High-Intensity Low Frequency Pulsed Electromagnetic Fields Treatment Stimulates Fin Regeneration in Adult Zebrafish—A Preliminary Report
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
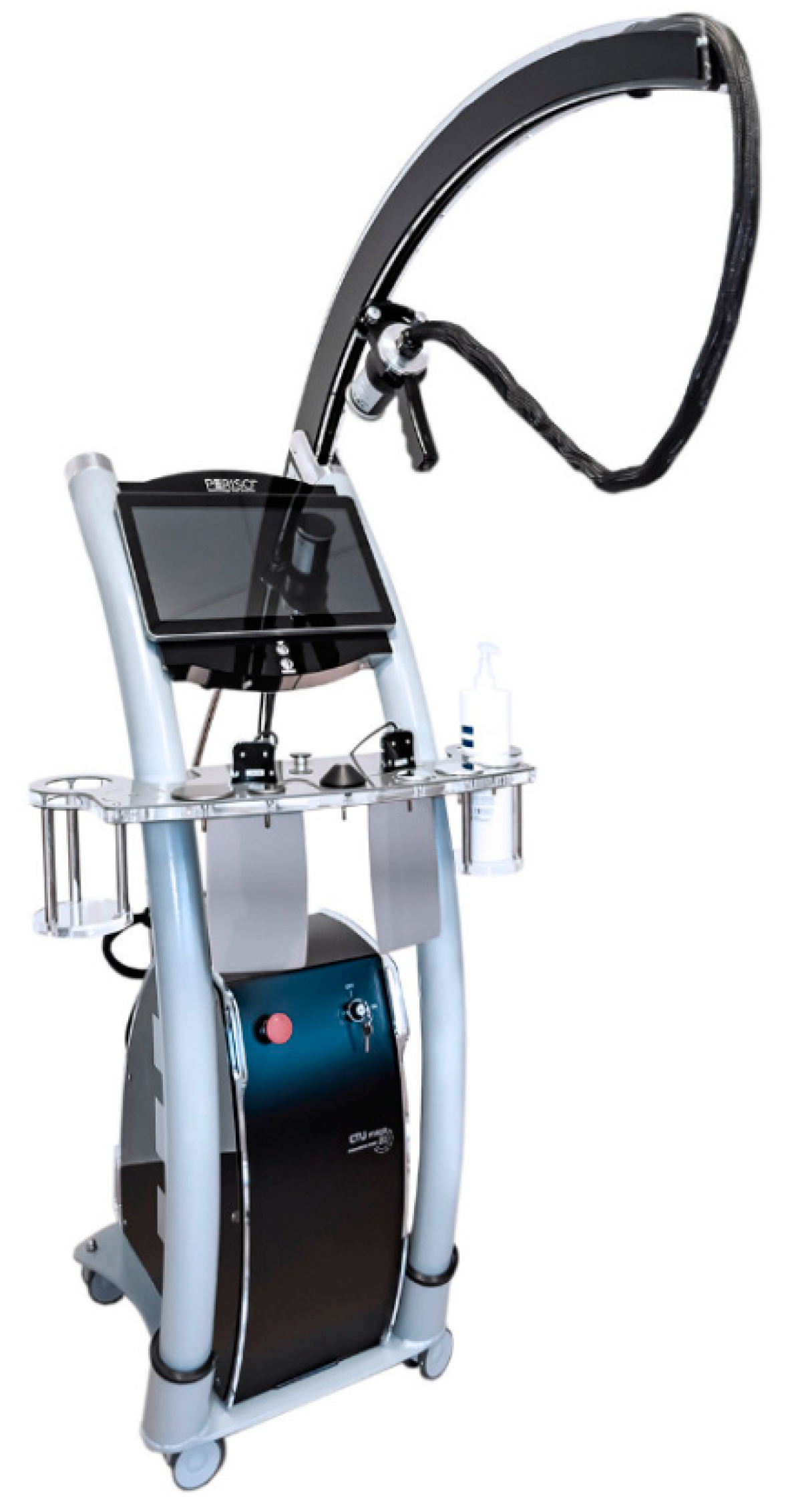
2.1. PEMF Generator
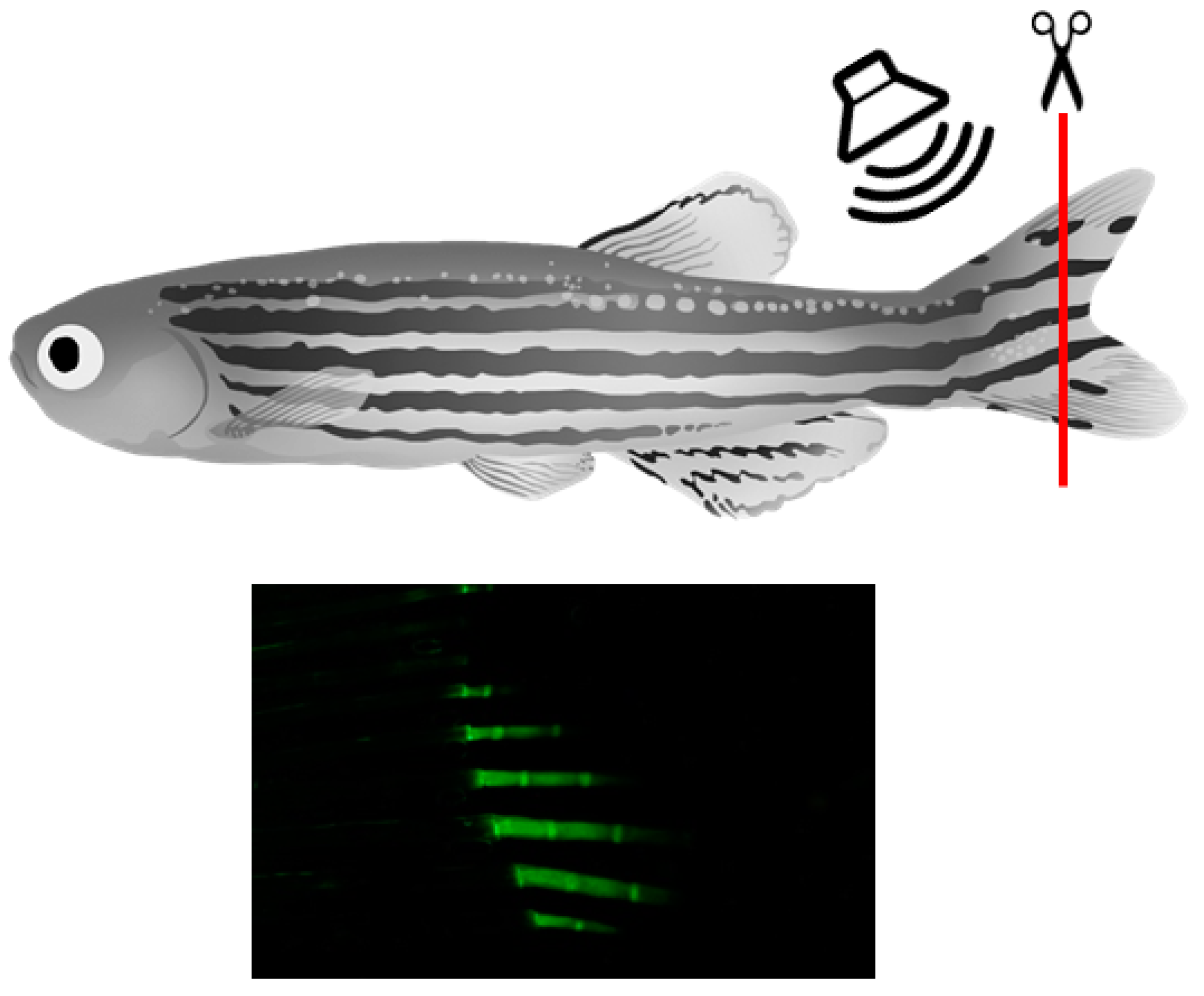
2.2. Animals and Treatments
2.3. Ethic Statement
2.4. Statistics
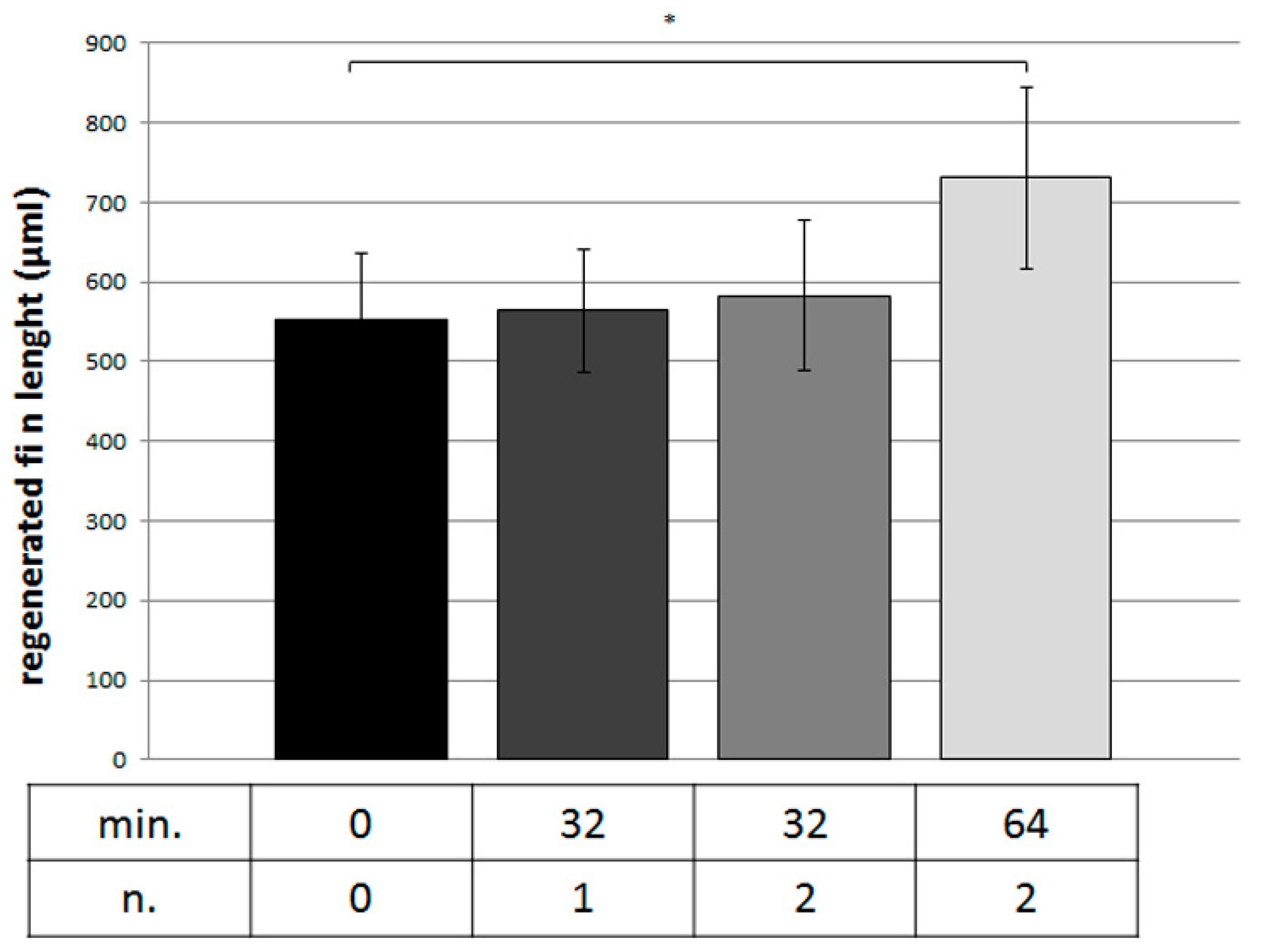
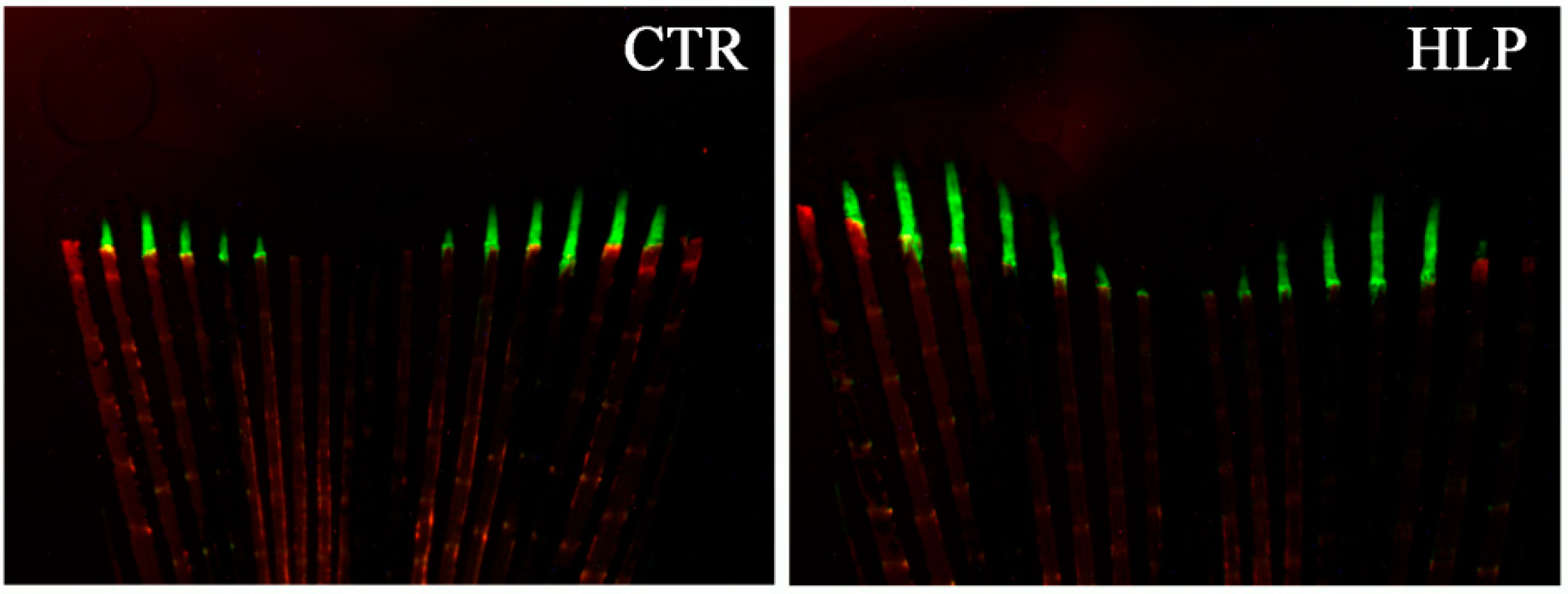
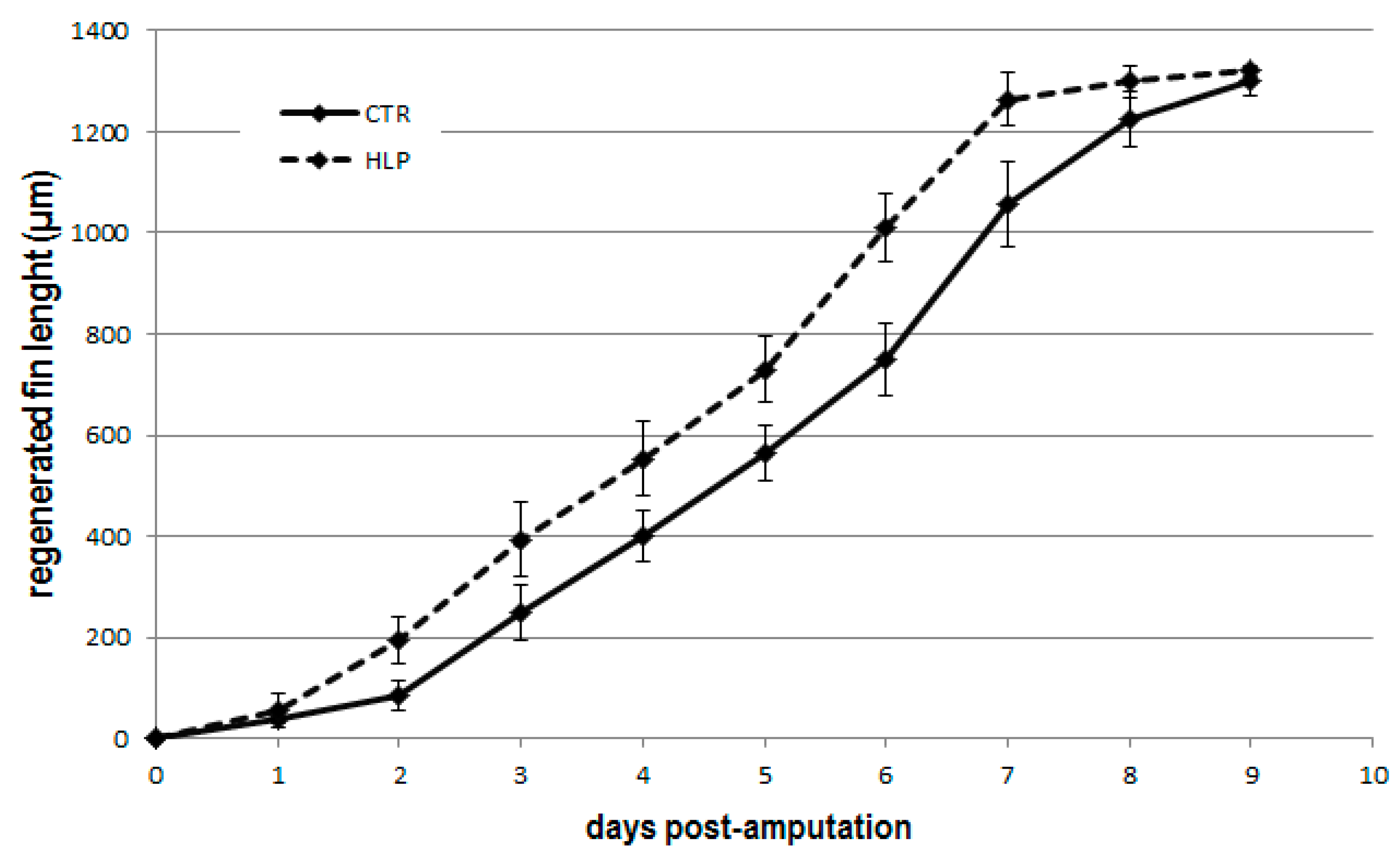
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bassett, C.A. Fundamental and practical aspects of therapeutic uses of pulsed electromagnetic fields (PEMFs). Crit. Rev. Biomed. Eng. 1989, 17, 451–529. [Google Scholar]
- Luben, R. Effects of low-energy electromagnetic fields (pulsed and DC) on membrane signal transduction processes in biological systems. Health Phys. Soc. 1991, 61, 15–28. [Google Scholar] [CrossRef]
- Ehnert, S.; Van Griensven, M.; Unger, M.; Scheffler, H.; Falldorf, K.; Fentz, A.K.; Seeliger, C.; Schröter, S.; Nussler, A.K.; Balmayor, E.R. Co-Culture with Human Osteoblasts and Exposure to Extremely Low Frequency Pulsed Electromagnetic Fields Improve Osteogenic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2018, 