Recent Advances in Biodeterioration and Biodegradation; Garg, K.L., Garg, N., Mujerki, K.G., Eds.; Naya Prokash: Calcutta, India, 1993; Volume 1, pp. 205–232. [Google Scholar]
- Saarela, M.; Alakomi, H.-L.; Suihko, M.-L.; Maunuksela, L.; Raaska, L.; Mattila-Sandholm, T. Heterotrophic Microorganisms in Air and Biofilm Samples from Roman Catacombs, with Special Emphasis on Actinobacteria and Fungi. Int. Biodeterior. Biodegrad. 2004, 54, 27–37. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, H.; Du, Y.; Tian, T.; Xiang, T.; Liu, X.; Wu, F.; An, L.; Wang, W.; Gu, J.-D.; et al. The Community Distribution of Bacteria and Fungi on Ancient Wall Paintings of the Mogao Grottoes. Sci. Rep. 2015, 5, 7752. [Google Scholar] [CrossRef] [PubMed]
- Pangallo, D.; Kraková, L.; Chovanová, K.; Šimonovičová, A.; De Leo, F.; Urzì, C. Analysis and Comparison of the Microflora Isolated from Fresco Surface and from Surrounding Air Environment through Molecular and Biodegradative Assays. World J. Microbiol. Biotechnol. 2012, 28, 2015–2027. [Google Scholar] [CrossRef]
- Nugari, M.P.; Realini, M.; Roccardi, A. Contamination of Mural Paintings by Indoor Airborne Fungal Spores. Aerobiologia 1993, 9, 131–139. [Google Scholar] [CrossRef]
- Milanesi, C.; Baldi, F.; Borin, S.; Brusetti, L.; Ciampolini, F.; Iacopini, F.; Cresti, M. Deterioration of Medieval Painting in the Chapel of the Holy Nail, Siena (Italy) Partially Treated with Paraloid B72. Int. Biodeterior. Biodegrad. 2009, 63, 844–850. [Google Scholar] [CrossRef]
- Trovão, J.; Tiago, I.; Catarino, L.; Gil, F.; Portugal, A. In Vitro Analyses of Fungi and Dolomitic Limestone Interactions: Bioreceptivity and Biodeterioration Assessment. Int. Biodeterior. Biodegrad. 2020, 155, 105107. [Google Scholar] [CrossRef]
- Hu, H.; Ding, S.; Katayama, Y.; Kusumi, A.; Li, S.X.; de Vries, R.P.; Wang, J.; Yu, X.-Z.; Gu, J.-D. Occurrence of Aspergillus allahabadii on Sandstone at Bayon Temple, Angkor Thom, Cambodia. Int. Biodeterior. Biodegrad. 2013, 76, 112–117. [Google Scholar] [CrossRef]
- Li, C. Biodeterioration of Acrylic Polymers Paraloid B-72 and B-44: Report on Field Trials. AAS 2012, 15, 283–290. Available online: http://www.jiaa-kaman.org/pdfs/aas_15/AAS_15_Li_C_pp_283_290.pdf (accessed on 19 June 2022).
- Shivani, D.; Kumar, J.S. Extracellular Enzymatic Profile of Fungal Deteriogens of Historical Palace of Ujjain. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 122–132. [Google Scholar]
- Rojas, T.I.; Aira, M.J.; Batista, A.; Cruz, I.L.; González, S. Fungal Biodeterioration in Historic Buildings of Havana (Cuba). Grana 2012, 51, 44–51. [Google Scholar] [CrossRef]
- Bellucci, R.; Cremonesi, P.; Pignagnoli, G. A Preliminary Note on the Use of Enzymes in Conservation: The Removal of Aged Acrylic Resin Coatings with Lipase. Stud. Conserv. 1999, 44, 278–281. [Google Scholar] [CrossRef]
- Cappitelli, F.; Vicini, S.; Piaggio, P.; Abbruscato, P.; Princi, E.; Casadevall, A.; Nosanchuk, J.D.; Zanardini, E. Investigation of Fungal Deterioration of Synthetic Paint Binders Using Vibrational Spectroscopic Techniques. Macromol. Biosci. 2005, 5, 49–57. [Google Scholar] [CrossRef]
- Duan, Y.; Wu, F.; Wang, W.; Gu, J.-D.; Li, Y.; Feng, H.; Chen, T.; Liu, G.; An, L. Differences of Microbial Community on the Wall Paintings Preserved in Situ and Ex Situ of the Tiantishan Grottoes, China. Int. Biodeterior. Biodegrad. 2018, 132, 102–113. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).