Inorganic–Organic Hybrid Electrolytes Based on Al-Doped Li7La3Zr2O12 and Ionic Liquids
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
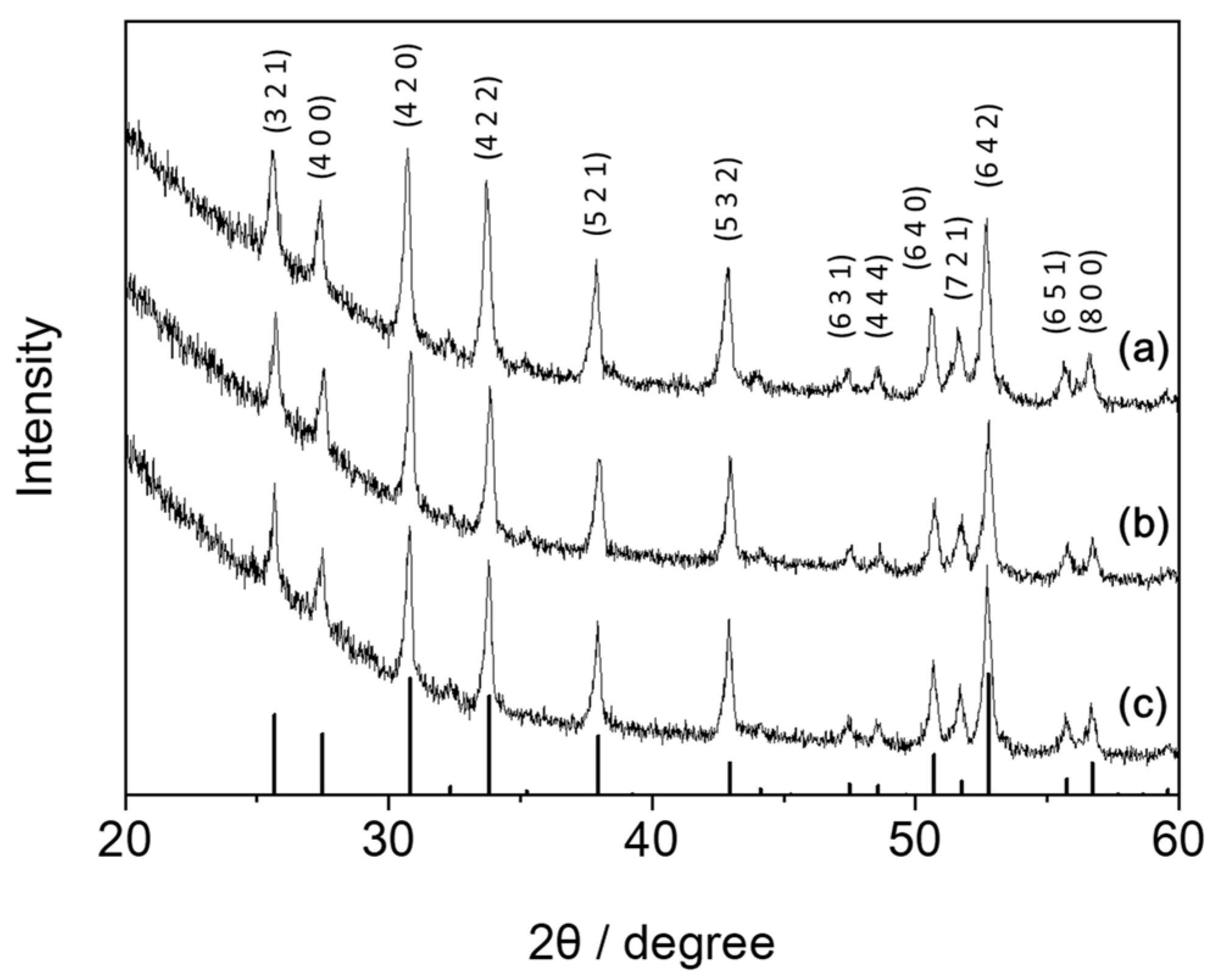
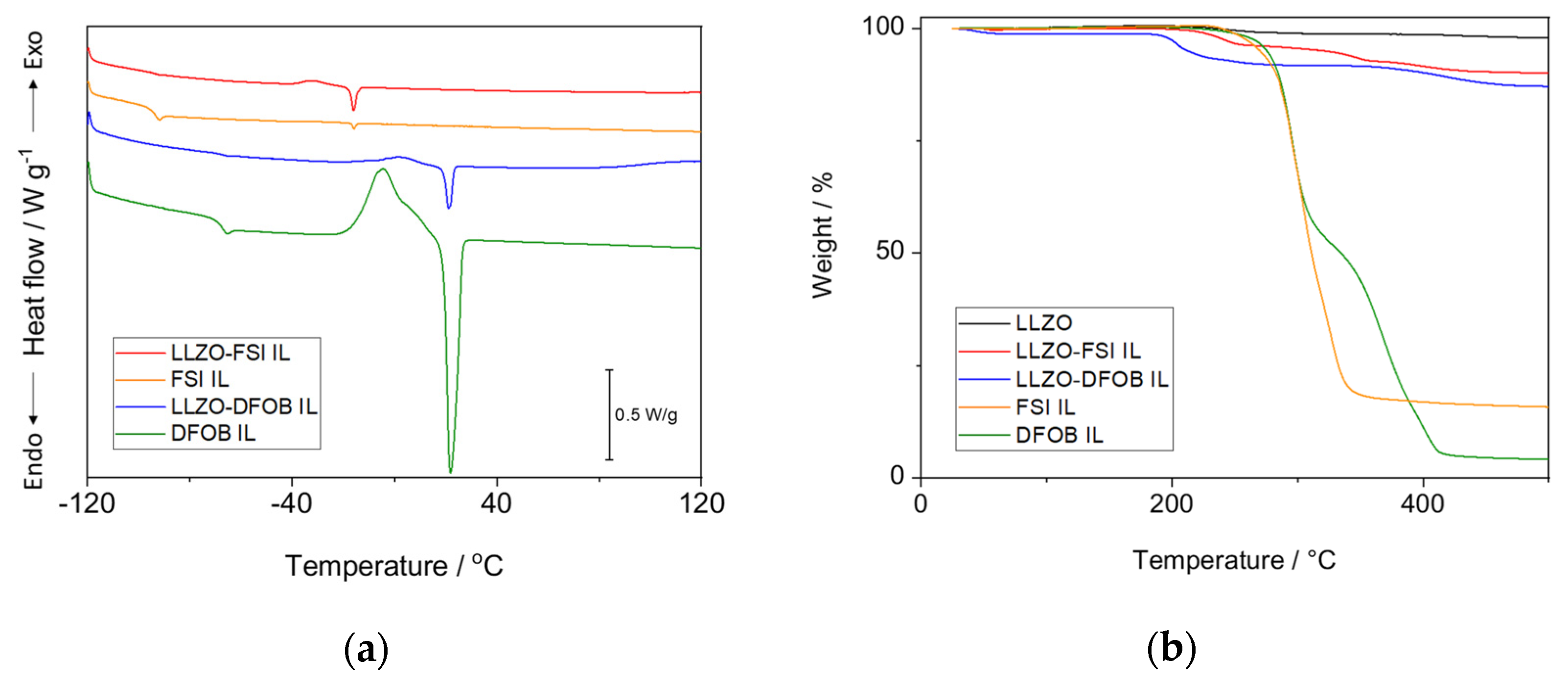
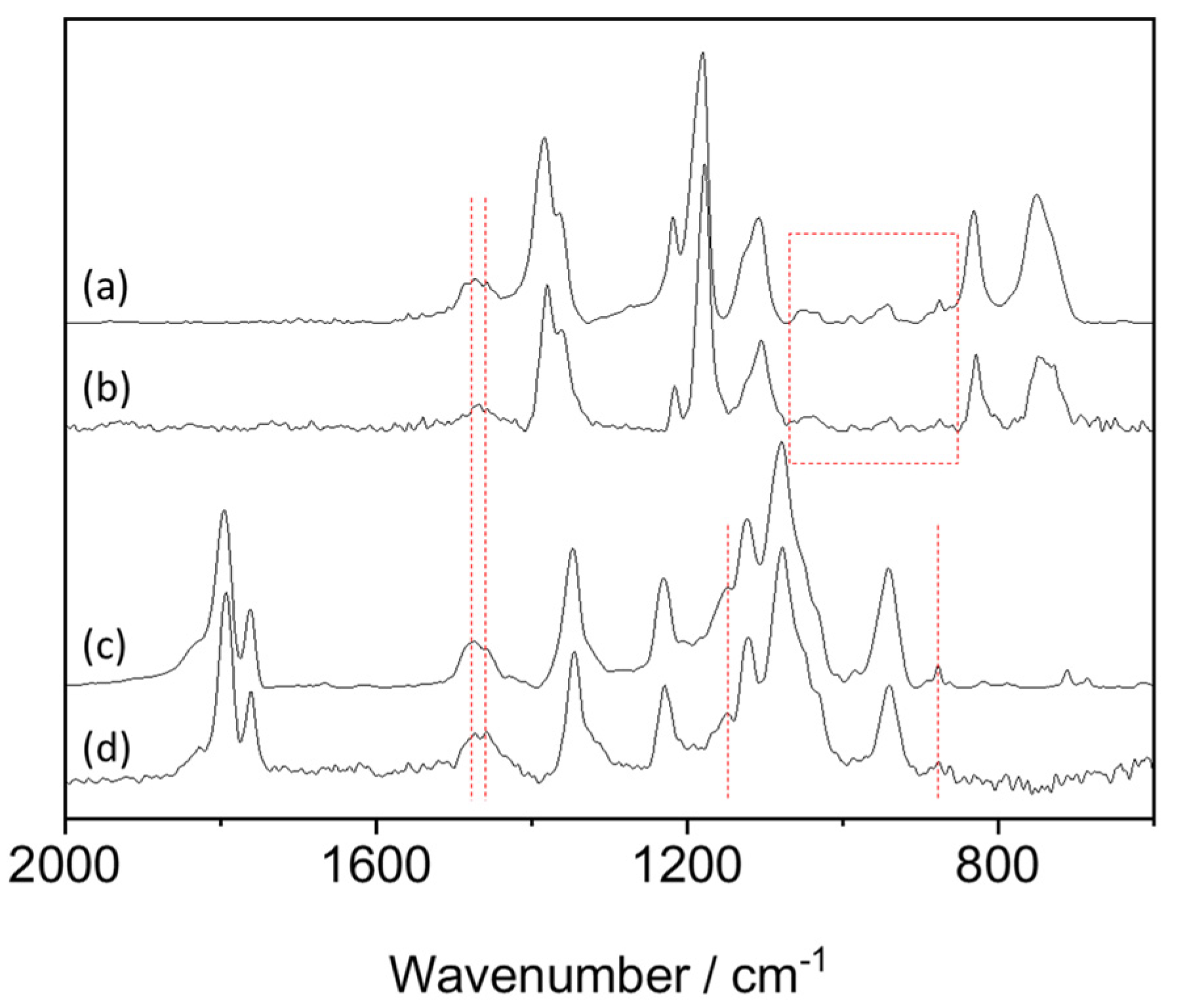
3.1. Fundamental Properties of LLZO–IL Composites
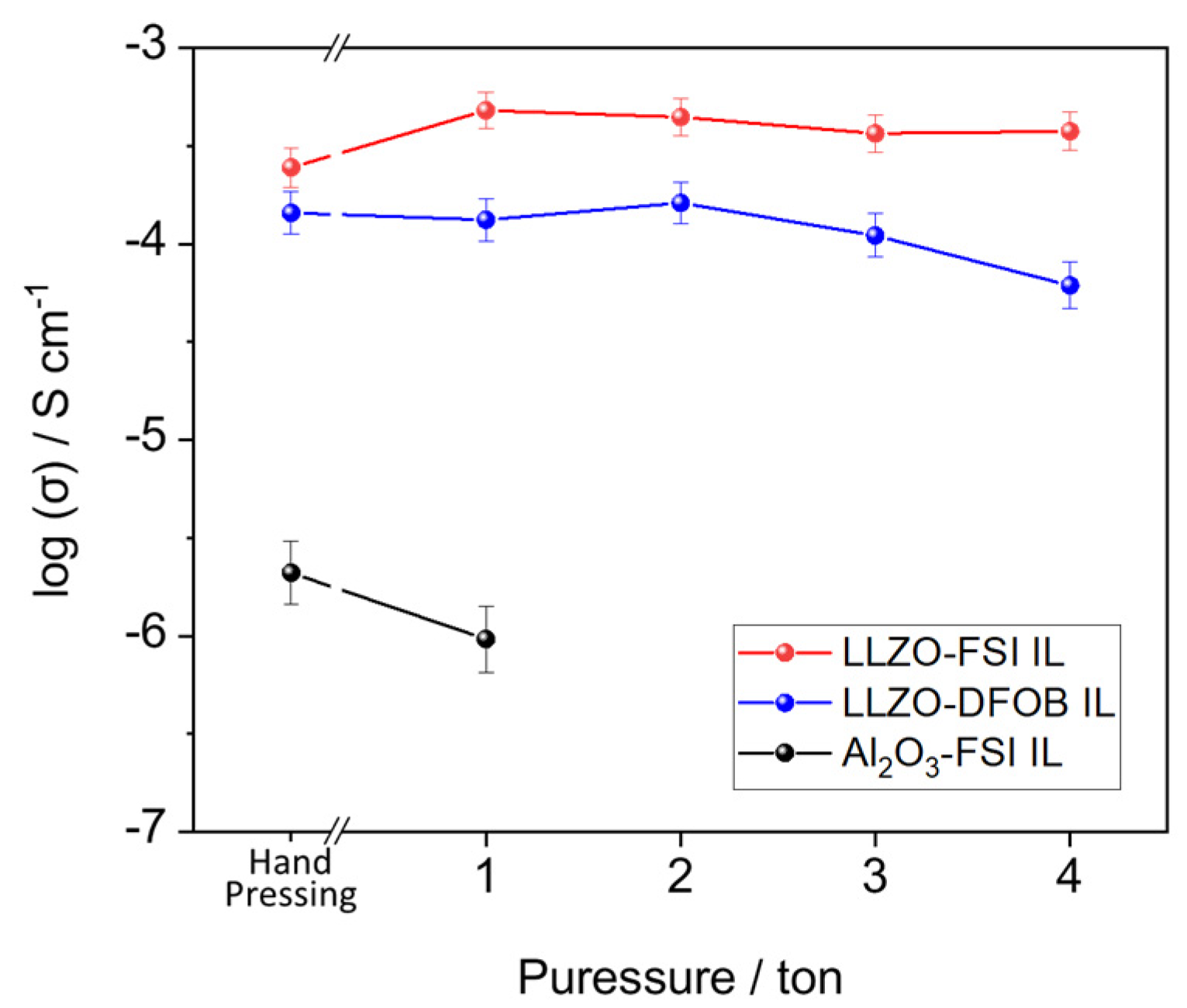
3.2. Ionic Conductivity of LLZO–IL Composites
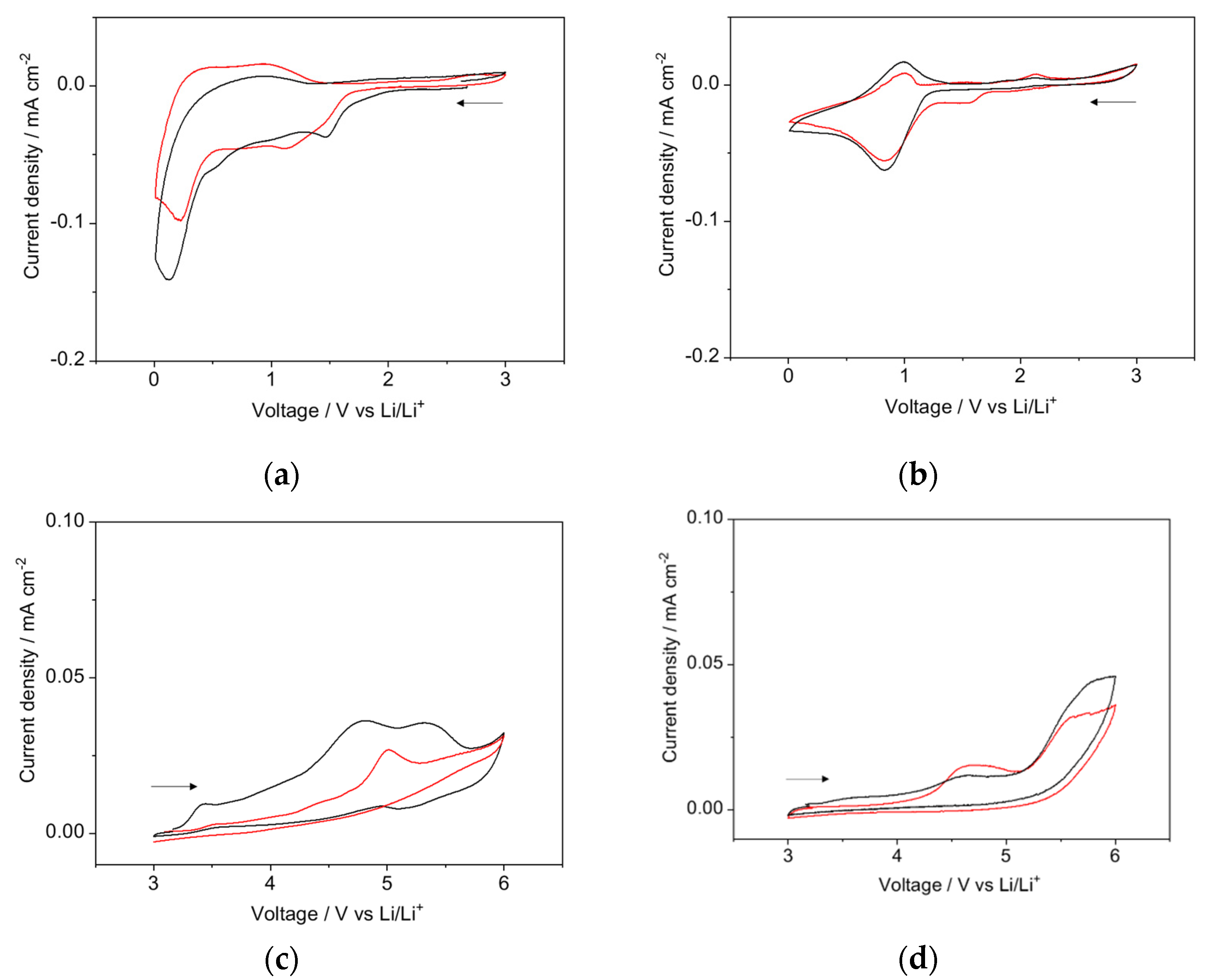
3.3. Electrochemical Stability of LLZO–IL Composites
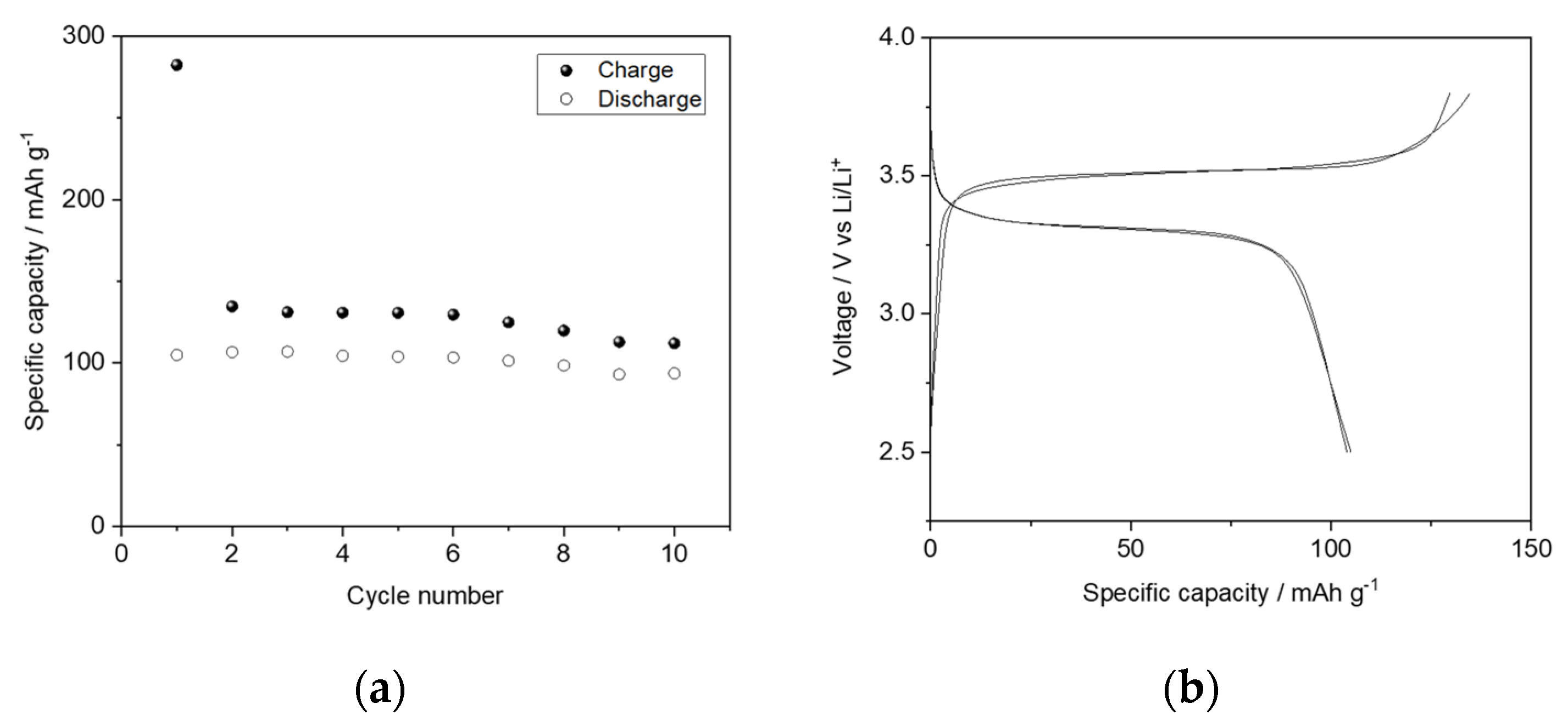
3.4. Performance of Battery with LLZO–IL Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Wen, K.; Chen, T.; Zhang, X.; Armand, M.; Chen, S. Recent progress in all-solid-state lithium batteries: The emerging strategies for advanced electrolytes and their interfaces. Energy Storage Mater. 2020, 31, 401–433. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, H.; Fu, L.; Ye, F.; Zhang, Y.; Luo, W.; Huang, Y. Promises, Challenges, and Recent Progress of Inorganic Solid-State Electrolytes for All-Solid-State Lithium Batteries. Adv. Mater. 2018, 30, e1705702. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Xue, C.; Hu, B.; Xu, B.; Li, L.; Nan, C.-W. Oxygen- and dendrite-resistant ultra-dry polymer electrolytes for solid-state Li–O2 batteries. Energy Storage Mater. 2020, 27, 244–251. [Google Scholar] [CrossRef]