19, 994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, W.H.; Chen, L.; Sun, J.; Lin, F. Effect of pulse-burst electromagnetic field stimulation on osteoblast cell activities. Bioelectromagnetics 2004, 25, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Ehnert, S.; Schröter, S.; Aspera-Werz, R.H.; Eisler, W.; Falldorf, K.; Ronniger, M.; Nussler, A.K. Translational Insights into Extremely Low Frequency Pulsed Electromagnetic Fields (ELF-PEMFs) for Bone Regeneration after Trauma and Orthopedic Surgery. J. Clin. Med. 2019, 8, 2028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.; Xin, F.; Jiang, W. Underlying Signaling Pathways and Therapeutic Applications of Pulsed Electromagnetic Fields in Bone Repair. Cell Physiol. Biochem. 2018, 46, 1581–1594. [Google Scholar] [CrossRef] [PubMed]
- Novickij, V.; Grainys, A.; Kučinskaite-Kodze, I.; Žvirbliene, A.; Novickij, J. Magneto-permeabilization of viable cell membrane using high pulsed magnetic field. IEEE Trans. Magn. 2015, 51, 5000505. [Google Scholar] [CrossRef]
- Kranjc, S.; Kranjc, M.; Scancar, J.; Jelenc, J.; Sersa, G.; Miklavcic, D. Electrochemotherapy by pulsed electromagnetic field treatment (PEMF) in mouse melanoma B16F10 in vivo. Radiol. Oncol. 2016, 50, 39–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miklavcic, D.; Novickij, V.; Kranjc, M.; Polajzer, T.; Meglic, S.H.; Napotnik, T.B.; Romih, R.; Lisjak, D. Contactless electroporation induced by high intensity pulsed electromagnetic fields via distributed nanoelectrodes. Bioelectrochemistry 2020, 132, 107440. [Google Scholar] [CrossRef]
- Novickij, V.; Dermol, J.; Grainys, A.; Kranjc, M.; Miklavčič, D. Membrane permeabilization of mammalian cells using bursts of high magnetic field pulses. PeerJ 2017, 4, e3267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Towhidi, L.; Firoozabadi, S.M.P.; Mozdarani, H.; Miklavcic, D. Lucifer Yellow uptake by CHO cells exposed to magnetic and electric pulses. Radiol. Oncol. 2012, 46, 119–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Premi, E.; Benussi, A.; La Gatta, A.; Visconti, S.; Costa, A.; Gilberti, N.; Cantoni, V.; Padovani, A.; Borroni, B.; Magoni, M. Modulation of long-term potentiation-like cortical plasticity in the healthy brain with low frequency-pulsed electromagnetic fields. BMC Neurosci. 2018, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Hammer, B.E.; Kidder, L.S.; Williams, P.C.; Xu, W.W. Magnetic Levitation of MC3T3 Osteoblast Cells as a Ground-Based Simulation of Microgravity. Microgravity Sci. Technol. 2009, 21, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Zhadin, M.N. Combined action of static and alternating magnetic fields on ion motion in a macromolecule: Theoretical aspects. Bioelectromagnetics 1998, 19, 279–292. [Google Scholar] [CrossRef]
- Zablotskii, V.; Polyakova, T.; Lunov, O.; Dejneka, A. How a High-Gradient Magnetic Field Could Affect Cell Life. Sci. Rep. 2017, 6, 37407. [Google Scholar] [CrossRef] [PubMed]
- Renn, J.; Winkler, C.; Schartl, M.; Fischer, R.; Goerlich, R. Zebrafish and medaka as models for bone research including implications regarding space-related issues. Protoplasma 2006, 229, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Uemoto, T.; Abe, G.; Tamura, K. Regrowth of zebrafish caudal fin regeneration is determined by the amputated length. Sci. Rep. 2020, 10, 649. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.M.; Lim, H.M.; Ro, H.S.; Ki, G.E.; Seo, Y.K. Pulsed Electromagnetic Fields Increase Pigmentation through the p-ERK/p-p38 Pathway in Zebrafish (Danio rerio). Int. J. Mol. Sci. 2018, 19, 3211. [Google Scholar] [CrossRef] [Green Version]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio), 5th ed.; University of Oregon Press: Eugene, OR, USA, 2007. [Google Scholar]