- Zhu, H.; MacFarlane, D.R.; Pringle, J.M.; Forsyth, M. Organic Ionic Plastic Crystals as Solid-State Electrolytes. Trends Chem. 2019, 1, 126–140. [Google Scholar] [CrossRef] [Green Version]
- Famprikis, T.; Canepa, P.; Dawson, J.A.; Islam, M.S.; Masquelier, C. Fundamentals of inorganic solid-state electrolytes for batteries. Nat. Mater. 2019, 18, 1278–1291. [Google Scholar] [CrossRef]
- Betz, J.; Bieker, G.; Meister, P.; Placke, T.; Winter, M.; Schmuch, R. Theoretical versus Practical Energy: A Plea for More Transparency in the Energy Calculation of Different Rechargeable Battery Systems. Adv. Energy Mater. 2018, 9, 1803170. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Li, H.; Chen, L.; Wu, F. Progress in thermal stability of all-solid-state-Li-ion-batteries. InfoMat 2021, 3, 827–853. [Google Scholar] [CrossRef]
- Nam, Y.J.; Oh, D.Y.; Jung, S.H.; Jung, Y.S. Toward practical all-solid-state lithium-ion batteries with high energy density and safety: Comparative study for electrodes fabricated by dry- and slurry-mixing processes. J. Power Sources 2018, 375, 93–101. [Google Scholar] [CrossRef]
- Viallet, V.; Seznec, V.; Hayashi, A.; Tatsumisago, M.; Pradel, A. Glasses and Glass-Ceramics for Solid-State Battery Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1697–1754. [Google Scholar] [CrossRef]
- Chen, X.; Guan, Z.; Chu, F.; Xue, Z.; Wu, F.; Yu, Y. Air-stable inorganic solid-state electrolytes for high energy density lithium batteries: Challenges, strategies, and prospects. InfoMat 2021, 4, e12248. [Google Scholar] [CrossRef]
- Xu, Q.; Tsai, C.-L.; Song, D.; Basak, S.; Kungl, H.; Tempel, H.; Hausen, F.; Yu, S.; Eichel, R.-A. Insights into the reactive sintering and separated specific grain/grain boundary conductivities of Li1.3Al0.3Ti1.7(PO4)3. J. Power Sources 2021, 492, 229631. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, Z.; Ganapathy, S.; Wang, C.; Haverkate, L.A.; Tułodziecki, M.; Unnikrishnan, S.; Wagemaker, M. Tandem Interface and Bulk Li-Ion Transport in a Hybrid Solid Electrolyte with Microsized Active Filler. ACS Energy Lett. 2019, 4, 2336–2342. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.-Z.; He, H.; Nan, C.-W. Tailoring inorganic–polymer composites for the mass production of solid-state batteries. Nat. Rev. Mater. 2021, 6, 1003–1019. [Google Scholar] [CrossRef]
- de la Torre-Gamarra, C.; Appetecchi, G.B.; Ulissi, U.; Varzi, A.; Varez, A.; Passerini, S. Na3Si2Y0.16Zr1.84PO12-ionic liquid hybrid electrolytes: An approach for realizing solid-state sodium-ion batteries? J. Power Sources 2018, 383, 157–163. [Google Scholar] [CrossRef]