- Molagoda, I.M.N.; Karunarathne, W.A.H.M.; Choi, Y.H.; Park, E.K.; Jeon, Y.J.; Lee, B.J.; Kang, C.H.; Kim, G.Y. Fermented Oyster Extract Promotes Osteoblast Differentiation by Activating the Wnt/β-Catenin Signaling Pathway, Leading to Bone Formation. Biomolecules 2019, 9, 711. [Google Scholar] [CrossRef] [Green Version]
- Cadossi, R.; Massari, L.; Racine-Avila, J.; Aaron, R.K. Pulsed Electromagnetic Field Stimulation of Bone Healing and Joint Preservation: Cellular Mechanisms of Skeletal Response. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2020, 4, e1900155. [Google Scholar] [CrossRef]
- Lin, H.Y.; Lin, Y.J. In vitro effects of low frequency electromagnetic fields on osteoblast proliferation and maturation in an inflammatory environment. Bioelectromagnetics 2011, 32, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Loo, S.T.; Liu, H.L.; Fang, H.W.; Lin, H.Y. Can low frequency electromagnetic field help cartilage tissue engineering? J. Biomed. Mater. Res. A 2010, 92, 843–851. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnovali, M.; Stefanetti, N.; Galluzzo, A.; Romeo, P.; Mariotti, M.; Sansone, V. High-Intensity Low Frequency Pulsed Electromagnetic Fields Treatment Stimulates Fin Regeneration in Adult Zebrafish—A Preliminary Report. Appl. Sci. 2022, 12, 7768. https://doi.org/10.3390/app12157768
Carnovali M, Stefanetti N, Galluzzo A, Romeo P, Mariotti M, Sansone V. High-Intensity Low Frequency Pulsed Electromagnetic Fields Treatment Stimulates Fin Regeneration in Adult Zebrafish—A Preliminary Report. Applied Sciences. 2022; 12(15):7768. https://doi.org/10.3390/app12157768
Chicago/Turabian StyleCarnovali, Marta, Noemi Stefanetti, Alessandro Galluzzo, Pietro Romeo, Massimo Mariotti, and Valerio Sansone. 2022. "High-Intensity Low Frequency Pulsed Electromagnetic Fields Treatment Stimulates Fin Regeneration in Adult Zebrafish—A Preliminary Report" Applied Sciences 12, no. 15: 7768. https://doi.org/10.3390/app12157768
APA StyleCarnovali, M., Stefanetti, N., Galluzzo, A., Romeo, P., Mariotti, M., & Sansone, V. (2022). High-Intensity Low Frequency Pulsed Electromagnetic Fields Treatment Stimulates Fin Regeneration in Adult Zebrafish—A Preliminary Report. Applied Sciences, 12(15), 7768. https://doi.org/10.3390/app12157768