- Kim, H.W.; Manikandan, P.; Lim, Y.J.; Kim, J.H.; Nam, S.C.; Kim, Y. Hybrid solid electrolyte with the combination of Li7La3Zr2O12 ceramic and ionic liquid for high voltage pseudo-solid-state Li-ion batteries. J. Mater. Chem. A 2016, 4, 17025–17032. [Google Scholar] [CrossRef]
- Kim, K.; Park, J.; Jeong, G.; Yu, J.S.; Kim, Y.C.; Park, M.S.; Cho, W.; Kanno, R. Rational Design of a Composite Electrode to Realize a High-Performance All-Solid-State Battery. ChemSusChem 2019, 12, 2637–2643. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef]
- Pervez, S.A.; Kim, G.; Vinayan, B.P.; Cambaz, M.A.; Kuenzel, M.; Hekmatfar, M.; Fichtner, M.; Passerini, S. Overcoming the Interfacial Limitations Imposed by the Solid-Solid Interface in Solid-State Batteries Using Ionic Liquid-Based Interlayers. Small 2020, 16, e2000279. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhang, L.; Liu, Y.; Wang, H.; Yu, C.; Zeng, H.; Wang, L.M.; Xu, B. Interface-Engineered Li7La3Zr2O12-Based Garnet Solid Electrolytes with Suppressed Li-Dendrite Formation and Enhanced Electrochemical Performance. ChemSusChem 2018, 11, 3774–3782. [Google Scholar] [CrossRef] [Green Version]
- Xiong, S.; Liu, Y.; Jankowski, P.; Liu, Q.; Nitze, F.; Xie, K.; Song, J.; Matic, A. Design of a Multifunctional Interlayer for NASCION-Based Solid-State Li Metal Batteries. Adv. Funct. Mater. 2020, 30, 2001444. [Google Scholar] [CrossRef] [Green Version]
- Navarra, M.A.; Fujimura, K.; Sgambetterra, M.; Tsurumaki, A.; Panero, S.; Nakamura, N.; Ohno, H.; Scrosati, B. New Ether-functionalized Morpholinium- and Piperidinium-based Ionic Liquids as Electrolyte Components in Lithium and Lithium-Ion Batteries. ChemSusChem 2017, 10, 2496–2504. [Google Scholar] [CrossRef]
- Kerr, R.; Mazouzi, D.; Eftekharnia, M.; Lestriez, B.; Dupré, N.; Forsyth, M.; Guyomard, D.; Howlett, P.C. High-Capacity Retention of Si Anodes Using a Mixed Lithium/Phosphonium Bis(fluorosulfonyl)imide Ionic Liquid Electrolyte. ACS Energy Lett. 2017, 2, 1804–1809. [Google Scholar] [CrossRef]
- Tsurumaki, A.; Branchi, M.; Rigano, A.; Poiana, R.; Panero, S.; Navarra, M.A. Bis(oxalato)borate and difluoro(oxalato)borate-based ionic liquids as electrolyte additives to improve the capacity retention in high voltage lithium batteries. Electrochim. Acta 2019, 315, 17–23. [Google Scholar] [CrossRef]
- Tsurumaki, A.; Ohno, H.; Panero, S.; Navarra, M.A. Novel bis(fluorosulfonyl)imide-based and ether-functionalized ionic liquids for lithium batteries with improved cycling properties. Electrochim. Acta 2019, 293, 160–165. [Google Scholar] [CrossRef]
- Moreno, J.S.; Deguchi, Y.; Panero, S.; Scrosati, B.; Ohno, H.; Simonetti, E.; Appetecchi, G.B. N-Alkyl-N-ethylpyrrolidinium cation-based ionic liquid electrolytes for safer lithium battery systems. Electrochim. Acta 2016, 191, 624–630. [Google Scholar] [CrossRef]
- Montanino, M.; Alessandrini, F.; Passerini, S.; Appetecchi, G.B. Water-based synthesis of hydrophobic ionic liquids for high-energy electrochemical devices. Electrochim. Acta 2013, 96, 124–133. [Google Scholar] [CrossRef]
- Buschmann, H.; Dölle, J.; Berendts, S.; Kuhn, A.; Bottke, P.; Wilkening, M.; Heitjans, P.; Senyshyn, A.; Ehrenberg, H.; Lotnyk, A.; et al. Structure and dynamics of the fast lithium ion conductor “Li7La3Zr2O12”. Phys. Chem. Chem. Phys. 2011, 13, 19378–19392. [Google Scholar] [CrossRef] [Green Version]
- Shin, R.-H.; Son, S.I.; Han, Y.S.; Kim, Y.D.; Kim, H.-T.; Ryu, S.-S.; Pan, W. Sintering behavior of garnet-type Li7La3Zr2O12-Li3BO3 composite solid electrolytes for all-solid-state lithium batteries. Solid State Ion. 2017, 301, 10–14. [Google Scholar] [CrossRef]
- Yang, S.H.; Kim, M.Y.; Kim, D.H.; Jung, H.Y.; Ryu, H.M.; Han, J.H.; Lee, M.S.; Kim, H.-S. Ionic conductivity of Ga-doped LLZO prepared using Couette–Taylor reactor for all-solid lithium batteries. J. Ind. Eng. Chem. 2017, 56, 422–427. [Google Scholar] [CrossRef]
- Mekhemer, G.A.H.; Balboul, B.A.A. Thermal genesis course and characterization of lanthanum oxide. Colloids Surf. A Physicochem. Eng. Asp. 2001, 181, 19–29. [Google Scholar] [CrossRef]
- Palumbo, O.; Trequattrini, F.; Cimini, A.; Tsurumaki, A.; Navarra, M.A.; Paolone, A. Inter- and Intramolecular Interactions in Ether-Functionalized Ionic Liquids. J. Phys. Chem. B 2021, 125, 2380–2388. [Google Scholar] [CrossRef]
- Li, L.; Zhou, S.; Han, H.; Li, H.; Nie, J.; Armand, M.; Zhou, Z.; Huang, X. Transport and Electrochemical Properties and Spectral Features of Non-Aqueous Electrolytes Containing LiFSI in Linear Carbonate Solvents. J. Electrochem. Soc. 2011, 158, A74. [Google Scholar] [CrossRef]
- Tsurumaki, A.; Trequattrini, F.; Palumbo, O.; Panero, S.; Paolone, A.; Navarra, M.A. The effect of ether-functionalisation in ionic liquids analysed by DFT calculation, infrared spectra, and Kamlet-Taft parameters. Phys. Chem. Chem. Phys. 2018, 20, 7989–7997. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.L.; McOwen, D.W.; Delp, S.A.; Fox, E.T.; Dickmann, J.S.; Han, S.-D.; Zhou, Z.-B.; Jow, T.R.; Henderson, W.A. N-Alkyl-N-methylpyrrolidinium difluoro(oxalato)borate ionic liquids: Physical/electrochemical properties and Al corrosion. J. Power Sources 2013, 237, 104–111. [Google Scholar] [CrossRef]


| SE | Additive | SE:Additive Ratio in Weight | Mixing Procedure | Pressure for Pellet | Conductivity (S/cm) | Ref. | |
|---|---|---|---|---|---|---|---|
| r.t. | 60 °C | ||||||
| Li6.24La3Zr2Al0.24O11.98 | FSI IL | 85:15 | mortar mixing | 1 ton | 4.8 × 10−4 c | 2.1 × 10−3 | - d |
| Li6.24La3Zr2Al0.24O11.98 | DFOB IL | 85:15 | mortar mixing | 1 ton | 1.9 × 10−4 c | 1.0 × 10−3 | - d |
| Li7La3Zr2O12 | [Py14][TFSI]/LiTFSI a (19:1 wt/wt) | 80:20 | ball milling (40 rpm/1 h) | 2 ton | 4.0 × 10−4 | 1.6 × 10−3 | [15] |
| Li6.75La3Zr1.75Ta0.25O12 | [Py14][TFSI] a | 86:14 | mortar mixing | 15 MPa | 6.7 × 10−4 | ~3 × 10−3 | [19] |
| Li1.5Al0.5Ge1.5(PO4)3 | [bmim][FSI]/LiFSI b (9/1) | 50:50 | ball milling (200 rpm/0.5 h) | - | ~2 × 10−3 | ~5 × 10−3 | [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsurumaki, A.; Rettaroli, R.; Mazzapioda, L.; Navarra, M.A. Inorganic–Organic Hybrid Electrolytes Based on Al-Doped Li7La3Zr2O12 and Ionic Liquids. Appl. Sci. 2022, 12, 7318. https://doi.org/10.3390/app12147318
Tsurumaki A, Rettaroli R, Mazzapioda L, Navarra MA. Inorganic–Organic Hybrid Electrolytes Based on Al-Doped Li7La3Zr2O12 and Ionic Liquids. Applied Sciences. 2022; 12(14):7318. https://doi.org/10.3390/app12147318
Chicago/Turabian StyleTsurumaki, Akiko, Rossella Rettaroli, Lucia Mazzapioda, and Maria Assunta Navarra. 2022. "Inorganic–Organic Hybrid Electrolytes Based on Al-Doped Li7La3Zr2O12 and Ionic Liquids" Applied Sciences 12, no. 14: 7318. https://doi.org/10.3390/app12147318
APA StyleTsurumaki, A., Rettaroli, R., Mazzapioda, L., & Navarra, M. A. (2022). Inorganic–Organic Hybrid Electrolytes Based on Al-Doped Li7La3Zr2O12 and Ionic Liquids. Applied Sciences, 12(14), 7318. https://doi.org/10.3390/app12147318







